
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adnan Iqbal | -- | 1071 | 2023-08-25 14:10:53 | | | |
| 2 | Dean Liu | Meta information modification | 1071 | 2023-08-28 03:59:38 | | |
Video Upload Options
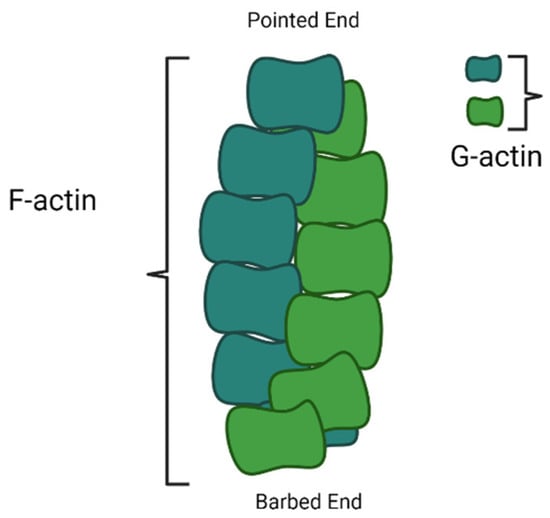
Cotton fiber development largely depends on cell wall biosynthesis and cytoskeleton arrangement. Cytoskeleton dynamics control many cellular processes, such as the movement of organelles, cell wall formation, and cell division. Microfilaments (actin-filament), microtubules, and intermediate filaments are the main constituents of the cytoskeleton. In most cells, actin filaments are involved in secretory vesicle transportation to the cell membrane and cell wall, enhancing cell expansion. The actin cytoskeleton also regulates tip growth and cell elongation. Dozens express actin proteins to hundreds of genes in the ACTIN family. Arabidopsis has 10 actin genes, of which 8 are functional, and 2 are categorized as pseudogenes, while cotton plants have been identified with 16 actin genes.
1. Actin Filament Development Pathway and Actin-Binding Proteins (ABPs)
2. Nucleation of Actin Filaments
3. Polymerization and Capping of Microfilament
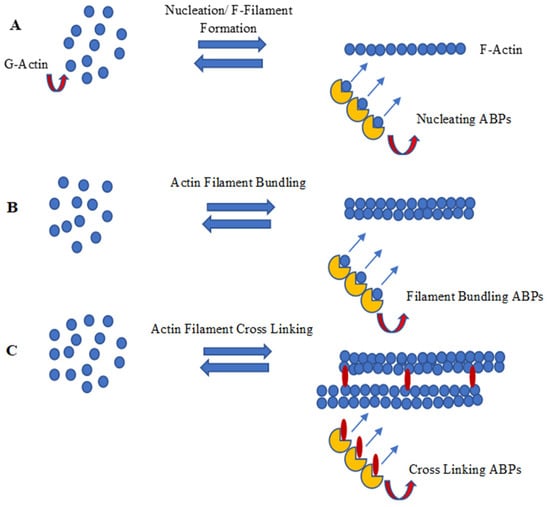
4. F-Actin Bundling and Cross Linking
5. Plant LIM, an Actin-Bundling Protein
References
- Komis, G.; Luptovciak, I.; Doskocilova, A.; Samaj, J. Biotechnological aspects of cytoskeletal regulation in plants. Biotechnol. Adv. 2015, 33, 1043–1062.
- Paavilainen, V.O.; Bertling, E.; Falck, S.; Lappalainen, P. Regulation of cytoskeletal dynamics by actin-monomer-binding proteins. Trends Cell Biol. 2004, 14, 386–394.
- Yanagisawa, M.; Zhang, C.; Szymanski, D.B. ARP2/3-dependent growth in the plant kingdom: SCARs for life. Front. Plant Sci. 2013, 4, 166.
- Winder, S.J.; Ayscough, K.R. Actin-binding proteins. J. Cell Sci. 2005, 118, 651–654.
- Goode, B.L.; Eck, M.J. Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem. 2007, 76, 593–627.
- Allingham, J.S.; Miles, C.O.; Rayment, I. A structural basis for regulation of actin polymerization by pectenotoxins. J. Mol. Biol. 2007, 371, 959–970.
- Winder, S.J.; Jess, T.; Ayscough, K.R. SCP1 encodes an actin-bundling protein in yeast. Biochem. J. 2003, 375, 287–295.
- Thomas, C.; Moreau, F.; Dieterle, M.; Hoffmann, C.; Gatti, S.; Hofmann, C.; Van Troys, M.; Ampe, C.; Steinmetz, A. The LIM domains of WLIM1 define a new class of actin bundling modules. J. Biol. Chem. 2007, 282, 33599–33608.
- Han, L.; Li, Y.; Sun, Y.; Wang, H.; Kong, Z.; Xia, G. The two domains of cotton WLIM1a protein are functionally divergent. Sci. China Life Sci. 2016, 59, 206–212.
- Zheng, Q.; Zhao, Y. The diverse biofunctions of LIM domain proteins: Determined by subcellular localization and protein—Protein interaction. Biol. Cell 2007, 99, 489–502.
- Li, L.; Li, Y.; Wang, N.N.; Li, Y.; Lu, R.; Li, X.B. Cotton LIM domain-containing protein Gh PLIM 1 is specifically expressed in anthers and participates in modulating F-actin. Plant Biol. 2015, 17, 528–534.
- Kadrmas, J.L.; Beckerle, M.C. The LIM domain: From the cytoskeleton to the nucleus. Nat. Rev. Mol. Cell Biol. 2004, 5, 920.
- Arnaud, D.; Dejardin, A.; Leple, J.C.; Lesage-Descauses, M.C.; Boizot, N.; Villar, M.; Benedetti, H.; Pilate, G. Expression analysis of LIM gene family in poplar, toward an updated phylogenetic classification. BMC Res. Notes 2012, 5, 102.
- Li, Y.; Jiang, J.; Li, L.; Wang, X.-L.; Wang, N.-N.; Li, D.-D.; Li, X.-B. A cotton LIM domain-containing protein (GhWLIM5) is involved in bundling actin filaments. Plant Physiol. Biochem. 2013, 66, 34–40.
- Han, L.-B.; Li, Y.-B.; Wang, H.-Y.; Wu, X.-M.; Li, C.-L.; Luo, M.; Wu, S.-J.; Kong, Z.-S.; Pei, Y.; Jiao, G.-L. The dual functions of WLIM1a in cell elongation and secondary wall formation in developing cotton fibers. Plant Cell 2013, 25, 4421–4439.




