Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Song Gao | -- | 1694 | 2023-08-23 10:23:11 | | | |
| 2 | Sirius Huang | Meta information modification | 1694 | 2023-08-24 03:10:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gao, S.; Guo, Z.; Liu, Z. Signal-Amplifying Substrates for Surface-Enhanced Raman Scattering-Based Bioassays. Encyclopedia. Available online: https://encyclopedia.pub/entry/48358 (accessed on 05 March 2026).
Gao S, Guo Z, Liu Z. Signal-Amplifying Substrates for Surface-Enhanced Raman Scattering-Based Bioassays. Encyclopedia. Available at: https://encyclopedia.pub/entry/48358. Accessed March 05, 2026.
Gao, Song, Zhanchen Guo, Zhen Liu. "Signal-Amplifying Substrates for Surface-Enhanced Raman Scattering-Based Bioassays" Encyclopedia, https://encyclopedia.pub/entry/48358 (accessed March 05, 2026).
Gao, S., Guo, Z., & Liu, Z. (2023, August 23). Signal-Amplifying Substrates for Surface-Enhanced Raman Scattering-Based Bioassays. In Encyclopedia. https://encyclopedia.pub/entry/48358
Gao, Song, et al. "Signal-Amplifying Substrates for Surface-Enhanced Raman Scattering-Based Bioassays." Encyclopedia. Web. 23 August, 2023.
Copy Citation
Surface-enhanced Raman spectroscopy (SERS) has become a powerful detection scheme for many applications, particularly bioassays, due to its unique strengths, such as its ultrasensitive performance. Due to the development of various SERS substrates, more SERS-based bioassays with improved sensitivity and reproducibility have been designed and manufactured.
Raman spectroscopy
SERS substrate
rational design
bioassays
high-sensitivity analysis
1. Introduction
Surface-enhanced Raman scattering (SERS) has emerged as a powerful analytical technique across many application domains. It combines Raman spectroscopy with nanostructured metal surfaces to achieve significant enhancement of the Raman signal. The conventional Raman spectroscopy, discovered by Raman and Krishnan in 1928 [1], has served as an analytical tool for a wide range of applications. Raman scattering provides insights into molecular structures by revealing their vibrational spectra. It provides compound-specific information and molecular-level fingerprinting without the need for complex instruments. This characteristic makes it promising for sensing various analyte molecules, and it has found extensive use in biological applications [2][3][4][5]. For example, it has been employed in identifying cancer cells [6], investigating biomolecular structures [7], and diagnosing diseases and pathologies [8][9]. Despite its utility, Raman scattering intensity is typically weak, as the process suffers from low efficiency. Upon light-matter interactions, only one in every 108 photons would undergo an inelastic scattering as estimated [10][11]. This limitation has hindered its application in trace analysis, necessitating methods to enhance the Raman signal. Fortunately, Fleishmann, Hendra, and McQuilian first observed unexpectedly strong Raman signals in 1974 from pyridine adsorbed on roughened silver electrodes [12]. Later, in 1977, Jeanmaire and Van Duyne discovered that placing a Raman-active species on a roughened noble metal surface significantly amplified the Raman signal, surpassing the limitations of conventional Raman scattering [13]. This phenomenon, known as surface-enhanced Raman scattering, has revolutionized the sensitivity of Raman spectroscopy. Now SERS is one of the key technologies to dramatically amplify the Raman scattering signal in order to bring several advantages, including ultrahigh sensitivity, less susceptibility to sample environment, rapid readout speed, and the possibility of on-site or field detection [14].
Bioassays are crucial tools in biological and pharmaceutical research, providing valuable information about the role and effects of substances in living organisms. They facilitate the evaluation of biological activity [15], assist early disease diagnostics [16], aid in drug discovery and development [17], ensure quality control [18], assess toxicity [19], monitor environmental impact [20], and contribute to expanding our understanding of living systems [21]. Both labeled and label-free bioassays have gained prominence as powerful tools for studying and analyzing biological systems. Labeled bioassays involve the use of molecular probes or labels, such as fluorescent dyes and radioisotopes, to detect and quantify the biomolecules of interest that have been specifically captured. These labels enable the direct measurement and visualization of the target, providing specific and sensitive results. Label-free bioassays, on the other hand, rely on the direct measurement of intrinsic properties of the captured biomolecule, such as mass, absorbance, and Raman response, without the use of exogenous labels. In general, labeled assays provide high sensitivity and versatility, but require sample manipulation and may introduce biases. Conversely, label-free assays offer minimal sample processing and real-time analysis but may lack sensitivity and pose challenges in data interpretation. As technology continues to advance, the development of hybrid strategies and improved detection methods may bridge the gap between these two bioassay paradigms, enabling researchers to harness the strengths of both approaches in their scientific investigations.
2. Basic Theory of SERS
It is now generally accepted that the Raman scattering signal is amplified through a combination of electromagnetic (EM) enhancement mechanism, and chemical (CM) enhancement mechanism. In brief, electromagnetic enhancement arises from the excitation of localized surface plasmons, which are collective oscillations of conduction electrons on the metal surface. These plasmons generate intense electromagnetic fields at the metal surface, leading to increased Raman scattering intensity. The chemical enhancement, on the other hand, results from the charge transfer between the molecule and the metal surface, further enhancing the Raman signal. Chemical enhancement is highly dependent on the specific molecule–substrate interactions and can vary for different analytes.
2.1. Electromagnetic Enhancement
The underlying principle of SERS involves the amplification of the incident and scattered electromagnetic fields when a nanostructured surface of the metal is irradiated with the frequency of localized surface plasmons resonance (LSPR) on the metal surface. This phenomenon is explained by considering a metal nanosphere subjected to an external electric field (Figure 1A). The oscillating electric field (𝐸0) of the incident light interacts with the metal nanoparticle, inducing a polarization of charge and creating a dipolar localized surface plasmon resonance. This polarization generates an induced dipole moment (𝜇ind) determined by the metal polarizability (𝛼met) and the incident electric field (𝐸0(𝜔inc)) [22].
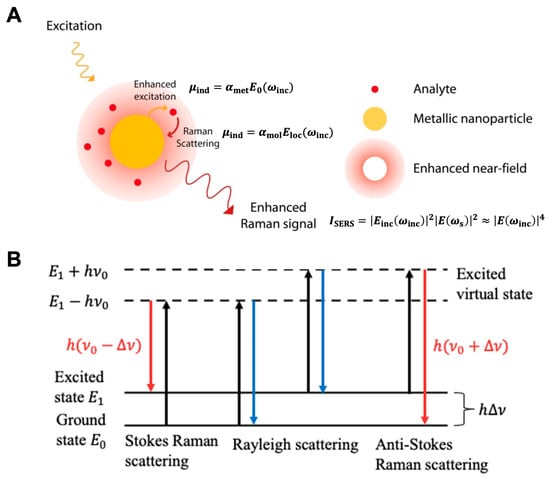
Figure 1. (A) Principal of surface-enhanced Raman scattering (reproduced under the terms of CC BY 4.0 from Ref. [23], copyright 2015, the authors). (B) Rayleigh scattering and Raman scattering energy-level diagram (reproduced under the terms of CC BY 4.0 from Ref. [24], copyright 2022, the authors).
As a Raman scattering arises on a molecule, a dipole moment is first created by the incident light. After being scattered, the photons are detected as the Raman signal. SERS follows a similar process but with the enhancement of the local electromagnetic field due to hotspots on the metal surface. In SERS, the inelastic scattering of 𝐸0 generates an enhanced local electric field (𝐸loc) near the metal surface. The interaction between the local electric field and the molecule adsorbed on the surface creates an induced dipole moment (𝜇ind) determined by the molecular polarizability (𝛼mol) and the enhanced local electric field (𝐸loc(𝜔inc)).
According to the vibrational spectrum theory in quantum mechanics, the presence of inelastic scattering for a vibrating molecule is explained by the incident electric field (𝐸inc) and the eigenvalue of angular frequency (𝜔vib) of the vibrating molecule. This inelastic scattering gives rise to three dipole components: Rayleigh scattering 𝜇ind(𝜔inc), Stokes scattering 𝜇ind(𝜔inc−𝜔vib), and anti-Stokes scattering 𝜇ind(𝜔inc+𝜔vib) (Figure 1B). The resonance frequency of surface plasmons on the metal surface determines the enhancement of the scattered Stokes field. Taking into account the incident and scattered field intensities, the overall SERS enhancement intensity can be described by an equation that involves the local electric field (𝐸inc(𝜔inc)) at the incident frequency (𝜔inc) and the electric field (𝐸(𝜔s)) at the Stokes-shifted frequency (𝜔s=𝜔inc−𝜔vib) [22].
When the electric field values at the incident frequency (𝐸inc(𝜔inc)) and the Stokes shifted frequency (𝐸(𝜔s)) are in proximity, the resulting enhancement derived from the electromagnetic mechanism follows the relationship where the SERS enhancement factor is directly proportional to the fourth power of the induced electric field enhancement value (𝐸(𝜔inc)).
2.2. Chemical Enhancement
The chemical effect is another significant mechanism contributing to SERS enhancement, and it requires direct contact between the SERS substrate and the analyte. This effect involves the interaction of the adsorbate and the surface to form a complex through electronic coupling. During this interaction, electrons on the substrate surface transfer from the Fermi level to the lowest unoccupied molecular orbital of the analyte molecule. This process leads to the creation of charge transfer intermediates, which demonstrate higher Raman cross-sections in comparison to the free molecule. If the incident photon at frequency 𝜔inc resonates with the charge transfer transition of the formed complex, the scattered Stokes intensity contains information about the vibrational state of the molecule. In other words, the Raman spectrum of the analyte molecule is enhanced when it forms a chemical bond or interacts strongly with the SERS-active metal surface.
The magnitude of the chemical enhancement effect is typically in the range of 100100 to 102102, which is weaker compared to the electromagnetic enhancement. However, the chemical effect can provide additional information about the chemical interactions between the analyte molecule and the metal surface, allowing for a more detailed characterization of the molecular species.
3. Substrates in Colloidal Suspension
SERS substrates designed for practical applications and problem-solving purposes have been extensively explored. These substrates in colloids can be broadly categorized into two groups: dispersed particles and aggregated systems. Dispersed particle systems in solution are relatively simple to prepare, making them widely used. Furthermore, advancements in producing increasingly uniform particles have improved the reproducibility of SERS measurements. Surface functionalization of colloidal particles is also often straightforward, allowing for a wide range of targets and applications [25][26][27]. However, these systems suffer from some drawbacks, with colloidal stability being a primary concern, as colloidal systems tend to be unstable and prone to irreversible aggregation, particularly in complex environments.
Highly enhancing SERS substrates often consist of aggregated or assembled nanoparticles. While yielding significant SERS enhancement factors, reproducibility can sometimes be challenging due to the difficulty in controlling aggregation. Controlled particle assembly offers a solution to this issue, allowing for precise nanoengineering and reducing the irreproducibility associated with aggregated particles. The outcome of the assembly process is influenced by surface functionalization and nanoparticle geometry.
4. Substrates on a Solid Support
Compared to traditional colloid-based systems, solid-supported SERS substrates offer numerous advantages. Nanostructures embedded on the surface exhibit high uniformity, provide compatibility with ultra-low volumes, enable single-nanoparticle measurements, and facilitate the preparation process. Similar to colloidal systems, various solid-supported substrates have been prepared. Colloidal nanoparticles, assemblies, or aggregates can be deposited onto surfaces [28][29][30]. A chemical layer can be used to functionalize surfaces and direct the adsorption of colloidal particles. [29][31]. By adjusting nanoparticle concentration and deposition time, the particle density on the surface can be optimized. These substrates offer similar advantages to colloidal systems while enabling comprehensive optical and structural characterization of individual particles during usage, which is particularly advantageous for single-molecule studies.
Apart from surface self-assembly methods, nanolithography is a commonly employed technique for fabricating surfaces with highly enhancing metal nanostructures. Colloidal lithography [32], and electron beam lithography (EBL) [33][34][35], are among the most prevalent lithographic methods used in SERS applications. These methods vary in terms of ease and cost of fabrication. Although lithography allows for the preparation of various highly sensitive structures, one drawback of deposited metal nanoparticles is their vulnerability to annealing, as structural changes in the nanoparticles can lead to shifts in LSPR position and alter SERS capabilities, sometimes even during the course of a measurement. Advancements in synthesis and nanolithography methods have contributed to higher field enhancements, improved spatial control, and greater uniformity and reproducibility.
References
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502.
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-enhanced Raman spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980.
- Howes, P.D.; Chandrawati, R.; Stevens, M.M. Colloidal nanoparticles as advanced biological sensors. Science 2014, 346, 1247390.
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skládal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042.
- Lane, L.A.; Qian, X.; Nie, S. SERS nanoparticles in medicine: From label-free detection to spectroscopic tagging. Chem. Rev. 2015, 115, 10489–10529.
- Kallaway, C.; Almond, L.M.; Barr, H.; Wood, J.; Hutchings, J.; Kendall, C.; Stone, N. Advances in the clinical application of Raman spectroscopy for cancer diagnostics. Photodiagn. Photodyn. Ther. 2013, 10, 207–219.
- Krafft, C.; Popp, J. The many facets of Raman spectroscopy for biomedical analysis. Anal. Bioanal. Chem. 2015, 407, 699–717.
- Stone, N.; Matousek, P. Advanced transmission Raman spectroscopy: A promising tool for breast disease diagnosis. Cancer Res. 2008, 68, 4424–4430.
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541.
- Mosca, S.; Conti, C.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy. Nat. Rev. Methods Primers 2021, 1, 21.
- Ellis, D.I.; Cowcher, D.P.; Ashton, L.; O’Hagan, S.; Goodacre, R. Illuminating disease and enlightening biomedicine: Raman spectroscopy as a diagnostic tool. Analyst 2013, 138, 3871–3884.
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166.
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20.
- Ye, J.; Chen, Y.; Liu, Z. A boronate affinity sandwich assay: An appealing alternative to immunoassays for the determination of glycoproteins. Angew. Chem. Int. Ed. 2014, 53, 10386–10389.
- Ahn, K.C.; Zhao, B.; Chen, J.; Cherednichenko, G.; Sanmarti, E.; Denison, M.S.; Lasley, B.; Pessah, I.N.; Kültz, D.; Chang, D.P.; et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 116, 1203–1210.
- Fan, Y.; Wang, S.; Zhang, F. Optical Multiplexed Bioassays for Improved Biomedical Diagnostics. Angew. Chem. Int. Ed. 2019, 58, 13208–13219.
- Kerns, E.H.; Di, L.; Carter, G.T. In vitro solubility assays in drug discovery. Curr. Drug Metab. 2008, 9, 879–885.
- Johnson, I.; Hutchings, M.; Benstead, R.; Thain, J.; Whitehouse, P. Bioassay selection, experimental design and quality control/assurance for use in effluent assessment and control. Ecotoxicology 2004, 13, 437–447.
- Sprague, J. Measurement of pollutant toxicity to fish I. Bioassay methods for acute toxicity. Water Res. 1969, 3, 793–821.
- Schuetzle, D.; Lewtas, J. Bioassay-directed chemical analysis in environmental research. Anal. Chem. 1986, 58, 1060A–1075A.
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360.
- Schlücker, S. Surface-Enhanced raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795.
- Boujday, S.; Lamy de la Chapelle, M.; Srajer, J.; Knoll, W. Enhanced vibrational spectroscopies as tools for small molecule biosensing. Sensors 2015, 15, 21239–21264.
- Ding, H.; Hu, D.J.J.; Yu, X.; Liu, X.; Zhu, Y.; Wang, G. Review on all-fiber online raman sensor with hollow core microstructured optical fiber. Photonics 2022, 9, 134.
- Jorgenson, E.; Ianoul, A. Biofunctionalization of plasmonic nanoparticles with short peptides monitored by SERS. J. Phys. Chem. B 2017, 121, 967–974.
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319.
- He, J.; Unser, S.; Bruzas, I.; Cary, R.; Shi, Z.; Mehra, R.; Aron, K.; Sagle, L. The facile removal of CTAB from the surface of gold nanorods. Colloids Surf. 2018, 163, 140–145.
- Rodríguez-Lorenzo, L.; Alvarez-Puebla, R.A.; Pastoriza-Santos, I.; Mazzucco, S.; Stéphan, O.; Kociak, M.; Liz-Marzán, L.M.; García de Abajo, F.J. Zeptomol detection through controlled ultrasensitive surface-enhanced Raman scattering. J. Am. Chem. Soc. 2009, 131, 4616–4618.
- Su, Q.; Ma, X.; Dong, J.; Jiang, C.; Qian, W. A reproducible SERS substrate based on electrostatically assisted APTES-functionalized surface-assembly of gold nanostars. ACS Appl. Mater. Interfaces 2011, 3, 1873–1879.
- Fortuni, B.; Fujita, Y.; Ricci, M.; Inose, T.; Aubert, R.; Lu, G.; Hutchison, J.A.; Hofkens, J.; Latterini, L.; Uji-i, H. A novel method for in situ synthesis of SERS-active gold nanostars on polydimethylsiloxane film. Chem. Commun. 2017, 53, 5121–5124.
- Lee, J.; Hua, B.; Park, S.; Ha, M.; Lee, Y.; Fan, Z.; Ko, H. Tailoring surface plasmons of high-density gold nanostar assemblies on metal films for surface-enhanced Raman spectroscopy. Nanoscale 2014, 6, 616–623.
- Zrimsek, A.B.; Henry, A.I.; Van Duyne, R.P. Single molecule surface-enhanced Raman spectroscopy without nanogaps. J. Phys. Chem. Lett. 2013, 4, 3206–3210.
- Das, G.; Chirumamilla, M.; Toma, A.; Gopalakrishnan, A.; Zaccaria, R.P.; Alabastri, A.; Leoncini, M.; Di Fabrizio, E. Plasmon based biosensor for distinguishing different peptides mutation states. Sci. Rep. 2013, 3, 1792.
- Chirumamilla, M.; Toma, A.; Gopalakrishnan, A.; Das, G.; Zaccaria, R.P.; Krahne, R.; Rondanina, E.; Leoncini, M.; Liberale, C.; De Angelis, F.; et al. 3D nanostar dimers with a sub-10-nm gap for single-/few-molecule surface-enhanced Raman scattering. Adv. Mater. 2014, 26, 2353–2358.
- Gopalakrishnan, A.; Chirumamilla, M.; De Angelis, F.; Toma, A.; Zaccaria, R.P.; Krahne, R. Bimetallic 3D nanostar dimers in ring cavities: Recyclable and robust surface-enhanced Raman scattering substrates for signal detection from few molecules. ACS Nano 2014, 8, 7986–7994.
More
Information
Subjects:
Spectroscopy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
617
Revisions:
2 times
(View History)
Update Date:
24 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





