Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vinay Kumar Pandey | -- | 3975 | 2023-08-23 09:09:51 | | | |
| 2 | Jason Zhu | Meta information modification | 3975 | 2023-08-24 03:50:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tripathi, A.; Pandey, V.K.; Tiwari, V.; Mishra, R.; Dash, K.K.; Harsányi, E.; Kovács, B.; Shaikh, A.M. Lactobacillaceae-Originated Probiotics in Preventive Measures of Alzheimer’s Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/48354 (accessed on 07 February 2026).
Tripathi A, Pandey VK, Tiwari V, Mishra R, Dash KK, Harsányi E, et al. Lactobacillaceae-Originated Probiotics in Preventive Measures of Alzheimer’s Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/48354. Accessed February 07, 2026.
Tripathi, Anjali, Vinay Kumar Pandey, Vivek Tiwari, Rashi Mishra, Kshirod Kumar Dash, Endre Harsányi, Béla Kovács, Ayaz Mukarram Shaikh. "Lactobacillaceae-Originated Probiotics in Preventive Measures of Alzheimer’s Disease" Encyclopedia, https://encyclopedia.pub/entry/48354 (accessed February 07, 2026).
Tripathi, A., Pandey, V.K., Tiwari, V., Mishra, R., Dash, K.K., Harsányi, E., Kovács, B., & Shaikh, A.M. (2023, August 23). Lactobacillaceae-Originated Probiotics in Preventive Measures of Alzheimer’s Disease. In Encyclopedia. https://encyclopedia.pub/entry/48354
Tripathi, Anjali, et al. "Lactobacillaceae-Originated Probiotics in Preventive Measures of Alzheimer’s Disease." Encyclopedia. Web. 23 August, 2023.
Copy Citation
Alzheimer’s disease (AD) is an ascending, neurodegenerative disorder that attacks the brain’s nerve cells, i.e., neurons, resulting in loss of memory, language skills, and thinking and behavioural changes. It is one of the most common causes of dementia, a group of disorders that is marked by the decline of cognitive functioning. Probiotics are living microorganisms that are beneficial for human well-being.
probiotics
Alzheimer’s disease
nervous system
1. Introduction
The concept of functional food is discussed thousands of times on different platforms [1]. Although the accurate definition is still a mystery, experts normally concur that in general, additives are involved in functional foods (containing microbes such as probiotics, mentioned underneath), which hold the benefit of health along with the basic nutrient content of the food as well (Figure 1). Functional foods have the potential to improve physiological mechanisms and enhance neuronal functioning at the level of the gastrointestinal tract (GIT) via altering biochemical parameters. Probiotics are considered one of the primary pathways for health benefits and are included in functional foods. It has been demonstrated that the host’s energy supply is significantly affected by the activities of microbiota present in the gut. The GI microbiota is the total population attributed to live microorganisms that inhabit a host organism’s GI tract [2].
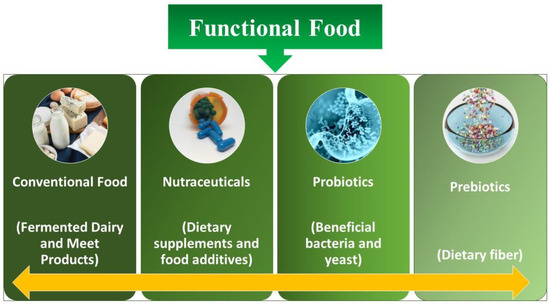
Figure 1. Different kinds of functional food.
Jäger et al. [3] stated that probiotics comprises of living microbes that, once ingested in sufficient quantities, are good for human health. The Greek words “pro” and “biotic”, which both imply “life”, are the origin of the phrase “probiotic” [3]. These microscopic organisms, which are primarily bacteria but can also incorporate certain yeasts, exist naturally in own bodies as well as in certain foods and dietary supplements. The microbial community or microbiome, which consists of billions of bacteria, inhabits in the human body. It also exists in other parts, including the mouth, genital tract, and skin, but microbes are mostly found in digestive system. General health is maintained by this intricate ecology [4]. The cornerstone of health is made up of these live nutrients. Dental disease, yeast infections, eczema, and food allergies are prevented and treated by maintaining a healthy microbiome. Probiotics are a broad category of microbes. All of them have unique advantages, although the majority belong to just two groups. Lactobacillaceae is the most widely used probiotic. It is found in yoghurt and other fermented foods. Countless varieties may aid those who struggle to digest the milk sugar lactose as well as those who experience diarrhoea [5]. Bifidobacterium is frequently discovered in various dairy products and could lessen the symptoms and signs of irritable bowel syndrome (IBS) and a few other disorders [6][7]. Probiotics encompass the yeast Saccharomyces boulardii. It seems to aid in the prevention of diarrhoea and other intestinal problems. Slightly over 4 million (1.6%) American adults reported using probiotics or prebiotics in the previous 30 days, according to the 2012 National Health Interview Survey (NHIS). Probiotics and prebiotics were the third most popular dietary supplement among adults beside vitamins and minerals. From 2007 to 2012, consumption of probiotics in adults increased by four times. The 2012 NHIS also revealed that 300,000 children between the age of 4 and 17 (or 0.5% of the population) reported using probiotics or prebiotics in the 30 days prior to a questionnaire [8].
Probiotics have demonstrated potential for an assortment of medical conditions, such as the management of infant colic, the prevention of necrotizing enterocolitis and sepsis in premature infants, the treatment of periodontal disease, and the induction or maintenance of remission in ulcerative colitis [9]. An unbalanced microbiota in the gut that causes the alteration of the microbiome (dysbiosis) has been strongly associated with psychological disorders. The gut produces several neurotransmitters that are linked to anxiety and depression symptoms, including the neurotransmitters glutamate, serotonin, and GABA (gamma-aminobutyric acid). Numerous mental diseases are believed to be associated with gut dysbiosis, and there is strong evidence linking gut health with psychological wellness. One of the main characteristics and pathological traits linked to a wide range of neurodegenerative illnesses, including Alzheimer’s, Parkinson’s, and Huntington’s diseases, is neuroinflammation such as microgliosis and astrogliosis. Furthermore, owing to the constant presence of inflammation in the brain, classic neuroinflammatory illnesses like multiple sclerosis clearly highlight the significance of neuroinflammation in these neurodegenerative diseases. The most prevalent dementia amongst them is AD, which may be identified by an increase in neurofibrillary plaque build-up in the brain. There is strong evidence that GM (gastrointestinal microbiota) plays a role in the pathogenesis of AD, which is largely mediated by altered microglial activity in the brain. AD is just one neurodegenerative condition where microglial dysfunction has been identified [10].
The “gut–microbiota–brain axis” is a term coined for representing such relationship: a link uniting the gut microbiome and AD and also representing the role played by inflammation in the progression as well as onset of AD. The considerably discussed topic of the microbiota–gut-brain axis holds that the gut microbiota has an impact on the activities of central nervous system (CNS) [11][12][13]. By encouraging the development of advantageous microbes, a diet high in prebiotics supports the health of the digestive system. Throughout the fermentation of prebiotics, short-chain fatty acids (SCFAs) including butyrate, acetate, and propionate are created. They are crucial for maintaining metabolic and intestinal health. Likewise, according to the study, probiotics affect brain function and behaviour, and knowledge of these biological causes may help to improve psychological well-being in both healthy people and, possibly, those with mental illnesses [11]. Probiotics have been shown to enhance stress response, improve memory function, and even lessen anxiety and sadness in preclinical experiments with rodents [14][15].
Many theoretical concepts have been proposed regarding the contribution of the gut microbiota to AD chronic neuroinflammation. The highly significant concept includes the following ideas: (1) A neuroinflammatory condition in the brains of patients with AD that can be caused by direct microorganism infection; (2) an age-related dysbacteriosis that hypothesizes the aging immune system to be a cause of AD development; (3) a microbicidal safeguard theory indicating the immune reaction against the build-up of harmful bacteria as the cause of build-up of AD in brain; and A notable, promising result in the decrement of the progression of AD is observed when probiotics are taken as a sole supplement. The key driving force behind the exploration of overall therapeutic efficacy is now their clinically tested anti-inflammatory effects and antioxidant capabilities and their capacity to improve a patient’s cognitive function [16]. Fermented milk has the potential to be used in the treatment of mental and functional CNS disorders as well as modulating stress adaptivity and mood in disease and well-being [17]. Various foods with probiotic activity are being created from the dairy products that are fermented, including yogurt, dairy serums, and kefir containing Lactobacillaceae and Bifidobacterium [18][19]
Role of Psychobiotics in the Improving Mental Health
Probiotics, in particular psychobiotics, have demonstrated promise for treating AD and other mental health problems. These substances have a significant impact on cognitive and emotional functions as well as brain function. The gut microbiota has been linked to mental diseases including AD, and dysbiosis in the gut microbiota has been linked to brain maturation and neurogenesis. Psychobiotics can assist in re-establishing a healthy balance of gut flora, which may have a good impact on AD symptoms and cognitive performance [20]. Psychobiotics have been shown to have anti-inflammatory properties, and by controlling the immune system and blocking the production of inflammatory substances and chemicals, they can reduce neurological inflammation. By lowering neuroinflammation, psychobiotics may save brain cells and slow the onset of AD. It has been shown that neuroprotective substances like brain-derived neurotrophic factor (BDNF), which promotes the development and longevity of neurons, are produced by some psychobiotics. Since inadequate amounts of BDNF have been associated with AD, boosting BDNF synthesis by using psychobiotics may aid in preserving the health and functionality of neurons. The capacity of the stomach to create and employ neurotransmitters like serotonin and gamma-aminobutyric acid (GABA), which are essential for regulating emotions, cognition, and recollection, may be impacted by psychobiotics [21]. By altering neurotransmitter levels, psychobiotics may improve cognitive performance and emotional well-being in persons with AD. Increased intestinal permeability, a side effect of AD, facilitates the bloodstream entry of harmful substances and can lead to cognitive decline. Psychobiotics have been shown to boost the intestinal barrier’s capacity to prevent the entrance of contaminants by promoting the production of mucins and tight junction proteins. By enhancing gut barrier function, psychobiotics may aid in the reduction in systemic inflammation and may improve memory retention in AD [22]. Oxidative stress is one of the key elements in the development and progression of AD. Psychobiotics are antioxidants that can scavenge free radicals to decrease oxidative damage to brain cells. By reducing oxidative stress, psychobiotics may help to preserve neurons and decrease the neurodegenerative processes linked to AD [23]. Psychobiotics therapies may improve emotional behaviour and cognitive performance in children and adolescents, according to a comprehensive review by [24]. Another study by Cheng et al. [25] discovered that certain psychobiotic strains reduced cortisol levels and suppressed inflammation, which improved the signs and symptoms associated with anxiety and depression.
2. Importance of Probiotics for Mental Health
Probiotics are living microorganisms that are thought to gain health benefits when ingested. They are commonly found in certain foods such as yoghurt, kimchi, sauerkraut, and kefir. An emerging interest in the role of probiotics on mental health has been a burning topic recently. Studies have proposed the positive effect of probiotics on mental health via reducing inflammation, regulating the gut microbiota, and boosting stress response. There is evidence to suggest that probiotics may help reduce symptoms of depression and anxiety, minimize stress-associated eating conduct, and improve cognitive outcomes [26]. Probiotics may help improve sleep quality, reduce fatigue, and boost mood. Furthermore, while more research work is necessary to better acknowledge the mechanisms and effects of probiotics on mental health, current evidence suggests that probiotics may be beneficial for overall mental health. It has been observed that the microbiota residing in the gastrointestinal tract (GIT) show an effect on the synaptic or neural signals produced by the CNS [27]. The term gut-brain axis depicts the association between the gut microbiota and the CNS. This association describes a relationship of the gut whose basis of communication is performed through the endocrine system, inflammatory responses, neural system, etc. Several researchers performed clinical trials to clarify this mutual association and have shown that a nerve, namely the vagus nerve, is responsible for the maintenance of the microbiota–gut-brain axis and performs a significant function in neurotransmitter signalling. The microbiota–gut-brain axis is a two-way mechanism. The microbiota present in the gut potentially influence the cognitive functions performed by the brain, such as remembrance power, memory, creativity, and controlling the centre of emotions, feelings, and sensations [28]. A clinical trial of probiotics containing Lactobacillus spp. was performed on mice, which resulted in lowering their anxiety levels. Many types of research are ongoing to specify the role of probiotics in the body, especially in the neural system. Probiotics show a positive effect on the mental health of an organism, and they help in stress management, act as anti-depressants, and cure various diseases like Parkinson’s disease, AD, autism, etc. In current studies, it has been found that the diet crucially contributes to the cognitive processes controlled by brain. The CNS involves the hypothalamic region that regulates stress and responds to stimuli. Dysfunction of the hypothalamic region or CNS can lead to neurodegenerative disorders [29].
3. Aetiology of AD
Around the world, the chronic neurological condition AD (AD) accounts for about 80% of all cases of dementia, mainly in persons over 60 years of age. In accordance with a thorough investigation for the Global Burden of Disease study, in the year 2016, about 43.8 million people worldwide were affected by AD. It is expected that by the year 2050, around 131 million people worldwide will be suffering from AD, constituting it as one of the biggest worldwide health and hygiene challenges of the upcoming future generation. Significant memory, cognitive, and motor deficits are hallmarks of AD [30]. This is primarily caused by the deposition of beta-amyloid plaque protein outside the neuron or the existence of tau tangles inside the neurons. This may change the calcium balance and cause neuroinflammation, vascular degeneration, and ultimately neuronal death. AD is tightly linked to neuron loss, synaptic dysfunction, and neuropil threads. Amyloid precursor protein (APP), a crucial component involved in the system for digesting proteins, is strongly associated with the aetiology of AD [31]. The noteworthy factors contributing to the prior development of AD include transmutations in the presenilin 1 (PSEN1) and presenilin (PSEN 2) proteins and APP. A delayed start in AD is caused due to a variety of factors involving lifestyle, ageing, food, environment, and overexpression of the apolipoprotein (Apo) E4 gene. In AD, beta-amyloid accumulation is prevalent. Beta-amyloid is a peptide produced when APP is cleaved by a proteolytic enzyme. By clathrin-mediated endocytosis, amyloid precursor protein derived from trans-Golgi networks is delivered into the endosomal compartment. A portion of the APP is recycled back to the surface of the cell during this process in the endosome [32]. A non-amyloidogenic route controls APP on the cell surface and -secretase functions at N-terminal terminus in the A domain. These produce 83 membrane-tethered amino acids with the carboxy-terminal ends and APP as a result (CTF). As a result, -secretase will continue to cleave CTF-83 to produce the living intracellular domain of P3 fragment and APP [33].
In the endosomal compartment, the APP will take the amyloidogenic pathway. A non-amyloidogenic route controls APP on the cell surface and secretase functions on the N-terminal terminus in the A domain. It produces 83 membrane-tethered amino acids with carboxy terminal ends and APP as a result (CTF). As a result, -secretase will continue to cleave CTF-83 to produce the functional intracellular domain of P3 fragment and APP [33]. The APP will take the amyloidogenic pathway in the endosomal compartment. In this route, the -secretase binds with the APP’s extracellular domain, resulting in 99 membrane-bound amino acids made up of APP and CTF-(C99). To create soluble A fragment and the APP intracellular domain, -secretase will further cleave C99 [34]. In the presence of transition metal ions like Fe2+ and Cu2+, which create H2O2, the A peptide oligomerizes. By promoting lipid peroxidation, this will eventually lead to the formation of 4-hydroxynonenal (4HNE). In contrast, impaired glucose and glutamate transport effects in an expansion in inositol 1,4,5-triphosphate (IP3) production and Ca2+ influx led to the efflux of Ca2+ from the storage of the endoplasmic reticulum. When calpain, which is calcium-dependent, becomes activated, CDK5 is induced, which leads to tau hyperphosphorylation, neurofibrillary tangle development, impairment of axonal transport, and microtubule disassembly. In the end, it results in neuronal death and synaptic dysfunction [35]. The reactive oxygen species (ROS) and the increased inflow for Ca2+ ions into the mitochondria drive the production of mitochondrial cyclophilin D. As a result, proapoptotic substances such as apoptosis-inducing factor (AIF) and cytochrome C are released. An activation of the caspase cascade eventually causes the death of neuronal cells. Moreover, plaques cause a release of certain cytokines like IL-1, TNF, and IL-6 as well as a few chemokines like macrophage inflammatory protein-1 and IL8 from microglial cells. All of these in turn trigger the astrocytes’ production of acute-phase proteins, cytokines, and chemokines, which activates the microglial cells. The pathophysiology of AD is neuroinflammation, which is produced in the brain by activated astrocytes and microglia [36]. According to recent research, AD patients have an elevated concentration of immune cells that are peripheral, and these cells actively contribute to regional irritation. The pro-inflammatory cytokinin cells can penetrate through the blood–brain barrier (BBB) and furthermore incite some inflammatory responses like heat, soreness, and redness, which are particular to the brain but are nonetheless sources for peripheral inflammation, whether through obesity or systemic inflammation. As a result, the BBB becomes more permeable and porous, allowing peripheral immune cells to enter. Microglial cells will then become overactive. This will at first manifest in AD patients as reduced hippocampal-dependent learning [37].
4. Significance of Probiotics in Curing AD
Probiotics work through a wide range of processes depicted in Figure 2, albeit the precise way they do so is still not fully understood.
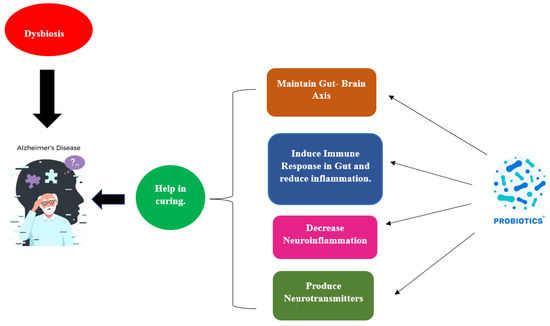
Figure 2. Influence of probiotics in curing AD.
The creation of short-chain fatty acids, bacteriocins, modulation, the struggle for nutrition, and the stimulation of the gut-brain axis’s function are all examples of how this happens. Figure 3 illustrates a probiotic’s potential mode of action in AD in relation to this. Based on the amount of fibre in the diet, The formation of short-chain fatty acids (SCFAs), which are generally saturated fatty acids, takes place in the gut. The fermentation process, which is mediated by the microbial species Lactobacillaceae, Clostridium, Bacteroides, Eubacterium, and Bifidobacterium, produces the metabolites acetate, butyrate, and propionate [38]. Three key mechanisms—neuronal routes, immune modulation, and endocrine factors—may all play a role in how SCFAs affect brain function. By enhancing barrier integrity and maintaining mucus production through immunological regulation, SFCAs can affect intestinal mucosa susceptibility and barrier function. SCFAs influence the release of cytokines, which influences the immune cells’ ability to proliferate and differentiate [39]. Because of this association, pro-inflammatory cytokines (such as IL-1, TNF, and IL-6) are suppressed, whereas an anti-inflammatory reaction is produced. Moreover, the expression of tight junction proteins is increased, and short SFCAs could impact the integrity of the BBB and traverse BBB using monocarboxylate transporters. By altering the function and form of neural microglia cells in the CNS, SCFAs have an impact on neuroinflammation, avoiding the death of neuronal cells. The ejection of gut hormones is modulated by SCFAs, which function as endocrine signalling molecules. SFCAs improve boundary strength and preserve mucus through immunological regulation. Propionate and acetate greatly increase the production of PYY and glucagon-like peptide-1 (GLP-1) in mouse colonic cells by activating the G-protein-coupled receptor. Neurons and intestinal enteroendocrine L-cells in the nucleus of the brainstem of the tractus solitarius generate and secrete GLP-1 [40]. Glucagon-like peptide-1 functions in preventing cell death and neuronal apoptosis and as a neuroprotective agent in the brain. PYY serves as a gut hormone that reduces appetite. Studies on animals have shown that the neuropeptide Y is highly raised in the hippocampus, which is part of the temporal lobe, of mice with AD and has neuroprotective effects by activating the PI3K-XBP1-induced Gip78/BiP path as well as hindering caspase-4 and caspase-3 actions. It also reduces oxidative stress by preventing the Aβ-induced lipid peroxidation that causes modifications at the level of the neurotransmitter glutamate and oxidative stress [41].
Figure 3. The microbiome–gut-brain axis in AD.
Furthermore, neurotransmitters and neurotrophic factors may be modulated by short-chain fatty acids SCFAs. Investigations have shown that the gut microbiota can either create antecedents for neurotransmitters or catalyse their production and distribution through food consumption or even both [41]. Via secretory enterochromaffin (EC) cells, neurotransmitter precursors encourage the release of some neurotransmitters like GABA and 5-HT. Butyrate as well as propionate influences the production of 5-HT host from the serum and colonic endoplasmic reticulum, according to the findings of [42]. Cells of the EC generate several neuroactive by-products such as tryptophan, peptide tyrosine tyrosine (PYY), and histamine. Certain neuroactive metabolites and precursors of neurotransmitters can penetrate through the blood–brain barrier (BBB) and participate in signalling in the CNS and in the brain, where neurotransmitter production takes place. Evident types of gut bacteria directly affect the signalling of the vagus nerve by stimulating the dorsal motor nucleus of the vagus nerve (DMV). An expanding body of evidence points to lengthy stress as a high-risk factor in the development of AD, which may quicken the progression of the disease. Stress and anxiety have a strong link to dementia risk [43][44]. Stress originating from outside or from the environment could cause psychological distress, which can be made worse by oxidative damage and inflammation. In response to psychological stress, the hypothalamic–pituitary–adrenal axis (HPA) is stimulated, which results in the discharge of glucocorticoids into the blood system and their passage through the blood–brain barrier into the brain for the activation of nutrient corticosteroid receptors in the mouse model and glucocorticoid receptors in humans, including both [45]. By reducing the hypothalamic–pituitary–adrenal axis’s hyperactivity that results from inflammatory processes and gut microbiota dysbiosis, probiotics have a pragmatic outcome on the microbiota–gut-brain axis. According to research by Mindus et al. [46] on the various Lactobacillus strains, Lactobacillaceae rhamnoses, a probiotic, reduced anxiety-like behaviour and decreased corticosterone levels in non-stressed mice. It is currently thought that stress and HPA dysregulation are related, but the precise mechanism is still unknown. In the case of a mouse model with ongoing anxiety and stress brought on via maternal separation, a probiotic, namely Bifidobacterium pseudocatenulatum, enhanced glucocorticoid responsiveness and reduced inflammation induced by stress [43]. By restoring the brain expression levels of important glucose transporters (GLUT3 and GLUT1) and insulin-like growth factor receptor along with the diminished phosphorylation of adenosine-monophosphate-activated protein kinase and protein-kinase B (Akt), the study showed that oral administration of probiotics improves glucose uptake in 3xTg-AD mice. Parallel to this, mice treated by Bonfili et al. [47] showed a reduction in phosphorylated tau clumps. In line with memory enhancement, probiotics prevent the time-dependent rise in glycated haemoglobin and the build-up of advanced glycation end products in AD mice.
There were 35 total research studies carried out by Ji and Shen [48] including 26 studies using animal models and 9 studies using humans. In the 26 animal model studies, mice were employed in 24 of them, whereas AD models from Caenorhabditis elegans and Drosophila melanogaster were used in two of them, respectively. Thirteen studies used single-strain probiotics, while the remaining studies used multi-strain probiotics (ranging from two to nine probiotic strains); four used probiotic-fermented milk or probiotic-fermented soybean; two studies used engineered probiotic strains; and four studies concentrated on the synergistic effect of probiotics with the AD drug memantine, selenium, or exercise. The most often used probiotics in the trials that were included were Bifidobacterium and Lactobacillaceae species. These investigations demonstrated that probiotic treatment had neuroprotective effects, could lessen cognitive impairments, and could control gut microbiota dysbiosis, which may be connected to oxidative and inflammatory pathways. Probiotics appear to be an appealing strategy to treat AD, which opens the door for further study through carefully planned, large-scale clinical research. The effectiveness and safety of Lactobacillaceae plantarium C29-fermented soybean (DW2009) as a dietary supplement for improving cognition were examined by Hwang et al. [49]. For 12 weeks, 100 people with mild cognitive impairment (MCI) were randomly assigned to receive either DW2009 (800 mg/day, n = 50) or a placebo (800 mg/day, n = 50). The change in the composite score of memory- and attention-related cognitive abilities as determined by computerised neurocognitive function assessments served as the major outcome measure. For every single therapy group, correlations between changes in serum brain-derived neurotrophic factor (BDNF) levels and cognitive function were assessed. The DW2009 group had larger gains in the total cognitive functions as opposed to the placebo group (z = 2.36, p for interaction = 0.02), particularly in the attention domain (z = 2.34, p for interaction = 0.02). Following ingestion of DW2009, elevated blood BDNF levels were linked to improved cognition (t = 2.83, p = 0.007).
5. Probiotics Safety Considerations
In accordance with the American Food and Drug Administration (FDA), probiotics are considered safe. The efficacy of Lactobacillaceae, Bifidobacterium, Clostridium, and Streptococcus species in AD has not yet been subject to any recorded data. Probiotics should not always be given to patients with ongoing AD, especially the ones who are accepting drugs that act as immunosuppressors, such as chemotherapy [50]. Many incidences of fungemia, bacteraemia, and sepsis demonstrated in people who received S. boulardii have been documented. Probiotic bacteria might occasionally include genes for antibiotic resistivity, which can be passed on to additional another bacterium, to potentially pathogenic strains that can lead to infection. Probiotic safety should consider the characteristics of the probiotic, the patient/consumer, and the manufacturing process. (Contaminated probiotics products represent a safety concern). While some can be determined right away, others require more research before any meaningful recommendations can be made [51].
References
- Monteiro, S.S.; Almeida, R.L.; Santos, N.C.; Pereira, E.M.; Silva, A.P.; Oliveira, H.M.L.; Pasquali, M.A.B. New Functional Foods with Cactus Components: Sustainable Perspectives and Future Trends. Foods 2023, 12, 2494.
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.M.; Dequenne, I.; de Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2019, 9, 454.
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62.
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103.
- Widyastuti, Y.; Febrisiantosa, A.; Tidona, F. Health-Promoting Properties of Lactobacilli in Fermented Dairy Products. Front. Microbiol. 2021, 12, 673890.
- Lopez-Santamarina, A.; Gonzalez, E.G.; Lamas, A.; Mondragon, A.D.C.; Regal, P.; Miranda, J.M. Probiotics as a Possible Strategy for the Prevention and Treatment of Allergies. A Narrative Review. Foods 2021, 10, 701.
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558.
- Lenoir-Wijnkoop, I.; Merenstein, D.; Korchagina, D.; Broholm, C.; Sanders, M.E.; Tancredi, D. Probiotics Reduce Health Care Cost and Societal Impact of Flu-Like Respiratory Tract Infections in the USA: An Economic Modeling Study. Front. Pharmacol. 2019, 10, 980.
- Barta, D.G.; Cornea-Cipcigan, M.; Margaoan, R.; Vodnar, D.C. Biotechnological Processes Simulating the Natural Fermentation Process of Bee Bread and Therapeutic Properties—An Overview. Front. Nutr. 2022, 9, 871896.
- Madabushi, J.S.; Khurana, P.; Gupta, N.; Gupta, M. Gut Biome and Mental Health: Do Probiotics Work? Cureus 2023, 15, e40293.
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain–Gut–Microbiome Axis. Biomolecules 2021, 11, 1000.
- Reis, D.J.; Ilardi, S.S.; Punt, S.E.W. The Anxiolytic Effect of Probiotics: A Systematic Review and Meta-analysis of the Clinical and Preclinical Literature. PLoS ONE 2018, 13, e0199041.
- Wang, H.; Lee, I.S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605.
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014, 34, 15490–15496.
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the Dairy Industry—Advances and Opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982.
- Arora, K.; Green, M.; Prakash, S. The Microbiome and Alzheimer’s Disease: Potential and Limitations of Prebiotic, Synbiotic, and Probiotic Formulations. Front. Bioeng. Biotechnol. 2020, 8, 537847.
- Nagaoka, S. Yogurt Production. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; pp. 45–54.
- Chen, W.; Narbad, A. Lactic Acid Bacteria in Foodborne Hazards Reduction; Springer: Singapore, 2018.
- Du, Y.; Xu, W.; Wu, T.; Li, H.; Hu, X.; Chen, J. Enhancement of Growth, Survival, Immunity and Disease Resistance in Litopenaeus Vannamei, by the Probiotic, Lactobacillus plantarum Ep-M17. Fish Shellfish Immunol. 2022, 129, 36–51.
- Basso, M.; Johnstone, N.; Knytl, P.; Nauta, A.; Groeneveld, A.; Cohen Kadosh, K. A Systematic Review of Psychobiotic Interventions in Children and Adolescents to Enhance Cognitive Functioning and Emotional Behavior. Nutrients 2022, 14, 614.
- Vasiliu, O. The Current State of Research for Psychobiotics Use in the Management of Psychiatric Disorders–A Systematic Literature Review. Front. Psychiatry 2023, 14, 1074736.
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 Relieves Anxiety, Depression, and Insomnia Symptoms in Test Anxious College via Modulating the Gut Microbiota and Its Metabolism. Front. Immunol. 2023, 14, 1158137.
- Roy, S.; Banerjee, S.; Bhowmick, P.; Choudhury, L. Psychobiotics: Deciphering Its Role in Neuropsychiatry. World J. Bio. Pharm. Health Sci. 2023, 13, 457–464.
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463.
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in Mental Health, Neurodegenerative and Neurodevelopmental Disorders. J. Food Drug Anal. 2019, 27, 632–648.
- Sivamaruthi, B.S.; Prasanth, M.I.; Kesika, P.; Chaiyasut, C. Probiotics in Human Mental Health and Diseases-A Minireview. Trop. J. Pharm. Res. 2019, 18, 889–895.
- Eastwood, J.; Walton, G.; Van Hemert, S.; Williams, C.; Lamport, D. The Effect of Probiotics on Cognitive Function Across the Human Lifespan: A Systematic Review. Neurosci. Biobehav. Rev. 2021, 128, 311–327.
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The Role of Microbiota-Gut-Brain Axis in Neuropsychiatric and Neurological Disorders. Pharmacol. Res. 2021, 172, 105840.
- Papalini, S.; Michels, F.; Kohn, N.; Wegman, J.; van Hemert, S.; Roelofs, K.; Arias-Vasquez, A.; Aarts, E. Stress Matters: Randomized Controlled Trial on the Effect of Probiotics on Neurocognition. Neurobiol. Stress 2019, 10, 100141.
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W.; Doerr, C. Alzheimer Disease (Nursing). In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32.
- Simoes, S.; Guo, J.; Buitrago, L.; Qureshi, Y.H.; Feng, X.; Kothiya, M.; Cortes, E.; Patel, V.; Kannan, S.; Kim, Y.H.; et al. Alzheimer’s Vulnerable Brain Region Relies on a Distinct Retromer Core Dedicated to Endosomal Recycling. Cell Rep. 2021, 37, 110182.
- Tan, J.Z.A.; Gleeson, P.A. The Role of Membrane Trafficking in the Processing of Amyloid Precursor Protein and Production of Amyloid Peptides in Alzheimer’s Disease. Biochim. Biophys. Acta Biomembr. 2019, 1861, 697–712.
- Evrard, C.; Gilet, A.L.; Colombel, F.; Dufermont, E.; Corson, Y. Now You Make False Memories; Now You Do Not: The Order of Presentation of Words in DRM Lists Influences the Production of the Critical Lure in Alzheimer’s Disease. Psychol. Res. 2018, 82, 429–438.
- Naomi, R.; Embong, H.; Othman, F.; Ghazi, H.F.; Maruthey, N.; Bahari, H. Probiotics for Alzheimer’s Disease: A Systematic Review. Nutrients 2021, 14, 20.
- Steiner, H.; Fukumori, A.; Tagami, S.; Okochi, M. Making the Final Cut: Pathogenic Amyloid-β Peptide Generation by γ-Secretase. Cell Stress 2018, 2, 292–310.
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimers. Dement. 2018, 4, 575–590.
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; van Tol, E.A.; Tuohy, K.M. Towards Microbial Fermentation Metabolites as Markers for Health Benefits of Prebiotics. Nutr. Res. Rev. 2015, 28, 42–66.
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25.
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. In Int. J. Obes. 2015, 39, 424–429.
- Chen, X.Y.; Du, Y.F.; Chen, L. Neuropeptides Exert Neuroprotective Effects in Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 493.
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease Induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, KC01–KC05.
- Moya-Pérez, A.; Perez-Villalba, A.; Benítez-Páez, A.; Campillo, I.; Sanz, Y. Bifidobacterium CECT 7765 Modulates Early Stress-Induced Immune, Neuroendocrine, and Behavioral Alterations in Mice. Brain Behav. Immun. 2017, 65, 43–56.
- Wilson, R.S.; Begeny, C.T.; Boyle, P.A.; Schneider, J.A.; Bennett, D.A. Vulnerability to Stress, Anxiety, and Development of Dementia in Old Age. Am. J. Geriatr. Psychiatry 2011, 19, 327–334.
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276.
- Mindus, C.; Ellis, J.; Van Staaveren, N.; Harlander-Matauschek, A. Lactobacillus-Based Probiotics Reduce the Adverse Effects of Stress in Rodents: A Meta-analysis. Front. Behav. Neurosci. 2021, 15, 642757.
- Bonfili, L.; Cecarini, V.; Gogoi, O.; Berardi, S.; Scarpona, S.; Angeletti, M.; Rossi, G.; Eleuteri, A.M. Gut Microbiota Manipulation Through Probiotics Oral Administration Restores Glucose Homeostasis in a Mouse Model of Alzheimer’s Disease. Neurobiol. Aging 2020, 87, 35–43.
- Ji, H.F.; Shen, L. Probiotics as Potential Therapeutic Options for Alzheimer’s Disease. Appl. Microbiol. Biotechnol. 2021, 105, 7721–7730.
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and Safety of Lactobacillus plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305.
- Dudek-Wicher, R.; Junka, A.; Paleczny, J.; Bartoszewicz, M. Clinical Trials of Probiotic Strains in Selected Disease Entities. Int. J. Microbiol. 2020, 2020, 8854119.
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging Issues in Probiotic Safety: 2023 Perspectives. Gut Microbes 2023, 15, 2185034.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
905
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
24 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




