Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ivan Šoša | -- | 1144 | 2023-08-24 07:48:55 | | | |
| 2 | Fanny Huang | Meta information modification | 1144 | 2023-08-28 07:05:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Šoša, I. Blood Ethanol Levels. Encyclopedia. Available online: https://encyclopedia.pub/entry/48407 (accessed on 09 February 2026).
Šoša I. Blood Ethanol Levels. Encyclopedia. Available at: https://encyclopedia.pub/entry/48407. Accessed February 09, 2026.
Šoša, Ivan. "Blood Ethanol Levels" Encyclopedia, https://encyclopedia.pub/entry/48407 (accessed February 09, 2026).
Šoša, I. (2023, August 24). Blood Ethanol Levels. In Encyclopedia. https://encyclopedia.pub/entry/48407
Šoša, Ivan. "Blood Ethanol Levels." Encyclopedia. Web. 24 August, 2023.
Copy Citation
In the interplay of the gut microbiota and endogenous ethanol production, the production of ethanol through the endogenous fermentation of carbohydrates is an inevitable result. “Alcohol-producing” processes may be succinctly abstracted to ethanol’s constant formation from acetaldehyde in the human body through various metabolic processes. In such cases where it is not introduced from the environment, it is called “endogenous ethanol”.
alcohol
bioreactor
fermentation
human body
blood
1. Introduction
Bioreactors are devices or systems maintaining a biologically active environment in which a chemical process that involves organisms or biochemically active substances is carried out [1]. Even though there is enough evidence to support the concept of auto-brewery syndrome (ABS; sometimes referred to as gut fermentation syndrome, endogenous ethanol fermentation, or drunkenness disease), parties in the legal process do not employ this strategy frequently [2][3][4][5]. In the interplay of the gut microbiota and endogenous ethanol production, the production of ethanol through the endogenous fermentation of carbohydrates is an inevitable result [6][7][8][9]. Even though ABS would seem to be a rarely diagnosed condition, legal experts should be aware that it exists and that it could require a different legal treatment. Greater attention should be paid to this entity after a report of the production of alcohol in a rather atypical part of the digestive system, i.e., the oral cavity [10].
Unexplained intoxication symptoms—such as disorientation, dizziness, and ataxia—are common presentations of ABS. Clearly, alcohol consumption must be excluded.
“Alcohol-producing” processes may be succinctly abstracted to ethanol’s constant formation from acetaldehyde in the human body through various metabolic processes [11]. In such cases where it is not introduced from the environment, it is called “endogenous ethanol” [9][12][13][14][15].
Alcohol metabolism is influenced by the host’s state of energy, nutrition, and hormones, but it essentially basically involves three simple steps. In the first step, ethanol is oxidized to the product acetaldehyde. Afterward, acetaldehyde is oxidized to acetate; generally speaking, much of the acetaldehyde produced from the oxidation of alcohol is oxidized in the liver to acetate (circulating levels of acetaldehyde are low under normal conditions). In peripheral tissues, this is activated by a key Acetyl CoA. Two key enzymes are included in these steps (Figure 1), namely, alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), which, in healthy individuals, are involved in the breakdown of ethanol and acetaldehyde into harmless acetate. Acetyl CoA is also the key metabolite produced from all major nutrients, i.e., carbohydrates, fat, and excess protein [16].
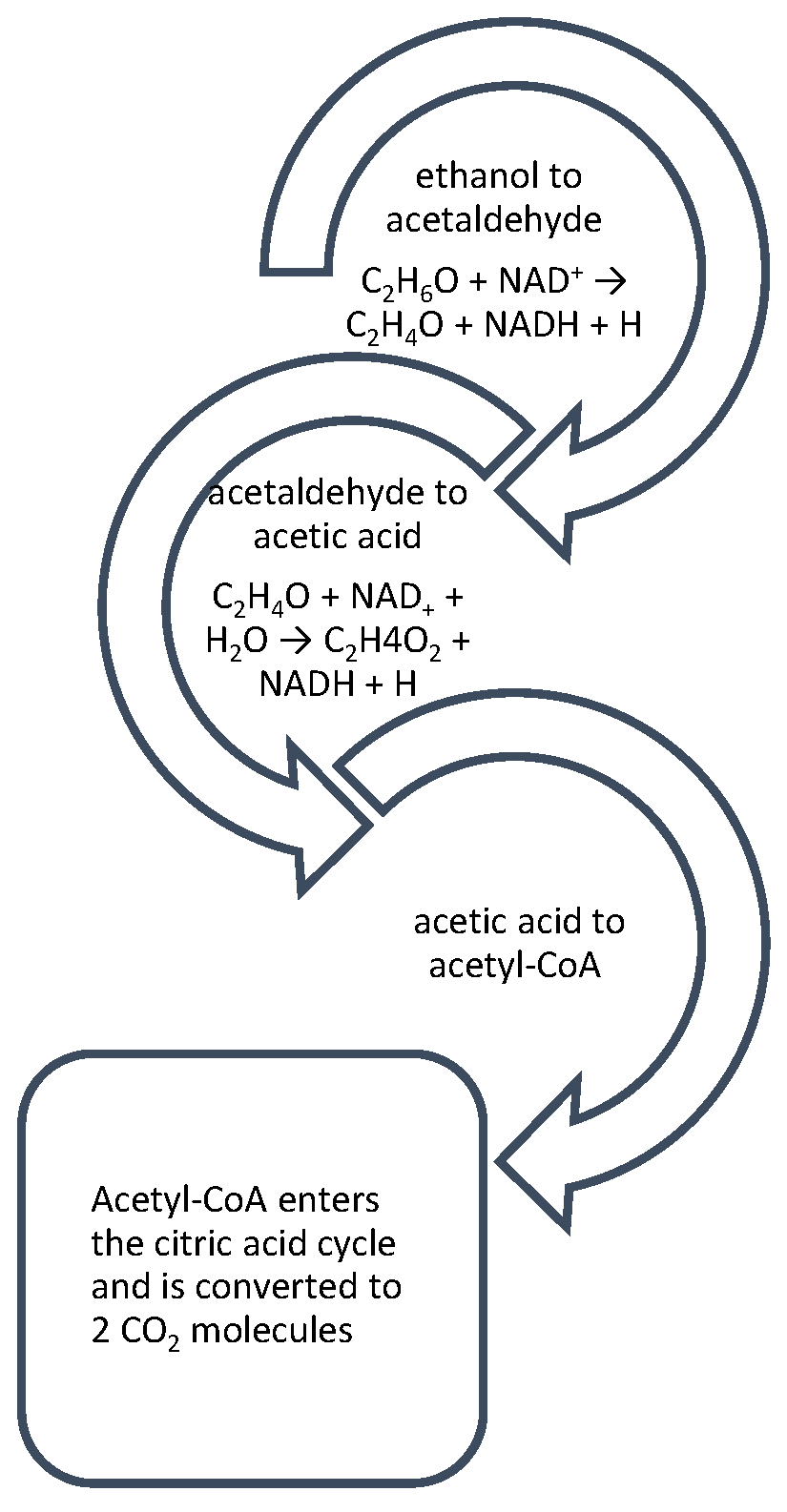
Figure 1. Alcohol metabolism in the human body comprises three crucial steps: after alcohol is metabolized by by ADH into a toxic acetaldehyde, it is then oxidized to acetic acid and acetyl-CoA. Acetyl-CoA is free to enter directly into the citric acid cycle. However, under alcoholic conditions, the citric acid cycle is stalled by the oversupply of NADH derived from ethanol oxidation.
Accordingly, the resulting carbon atoms from alcohol are the same products produced from the oxidation of carbohydrates, fat, and protein. Dietary recommendations for adult humans advise that carbohydrates make up 45% to 65% of total daily calories [17]. This quota is easy to accomplish since the molecules are found in a wide array of food products. However, there are carbohydrates available to organisms besides those in food [18].
2. Blood Ethanol Levels
Blood ethanol levels in healthy individuals may vary between 0 and 0.7 mmol/L, and in patients with diabetes or cirrhosis, even higher levels are observed [4][13][19][20][21][22]. The first reliable (chromatographic) article published on the concentrations of endogenously produced ethanol was that written by Lester from 1962. He established that the “normal” blood alcohol concentration (BAC) of humans, without exogenous intake, ranges from 0.0 to 0.071 µg/dL [20]. These concentrations result from the permanent production of acetaldehyde. The human body constantly forms ethanol acetaldehyde through various metabolic processes [13]. Later reports have claimed to find body fluids with abnormally high concentrations of ethanol in apparently healthy individuals; however, these reports happen to suffer from methodological deficiencies, a complete lack of appropriate control trials, or the use of overly comprehensive methods of analysis. The majority of the searched literature consisted of case reports or case series. Even case reports may be used to develop or write a systematic review. A special protocol for systematic reviews that use reports or studies of cases/case series was consulted [23]. This protocol is especially useful in disciplines like forensic medicine, where case reports/studies are a common practice.
For instance, besides Lester’s finding of endogenous ethanol production [20], more recent researchers, such as Al-Awadhi et al., provided some new perspectives on ABS and established that endogenous ethanol production could occur at 0.04 mg/dL [4]. Yet another large-sample study from Saudi Arabia yielded blood ethanol concentrations so modest that they were far too low to have any forensic significance [24].
With reliable means, e.g., gas chromatography (GC), it was determined that the concentrations of endogenous ethanol in the peripheral venous blood of healthy individuals, as well as those afflicted by specific metabolic conditions (diabetes, hepatitis, and cirrhosis), hardly surpassed 0.08 mg/dL [25].
What about all those other cases? Forensics and legal professionals often settle cases with any plausible drunk-driving defense strategy [3][5][6][8][26]; however, a consensus stating that this metabolic disturbance is rare should be reached [5][6]. Yeast, certain mold fungi species, and several bacterial strains are capable of producing lactic acid and ethanol when in anaerobic conditions (in particular, Candida albicans and Saccharomyces cerevisiae can anaerobically convert carbohydrates into endogenous ethanol and carbon dioxide) [6]. They employ the Entner–Doudoroff pathway (typically prokaryotic heterofermentation) [27][28]. The fermentation process begins via Embden–Meyerhof’s glycolysis, which is typical for eukaryotes [14]. Moreover, resulting from additional reactions involving the Ehrlich pathway, higher alcohols are produced [9][29]. Unfortunately, blood ethanol concentration is considered fundamental evidence in cases of driving under the influence of alcohol, regardless of its origin [5][26][30].
The gut fermentation of carbohydrates in the human body, acting as a bioreactor, in amounts sufficient to produce the effects of intoxication is relatively rare [8][21][31][32], but the gut microbiota is present even in the more subtle form of an endogenous brew [33][34][35]. In this biological process, which is called alcoholic fermentation, sugars are converted into energy for cells, with ethanol and carbon dioxide produced as by-products [36]. The average person passes about 0.5 L of gas a day, which is a by-product of fermentation; this value is far from enough to produce an intoxicating effect. As a rough estimate, increasing this value from 0.00 to 1.0 g/kg in two hours would require a person to pass approximately 20 L of gas during a given period [4][14][37][38]. Alcoholic beverages and ethanol fuel employ this very process [5][39][40].
Thus, it is reasonable to conclude that the microbiota has a range of hand-in-glove microorganisms that have learned to exist with us [35]. Interestingly, organisms known to ferment sugar and generate copious amounts of gas, particularly bacilli and Gram-negative cocci, were found in samples from an autopsy of a boy from Africa who died after the perforation of the back of the wall of the abdominal cavity due to extreme gas distention [41].
References
- Schmid, A.; Kreidl, E.; Bertschinger, M.; Vetsch, P. Benchtop Bioreactors in Mammalian Cell Culture: Overview and Guidelines. Methods Mol. Biol. 2022, 2436, 1–15.
- Seydi, M.; Boogar, I.R.; Talepasand, S. The Predictive Role of Gender, Age, and Personality Organization in Risky Driving; Eliva Press: Chișinău, Moldova, 2022.
- Akbaba, M. A medicolegal approach to the very rare Auto-Brewery (endogenous alcohol fermentation) syndrome. Traffic Inj. Prev. 2020, 21, 295–297.
- Al-Awadhi, A.; Wasfi, I.A.; Al Reyami, F.; Al-Hatali, Z. Autobrewing revisited: Endogenous concentrations of blood ethanol in residents of the United Arab Emirates. Sci. Justice J. Forensic Sci. Soc. 2004, 44, 149–152.
- Akhavan, B.J.; Ostrosky-Zeichner, L.; Thomas, E.J. Drunk without Drinking: A Case of Auto-Brewery Syndrome. ACG Case Rep. J. 2019, 6, e00208.
- Dinis-Oliveira, R.J. The Auto-Brewery Syndrome: A Perfect Metabolic “Storm” with Clinical and Forensic Implications. J. Clin. Med. 2021, 10, 4637.
- Chen, G.; Shi, F.; Yin, W.; Guo, Y.; Liu, A.; Shuai, J.; Sun, J. Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions. Front. Microbiol. 2022, 13, 916765.
- Bayoumy, A.B.; Mulder, C.J.J.; Mol, J.J.; Tushuizen, M.E. Gut fermentation syndrome: A systematic review of case reports. United Eur. Gastroenterol. J. 2021, 9, 332–342.
- Jones, A.W. Alcohol, its analysis in blood and breath for forensic purposes, impairment effects, and acute toxicity. Wiley Interdiscip. Rev. Forensic Sci. 2019, 1, e1353.
- Smedra, A.; Trzmielak, M.; Goralska, K.; Dzikowiec, M.; Brzezianska-Lasota, E.; Berent, J. Oral form of auto-brewery syndrome. J. Forensic Leg. Med. 2022, 87, 102333.
- Brick, J.; Bennett, W. Alcohol Calculations in Emergency and Forensic Medicine. J. Addict Med. Ther. Sci. 2017, 3, 024–029.
- Ostrovsky Yu, M. Endogenous ethanol--its metabolic, behavioral and biomedical significance. Alcohol 1986, 3, 239–247.
- Simic, M.; Ajdukovic, N.; Veselinovic, I.; Mitrovic, M.; Djurendic-Brenesel, M. Endogenous ethanol production in patients with diabetes mellitus as a medicolegal problem. Forensic Sci. Int. 2012, 216, 97–100.
- Jones, A. Biochemical and physiological research on the disposition and fate of ethanol in the body. In Medico Legal Aspects of Alcohol, 5th ed.; Lawyers and Judges Publishing Company: Tucson, AZ, USA, 2008.
- Jones, A.W. Driving under the influence of alcohol. Handb. Forensic Med. 2022, 3, 1387–1408.
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685.
- Riccardi, G.; Giosue, A.; Calabrese, I.; Vaccaro, O. Dietary recommendations for prevention of atherosclerosis. Cardiovasc. Res. 2022, 118, 1188–1204.
- Kiely, L.J.; Hickey, R.M. Characterization and Analysis of Food-Sourced Carbohydrates. Methods Mol. Biol. 2022, 2370, 67–95.
- Hafez, E.M.; Hamad, M.A.; Fouad, M.; Abdel-Lateff, A. Auto-brewery syndrome: Ethanol pseudo-toxicity in diabetic and hepatic patients. Hum. Exp. Toxicol. 2017, 36, 445–450.
- Lester, D. The concentration of apparent endogenous ethanol. Q. J. Stud. Alcohol. 1962, 23, 17–25.
- Tameez Ud Din, A.; Alam, F.; Tameez-Ud-Din, A.; Chaudhary, F.M.D. Auto-Brewery Syndrome: A Clinical Dilemma. Cureus 2020, 12, e10983.
- Malik, F.; Wickremesinghe, P.; Saverimuttu, J. Case report and literature review of auto-brewery syndrome: Probably an underdiagnosed medical condition. BMJ Open Gastroenterol. 2019, 6, e000325.
- Nambiema, A.; Sembajwe, G.; Lam, J.; Woodruff, T.; Mandrioli, D.; Chartres, N.; Fadel, M.; Le Guillou, A.; Valter, R.; Deguigne, M.; et al. A Protocol for the Use of Case Reports/Studies and Case Series in Systematic Reviews for Clinical Toxicology. Front. Med. 2021, 8, 708380.
- Ragab, A.R.; Al-Mazroua, M.K.; Afify, M.M.; Al Saeed, I.; Katbai, C. Endogenous ethanol production levels in Saudi Arabia residents. J. Alcohol. Drug Depend. 2015, 3, 211.
- Logan, B.K.; Jones, A.W. Endogenous ethanol ‘auto-brewery syndrome’ as a drunk-driving defence challenge. Med. Sci. Law 2000, 40, 206–215.
- Beitel, G.A.; Sharp, M.C.; Glauz, W.D. Probability of arrest while driving under the influence of alcohol. Inj. Prev. 2000, 6, 158–161.
- Jamai, L.; Ettayebi, K.; El Yamani, J.; Ettayebi, M. Production of ethanol from starch by free and immobilized Candida tropicalis in the presence of alpha-amylase. Bioresour. Technol. 2007, 98, 2765–2770.
- Aruna, A.; Nagavalli, M.; Girijashankar, V.; Ponamgi, S.P.; Swathisree, V.; Rao, L.V. Direct bioethanol production by amylolytic yeast Candida albicans. Lett. Appl. Microbiol. 2015, 60, 229–236.
- Roerecke, M.; Vafaei, A.; Hasan, O.S.M.; Chrystoja, B.R.; Cruz, M.; Lee, R.; Neuman, M.G.; Rehm, J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1574–1586.
- Kovačić, Z.; Nestić, M.; Stemberga, V.; Bosnar, A.; Petrovečki, M.; Sutlović, D. Reliability of breath alcohol testing with Dräger Alcotest 7410Plus analyzer in a court process. Medica Jadertina 2008, 38, 47–51.
- Painter, K.; Cordell, B.; Sticco, K. Auto-Brewery Syndrome (Gut Fermentation); StatPearls Publishing LLC: Treasure Island, FL, USA, 2018.
- Malik, F.; Wickremesinghe, P.; Saleem, A. Auto-Brewery Syndrome: A Schematic for Diagnosis and Appropriate Treatment. Pract. Gastroenterol. 2021, 45, 10–20.
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103.
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032.
- Riccio, P.; Rossano, R. The human gut microbiota is neither an organ nor a commensal. FEBS Lett. 2020, 594, 3262–3271.
- Wang, L.; Hao, J.; Wang, C.; Li, Y.; Yang, Q. Carbohydrate-to-protein ratio regulates hydrolysis and acidogenesis processes during volatile fatty acids production. Bioresour. Technol. 2022, 355, 127266.
- Niemelä, O.; Aalto, M.; Bloigu, A.; Bloigu, R.; Halkola, A.S.; Laatikainen, T. Alcohol Drinking Patterns and Laboratory Indices of Health: Does Type of Alcohol Preferred Make a Difference? Nutrients 2022, 14, 4529.
- Center, N.T.L. Challenges and Defenses III. Responses to Common. Challenges and Defenses in Impaired Driving Case. Available online: https://www.tampermonkey.net/changelog.php?version=4.19.0&ext=dhdg&updated=true&old=4.18.1 (accessed on 19 December 2022).
- Rajeswari, S.; Baskaran, D.; Saravanan, P.; Rajasimman, M.; Rajamohan, N.; Vasseghian, Y. Production of ethanol from biomass—Recent research, scientometric review and future perspectives. Fuel 2022, 317, 123448.
- Chen, W.H.; Biswas, P.P.; Ong, H.C.; Hoang, A.T.; Nguyen, T.B.; Dong, C.D. A critical and systematic review of sustainable hydrogen production from ethanol/bioethanol: Steam reforming, partial oxidation, and autothermal reforming. Fuel 2023, 333, 126526.
- Ladkin, R.G.; Davies, J.N. Rupture of the stomach in an African child. Br. Med. J. 1948, 1, 644.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
28 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




