Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Akebe Luther King Abia | -- | 2851 | 2023-08-23 05:19:25 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 2853 | 2023-08-24 08:40:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Israel, E.; Ramganesh, S.; Abia, A.L.K.; Chikere, C.B. Quorum Sensing in Microorganisms. Encyclopedia. Available online: https://encyclopedia.pub/entry/48347 (accessed on 08 February 2026).
Israel E, Ramganesh S, Abia ALK, Chikere CB. Quorum Sensing in Microorganisms. Encyclopedia. Available at: https://encyclopedia.pub/entry/48347. Accessed February 08, 2026.
Israel, Edamkue, Selvarajan Ramganesh, Akebe Luther King Abia, Chioma Blaise Chikere. "Quorum Sensing in Microorganisms" Encyclopedia, https://encyclopedia.pub/entry/48347 (accessed February 08, 2026).
Israel, E., Ramganesh, S., Abia, A.L.K., & Chikere, C.B. (2023, August 23). Quorum Sensing in Microorganisms. In Encyclopedia. https://encyclopedia.pub/entry/48347
Israel, Edamkue, et al. "Quorum Sensing in Microorganisms." Encyclopedia. Web. 23 August, 2023.
Copy Citation
The marine environment possesses diverse and complex characteristics, representing a significant challenge for microbial survival. Therefore, bacteria must develop adaptive mechanisms to thrive in such environments. Quorum sensing (QS), a well-established phenomenon in microorganisms, involves the communication between cells through chemical signals, which depends on cell density. Extensive research has been conducted on this microbial ability, encompassing the early stages of understanding QS to the latest advancements in the identification and characterization of its mechanisms.
quorum sensing
microbial communication
biofilm formation
biogeochemical cycling
1. Introduction
Extensive research has significantly advanced the understanding of bacteria, particularly concerning their lifecycle. From the earliest investigations into basic phenomena, such as food fermentation, research has progressed to uncovering the presence of disease-causing pathogens in diverse ecological niches, including plant rhizosphere soil, deep ocean zones, alpine peaks, and other extreme environments [1]. Among the notable discoveries is the phenomenon of cell-to-cell communication, known as “quorum sensing,” which occurs within bacterial communities, both within and between species. Traditionally, quorum sensing (QS) was understood to predominantly regulate bacterial bioluminescence. The concept of quorum sensing was first observed over 25 years ago in two marine bacterial species: Vibrio fischeri and Vibrio harveyi. In these species, quorum sensing is controlled by various genes and regulons, including Lux genes (LuxCDABEG) and their respective autoinducers. In Vibrio fischeri, N-acyl homoserine lactone (AHL) acts as the autoinducer, while in Vibrio harveyi, autoinducer-2 (AI-2) plays QS role [2]. These autoinducers are synthesized when the bacterial population is low, but once the bacterial density reaches a threshold, these molecules bind to their respective receptor proteins, initiating a signaling cascade. This signaling alters the expression of lux genes, thus triggering the activation of bioluminescence, a phenomenon termed ‘quorum sensing’ [3][4][5]. Since this initial observation, researchers have identified homologs of these genes in various bacterial communities, which play a regulatory role in quorum sensing. This communication process has been found to influence a wide range of biological processes, including bioluminescence [6], biofilm formation [7], cell competency [8], horizontal gene transfer [9], virulence factor expression [10], symbiotic relationships [11], sporulation in fungi [12][13], pigment production [14], motility [15], toxin production [16], and even antibiotic production [17][18][19]. These observations highlight the intricate communication networks and coordinated behavioral patterns within bacterial communities, underscoring the complexity of microbial ecosystems. Investigations into QS have significantly enriched our understanding of the regulatory mechanisms governing bacterial interactions, thereby offering novel insights into microbial ecology and behavior. Furthermore, the study of QS has sparked interest in the potential for strategic manipulation of bacterial behavior. This field of study opens up promising avenues for application across a broad range of sectors, including healthcare for infection control, environmental management for bioremediation efforts, and various advancements in biotechnology.
To effectively engage in QS, bacteria must possess certain capabilities, including producing and secreting autoinducer signaling molecules, perceiving changes in their concentration, and respond by regulating gene transcription [20][21]. The process heavily relies on the diffusion mechanism of these signaling molecules, which are typically secreted at low concentrations by individual bacteria. These molecules may simply diffuse away from the cell in environments with low cell density. However, as cell density increases, the local concentration of signaling molecules can surpass a threshold level, triggering a shift in gene expression [22]. Numerous quorum sensing systems have been extensively studied and documented in various taxa and habitats (a PubMed keyword search for “quorum sensing” generates over 12,000 results) [23]. However, many taxa and habitats remain, including deep-sea microbes, where quorum sensing remains unidentified or poorly characterized. Further research is needed to explore and understand the prevalence and specific mechanisms of quorum sensing in these diverse environments
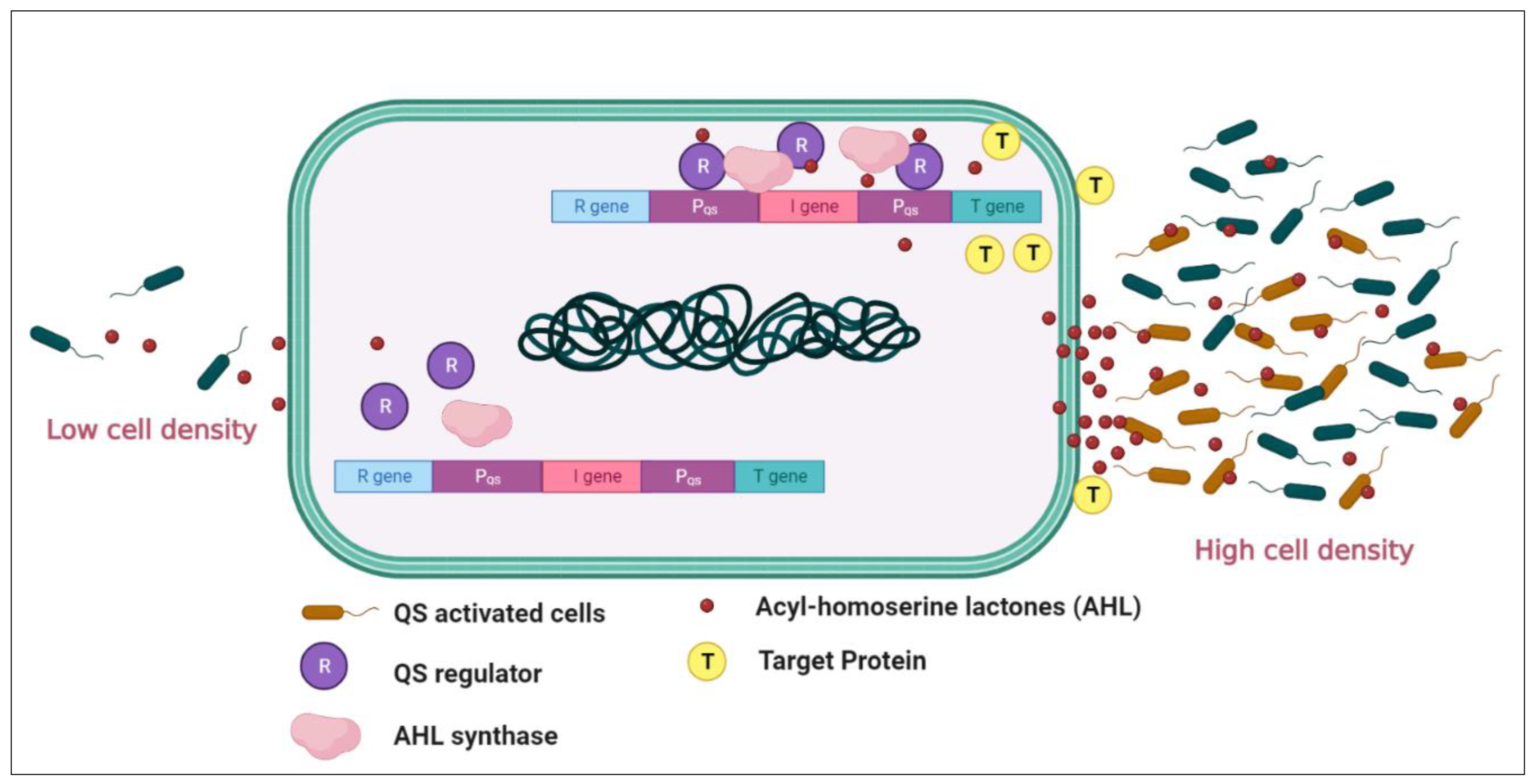
2. Bacterial Quorum Sensing in Oceanic Settings
Quorum sensing (QS) is crucial in bacterial intercommunication, particularly in marine ecosystems. Traditionally, QS has been associated with the regulation of bacterial bioluminescence. However, a study by Tanet et al. [24] presented a challenge to this conventional understanding. The researchers analyzed the bioluminescent strain Photobacterium phosphoreum ANT-2200, originating from deep-sea waters of the Mediterranean, and found it exhibited higher light emission rates at lower cell densities rather than higher ones. Additionally, high hydrostatic pressure did not affect QS gene transcription in this bacteria. Consequently, these findings suggest that in this particular strain, bioluminescence regulation may not be density-dependent and could be independent of conventional QS control mechanisms.
Quorum sensing (QS) has been discovered in diverse bacterial species and habitats, including marine ecosystems. N-acyl homoserine lactones typically serve as signaling molecules in quorum sensing for gram-negative bacteria, whereas gram-positive bacteria generally employ autoinducing peptides for intercellular communication. However, these broad categories are not all-encompassing. Numerous bacterial species are known to produce unique autoinducers, underlining the diversity and specificity inherent in bacterial communication systems [25]. In marine environments, the genera belonging to Proteobacteria that commonly produce autoinducers (Ais) predominantly include Pseudoalteromonas, Thalassomonas, Pseudomonas, Roseobacter, Aeromonas, and Vibrio. QS has also been identified in Epsilonproteobacteria, some of which are human pathogens. The thermal origin of this ability in mesophilic and pathogenic Epsilonproteobacteria has been traced back to ocean hydrothermal vents [26]. Among the different classes of signaling molecules, N-acyl homoserine lactones (AHLs), oligopeptides, and LuxS/autoinducer 2 (AI-2) have been extensively studied in marine environments [27]. For instance, some marine bacteria, such as Vibrio species, have been shown to employ multiple QS systems simultaneously, indicating a complex hierarchical organization of QS regulation that might differ from most terrestrial bacteria. Additionally, some marine bacteria are known to produce unique QS signal molecules, such as the boronated autoinducer AI-2 (BAI-2) in Vibrio harveyi, which is a derivative of the more common AI-2 signal and is believed to be more stable in the marine environment. Vibrio harveyi, a Gram-negative bacterium renowned for its bioluminescence, thrives predominantly in marine settings as a free-living organism. However, its versatility extends to engaging symbiotic and pathogenic relationships with diverse marine creatures [28]. The crux of V. harveyi’s ability to interact with its hosts lies in its quorum sensing system, a regulatory mechanism controlling its bioluminescence, biofilm development, and virulence factor expression. This complex system, which encompasses multiple signaling molecules, enables V. harveyi to adapt and react to changes within its host environment and microbial community. In the quorum sensing (QS) network of Vibrio harveyi, three autoinducers (Ais) are used, which differ based on whether they are for intra-species, intra-genera, or inter-species communication [29]. Another common QS network structure is observed in the Pseudomonas genus, particularly in Pseudomonas aeruginosa. In marine ecosystems, P. aeruginosa has been isolated from various niches, including coastal waters, marine sediments, and marine organisms [30]. It is known for its robust biofilm formation capabilities, enabling it to survive in challenging marine conditions. This bacterium possesses four known QS pathways that function independently or in a coordinated manner. Two of these pathways are of the LuxI/LuxR type, specifically the LasI/LasR and RhlI/RhlR systems. Additionally, P. aeruginosa uses the quinolone-based QS system (PQS, which uses the 2-heptyl3-hydroxy-4-quinolone signal) and more recently identified integrated QS system (IQS, utilizing the 2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde signal). These QS circuits are arranged hierarchically (Figure 1) [31].

Figure 1. An illustration of the working system of the autoinducer mechanism in Gram-negative (Pseudomonas aeruginosa). The bacteria secrete AHLs (red dots) that, in threshold concentrations, penetrate the cells, activate the AHL receptor, and induce the QS-regulated gene expression. ‘I’ represents the LuxI gene encoding for the enzyme that synthesizes the AHL signal, and ‘R’ represents the LuxR gene encoding for the protein that binds AHL and modulates the transcription of luciferase genes, quinolone-based QS system (Pqs). (Image created in Bio-render online platform).
To date, no AI-2 receptors have been identified in Epsilonproteobacteria. However, two main receptor categories, LuxP in Vibrio spp. And LrsB in enteric bacteria are known. In deep-sea vents, Epsilonproteobacteria act as early-stage colonizers, preparing the stage for colonizing other microbes through QS signaling involving adhesive polymeric secretion. This secretion also serves as a nutrient source, promoting the recruitment of additional microbial species. The extracellular matrix of P. aeruginosa is composed of extracellular DNA (eDNA), which aids biofilm assemblage in a reaction with extracellular calcium by promoting bacterial association when released via QS-dependent and independent pathways. eDNA affects the RNA composition of members of the biofilm, acting as a nutrient source through the contents of the membrane vesicle in which it is enclosed, as well as modulating the expression of genes influencing acidity and antimicrobial resistance [32].
3. Biofilm Formation
Biofilms play a crucial role in the adaptive strategies employed by bacteria in marine environments, including deep-sea ecosystems. These complex structures support bacterial survival and thriving within these environments. The unique characteristics of biofilms provide member cells with distinct advantages, such as heightened resistance against host immune responses, enhanced nutrient availability and utilization, and increased tolerance to antimicrobial agents [33]. Several studies focusing on Vibrio fischeri, Vibrio anguillarum, and Vibrio harveyi have demonstrated the involvement of QS in regulating bioluminescence, virulence, and biofilm formation in these species. Notably, biofilms from extreme environments are able to rapidly transition from early colonization stages to robust production of extracellular polymeric substances (EPS) that contribute to protective mechanisms [34].
In marine ecosystems, EPS production is a common trait observed among various microorganisms, including bacteria, cyanobacteria, and actinobacteria. These EPS molecules contribute to biofilms’ development and structural integrity in marine environments, facilitating microbial survival and adaptation [34]. Biofilm formation mechanisms vary among different bacterial species. In dominant oceanic bacteria, such as Vibrio species, N-acyl homoserine lactones (AHLs) regulate the secretion of Extracellular Polymeric Substances (EPS) to construct a biofilm matrix. Specific AHLs, such as C4-HSL and C6-HSL, elevate the expression of EPS-related genes by binding to AHL receptor proteins, thereby enhancing EPS production and forming a denser biofilm matrix [35]. In contrast, P. aeruginosa has identified five gene clusters believed to contribute to exopolysaccharide synthesis, which includes the alg biosynthetic genes, the psl and pel operons, and two additional gene clusters. Only the pel biosynthetic operon is definitively known to be subject to QS regulation [36]. The proteins encoded by the pel gene cluster (pelABCDEFG) produce a glucose-rich biofilm exopolysaccharide [37].
Furthermore, QS has been implicated in the regulation of motility and colonization in marine bacteria. In Vibrio cholerae, a pathogenic bacterium responsible for cholera, quorum sensing coordinates the expression of genes involved in flagellar motility and biofilm formation, enabling efficient colonization of aquatic environments and host surfaces [38]. Quorum sensing in marine environments is not limited to bacteria alone. Marine algae, such as diatoms, also employ QS mechanisms to coordinate their growth and reproductive processes [39]. This interkingdom communication between bacteria and algae further highlights the ecological significance of QS in marine ecosystems. The deep-sea environment presents unique challenges for microbial communities, including high pressure, low temperature, and limited nutrient availability. Quorum sensing allows bacteria to adapt to these extreme conditions by facilitating cooperative behaviors and resource sharing within biofilms. Moreover, studies have shown that deep-sea bacteria exhibit specific adaptations in their QS systems, likely influenced by the selective pressures of these environments [40][41]. Marine bacteria might adopt specific adaptations in their quorum sensing systems, including changes in signaling molecules [42], modifications of receptors [43], or alterations in downstream signal transduction pathways [44].
4. Biogeochemical Cycling and Quorum Sensing
Quorum sensing mechanisms have been found to play a significant role in regulating microbial activities involved in biogeochemical cycling in the ocean. Numerous marine bacterial species participate in quorum sensing and are crucial regulators of biogeochemical cycles, including Vibrio sp, Pseudomonas, Pseudoalteromonas, Marinobacter, Roseobacter, and some members of Cyanobacteria. In marine environments, QS-mediated gene regulation influences the cycling of essential elements, such as carbon, nitrogen, phosphorous, and sulfur. For example, QS controls the production of extracellular enzymes involved in the breakdown of complex organic matter, allowing bacteria to efficiently utilize these substrates for energy and nutrient acquisition [38][45]. The coordinated expression of genes involved in enzymatic activities enhances the degradation and cycling of organic compounds in the ocean. Vibrio and Pseudoalteromonas species are associated with the carbon cycle as they have the capability to break down chitin, a highly abundant N-acetylglucosamine polymer in the ocean [46]. These bacteria play a key role in the phosphorus cycle due to their ability to produce phosphatases, enzymes that cleave phosphate groups from organic molecules, making it bioavailable for themselves and other organisms [47]. Notably, Marinobacter, a Proteobacteria genus, is often found near oil spills [48], where it aids in hydrocarbon degradation, thereby participating in the carbon cycle. Quorum sensing also influences the nitrogen cycle by regulating nitrogen fixation, nitrification, and denitrification processes. Some marine bacteria, such as the genus Vibrio, utilize QS to control the expression of nitrogen fixation genes, enabling them to convert atmospheric nitrogen into biologically available forms [49]. Quorum sensing also regulates the expression of genes involved in nitrification and denitrification, affecting the balance of nitrogen species in marine ecosystems.
Sulfur cycling in the ocean is another biogeochemical process influenced by QS. QS-controlled gene expression in sulfur-oxidizing bacteria regulates the production of enzymes that oxidise reduced sulfur compounds, such as hydrogen sulfide, into sulfate [50][51]. This process is crucial for sulfur recycling in marine environments and contributes to the global sulfur cycle. The Roseobacter Clade, part of the Alphaproteobacteria, plays a key role in the sulfur cycle by breaking down dimethylsulfoniopropionate (DMSP), a prevalent sulfur compound in the ocean, subsequently leading to the production of dimethyl sulfide (DMS), a gas that has a significant impact on the climate [52].
5. Methods of Monitoring Quorum Sensing
Monitoring QS in the marine environment requires using specific techniques to capture the dynamic nature of QS interactions. Time-series methods are commonly employed to observe QS dynamics in deep-sea environments. These methods involve collecting samples at multiple time points and analyzing them using various techniques.
Omics approaches, such as metagenomics, metatranscriptomics, and metaproteomics, provide a comprehensive view of the microbial community and its gene expression patterns. These techniques enable the identification of QS-related genes, signaling molecules, and regulatory networks in the marine environment [53][54]. By analyzing the genetic information encoded in the collected samples, researchers can gain insights into the presence and activity of QS systems. Fluorescence in situ hybridization (FISH) is a microscopy-based technique that allows the visualization and identification of specific microbial populations in their natural habitat [55][56]. Using fluorescently labeled probes that target QS-related genes or specific microbial taxa, researchers can observe the spatial distribution and abundance of QS-active cells in marine samples [53]. This technique provides valuable information on the localization and dynamics of QS-mediated interactions. Bioengineered biosensors offer a powerful tool for studying QS dynamics in marine systems in real-time [57]. These biosensors are typically designed to detect and respond to specific QS signaling molecules, providing valuable information about the temporal and spatial distribution of these molecules within marine environments.
Raman microscopy is another powerful tool used in QS studies. It enables the non-destructive analysis of individual cells and their chemical composition. By detecting molecular vibrations, Raman microscopy can provide insights into cellular metabolism and QS-related molecule production in the marine environment [53][58]. Stimulated Raman Scattering (SRS) Microscopy is a technique that holds great potential for examining biofilms, especially in situ, due to its ability to swiftly provide details about the biofilm’s chemical composition and structure [59].
6. Biotechnological Applications
The biotechnological advancements in QS have led to the discovery and utilization of extracellular polymeric substances (EPS) produced by marine bacteria for various applications in different industries. One notable example is the EPS produced by Vibrio diabolicus and Alteromonas infernus, isolated from deep-sea hydrothermal vents, showing medicinal regenerative properties. These EPS contain glycosaminoglycans that are beneficial for tissue repair and remodeling due to their low immunogenicity. One such product derived from these EPS is Hyalurift®, which utilizes hyaluronic acid to promote tissue remodeling and aid in joint injuries and embryogenesis. The EPS consists of high molecular weight linear tetrasaccharidic repeating units of glucuronic acid and hexosamine (N-acetyl-glucosamine and N-acetyl-galactosamine), resembling a fusion of hyaluronan and non-sulfated chondroitin units [60]. Alteromonas macleodii subsp. Fijiensis, strain HYD657, is another species that produces EPS with protective properties for sensitive skin against chemicals and UV radiation. This EPS, marketed as Abyssine, offers potential applications in skin care products for its protective and rejuvenating effects [34].
In the food industry, EPS produced by Labrenzia sp. have gained attention due to their low viscosity and antioxidant properties. These EPS can be utilized in food and pharmaceutical products, providing viscosity control and antioxidant benefits (Di Donato et al., 2016). Deep-sea bacteria have also shown the production of extremozymes, which are enzymes capable of functioning under extreme conditions. These extremozymes have significant applications in bioremediation, particularly in degrading hazardous compounds, such as crude oil, polycyclic aromatic hydrocarbons (PAHs), and heavy metals, in the environment. They offer potential solutions for environmental clean-up and mitigation of pollution [61].
7. Conclusions
Studying QS in extreme environments, including deep-sea, poses unique challenges in fully comprehending its intricate systems. However, unraveling the functions, molecular components, and underlying mechanisms of QS is imperative. Deep-sea environments have served as habitats for ancestral microorganisms, making it crucial to investigate quorum sensing to gain a comprehensive understanding of its roles in microbial communities, as many of these microbes have contributed to the cultivation of various organisms observed today. In-depth studies have shed light on strategies to mitigate virulence by disrupting quorum sensing through QS techniques and enhancing therapeutic applications by inducing autoinducer molecules. It is essential to incorporate ecological considerations in microbial QS research, recognizing the influence of evolution within diverse bacterial communities across different environments.
References
- Sood, U.; Dhingra, G.G.; Anand, S.; Hira, P.; Kumar, R.; Kaur, J.; Verma, M.; Singhvi, N.; Lal, S.; Rawat, C.D.; et al. Microbial Journey: Mount Everest to Mars. Indian J. Microbiol. 2022, 62, 323–337.
- Bassler, B.L.; Losick, R. Bacterially Speaking. Cell 2006, 125, 237–246.
- Nealson, K.H.; Platt, T.; Hastings, J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970, 104, 313–322.
- Nealson, K.H. Autoinduction of bacterial luciferase: Occurrence, mechanism and significance. Arch. Microbiol. 1977, 112, 73–79.
- Engebrecht, J.; Nealson, K.; Silverman, M. Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell 1983, 32, 773–781.
- Wang, D.; Bai, L.; Li, S.; Yan, W. Similarities and Differences in Quorum Sensing-Controlled Bioluminescence between Photobacterium phosphoreum T3 and Vibrio qinghaiensis sp.-Q67. Appl. Sci. 2022, 12, 2066.
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e10.
- Shanker, E.; Federle, M.J. Quorum sensing regulation of competence and bacteriocins in Streptococcus pneumoniae and mutans. Genes 2017, 8, 15.
- van Gestel, J.; Bareia, T.; Tenennbaum, B.; Dal Co, A.; Guler, P.; Aframian, N.; Puyesky, S.; Grinberg, I.; D’Souza, G.G.; Erez, Z.; et al. Short-range quorum sensing controls horizontal gene transfer at micron scale in bacterial communities. Nat. Commun. 2021, 12, 2324.
- İnat, G.; Sırıken, B.; Başkan, C.; Erol, İ.; Yıldırım, T.; Çiftci, A. Quorum sensing systems and related virulence factors in Pseudomonas aeruginosa isolated from chicken meat and ground beef. Sci. Rep. 2021, 11, 15639.
- Kai, K. Bacterial quorum sensing in symbiotic and pathogenic relationships with hosts. Biosci. Biotechnol. Biochem. 2018, 82, 363–371.
- Albuquerque, P.; Casadevall, A. Quorum sensing in fungi—A review. Med. Mycol. 2012, 50, 337–345.
- Huang, M.; Hull, C.M. Sporulation: How to survive on planet Earth (and beyond) HHS Public Access Author manuscript. Curr. Genet. 2017, 63, 831–838.
- Joshi, C.; Kothari, V.; Patel, P. Importance of selecting appropriate wavelength, while quantifying growth and production of quorum sensing regulated pigments in bacteria. Recent Pat. Biotechnol. 2016, 10, 145–152.
- Bramhachari, P.V.; Yugandhar, N.M.; Prathyusha, A.; Mohana Sheela, G.; Naravula, J.; Venkateswarlu, N. Quorum sensing regulated swarming motility and migratory behavior in bacteria. In Implication of Quorum Sensing System in Biofilm Formation and Virulence; Springer: Berlin/Heidelberg, Germany, 2018; pp. 49–66.
- Butrico, C.E.; Cassat, J.E. Quorum sensing and toxin production in Staphylococcus aureus osteomyelitis: Pathogenesis and paradox. Toxins 2020, 12, 516.
- Armes, A.C.; Walton, J.L.; Buchan, A. Quorum Sensing and Antimicrobial Production Orchestrate Biofilm Dynamics in Multispecies Bacterial Communities. Microbiol. Spectr. 2022, 10, e02615-22.
- Liu, X.; Bimerew, M.; Ma, Y.; Müller, H.; Ovadis, M.; Eberl, L.; Berg, G.; Chernin, L. Quorum-sensing signaling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain of Serratia plymuthica. FEMS Microbiol. Lett. 2007, 270, 299–305.
- Sanchez, L.M.; Mannathan, S.; Lee, C.-K.; Merlo, M.E.; Meng, X.-F.; Nihira, T.; Takano, E.; Minnaard, A.J.; Dijkhuizen, L.; Petrusma, M. Identification and characterisation of enzymes involved in γ-butyrolactone biosynthesis in Streptomyces coelicolor. In Quorum Sensing in Streptomyces Coelicolor; University of Groningen: Groningen, The Netherlands, 2016; pp. 92–137.
- Pan, J.; Ren, D. Quorum sensing inhibitors: A patent overview. Expert Opin. Ther. Pat. 2009, 19, 1581–1601.
- Carradori, S.; Di Giacomo, N.; Lobefalo, M.; Luisi, G.; Campestre, C.; Sisto, F. Biofilm and quorum sensing inhibitors: The road so far. Expert Opin. Ther. Pat. 2020, 30, 917–930.
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587.
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320.
- Tanet, L.; Tamburini, C.; Baumas, C.; Garel, M.; Simon, G.; Casalot, L. Bacterial bioluminescence: Light Emission in Photobacterium phosphoreum Is Not under Quorum-Sensing Control. Front. Microbiol. 2019, 10, 365.
- Suresh, S.; Alva, P.P.; Premanath, R. Modulation of quorum sensing-associated virulence in bacteria: Carbohydrate as a key factor. Arch. Microbiol. 2021, 203, 1881–1890.
- Pérez-Rodríguez, I.; Bolognini, M.; Ricci, J.; Bini, E.; Vetriani, C. From deep-sea volcanoes to human pathogens: A conserved quorum-sensing signal in Epsilonproteobacteria. ISME J. 2015, 9, 1222–1234.
- Keller, L.; Surette, M.G. Communication in bacteria: An ecological and evolutionary perspective. Nat. Rev. Microbiol. 2006, 4, 249–258.
- Girard, L. Quorum sensing in Vibrio spp.: The complexity of multiple signalling molecules in marine and aquatic environments. Crit. Rev. Microbiol. 2019, 45, 451–471.
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588.
- Rehman, Z.U.; Leiknes, T.O. Quorum-quenching bacteria isolated from red sea sediments reduce biofilm formation by Pseudomonas aeruginosa. Front. Microbiol. 2018, 9, 1354.
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41.
- del Mar Cendra, M.; Torrents, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021, 49, 107734.
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586.
- Di Donato, P.; Poli, A.; Taurisano, V.; Abbamondi, G.R.; Nicolaus, B.; Tommonaro, G. Recent advances in the study of marine microbial biofilm: From the involvement of quorum sensing in its production up to biotechnological application of the polysaccharide fractions. J. Mar. Sci. Eng. 2016, 4, 34.
- Jamuna Bai, A.; Ravishankar Rai, V. Effect of small chain N acyl homoserine lactone quorum sensing signals on biofilms of food-borne pathogens. J. Food Sci. Technol. 2016, 53, 3609–3614.
- Sakuragi, Y.; Kolter, R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J. Bacteriol. 2007, 189, 5383–5386.
- Friedman, L.; Kolter, R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 2004, 51, 675–690.
- Zhu, J.; Mekalanos, J.J. Quorum Sensing-Dependent Biofilms Enhance Colonization in Vibrio cholerae), the phenomenon by which bacteria monitor their cell population density through the extracellular accumulation of signaling molecules called autoinduc. Dev. Cell 2003, 5, 647–656.
- Seyedsayamdost, M.R.; Carr, G.; Kolter, R.; Clardy, J. Roseobacticides: Small molecule modulators of an algal-bacterial symbiosis. J. Am. Chem. Soc. 2011, 133, 18343–18349.
- Krupke, A.; Hmelo, L.R.; Ossolinski, J.E.; Mincer, T.J.; Van Mooy, B.A.S. Quorum sensing plays a complex role in regulating the enzyme hydrolysis activity of microbes associated with sinking particles in the ocean. Front. Mar. Sci. 2016, 3, 55.
- Cude, W.N.; Mooney, J.; Tavanaei, A.A.; Hadden, M.K.; Frank, A.M.; Gulvik, C.A.; May, A.L.; Buchan, A. Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp. strain Y4I. Appl. Environ. Microbiol. 2012, 78, 4771–4780.
- Baysse, C.; Cullinane, M.; Dénervaud, V.; Burrowes, E.; Dow, J.M.; Morrissey, J.P.; Tam, L.; Trevors, J.T.; O’Gara, F. Modulation of quorum sensing in Pseudomonas aeruginosa through alteration of membrane properties. Microbiology 2005, 151, 2529–2542.
- Eldar, A. Social conflict drives the evolutionary divergence of quorum sensing. Proc. Natl. Acad. Sci. USA. 2011, 108, 13635–13640.
- Ball, A.S.; Chaparian, R.R.; Van Kessel, J.C. Quorum sensing gene regulation by LuxR/HapR master regulators in vibrios. J. Bacteriol. 2017, 199, 1110–1128.
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199.
- Raimundo, I.; Silva, R.; Meunier, L.; Valente, S.M.; Lago-Lestón, A.; Keller-Costa, T.; Costa, R. Functional metagenomics reveals differential chitin degradation and utilization features across free-living and host-associated marine microbiomes. Microbiome 2021, 9, 43.
- Stout, L.M.; Joshi, S.R.; Kana, T.M.; Jaisi, D.P. Microbial activities and phosphorus cycling: An application of oxygen isotope ratios in phosphate. Geochim. Cosmochim. Acta 2014, 138, 101–116.
- dos Santos, H.F.; Cury, J.C.; do Carmo, F.L.; dos Santos, A.L.; Tiedje, J.; van Elsas, J.D.; Rosado, A.S.; Peixoto, R.S. Mangrove Bacterial Diversity and the Impact of Oil Contamination Revealed by Pyrosequencing: Bacterial Proxies for Oil Pollution. PLoS ONE 2011, 6, e16943.
- Visick, K.L.; Ruby, E.G. Vibrio fischeri and its host: It takes two to tango. Curr. Opin. Microbiol. 2006, 9, 632–638.
- Weiland-Bräuer, N. Friends or foes—Microbial interactions in nature. Biology 2021, 10, 496.
- Sievert, S.M.; Vetriani, C. Chemoautotrophy at deep-sea vents: Past, present, and future. Oceanography 2012, 25, 218–233.
- Li, C.Y.; Mausz, M.A.; Murphy, A.; Zhang, N.; Chen, X.L.; Wang, S.Y.; Gao, C.; Aguilo-Ferretjans, M.M.; Silvano, E.; Lidbury, I.D.E.A.; et al. Ubiquitous occurrence of a dimethylsulfoniopropionate ABC transporter in abundant marine bacteria. ISME J. 2023, 17, 579–587.
- Nawaz, M.Z.; Sasidharan, R.S.; Alghamdi, H.A.; Dang, H. Understanding Interaction Patterns within Deep-Sea Microbial Communities and Their Potential Applications. Mar. Drugs 2022, 20, 108.
- Hualpa-Cutipa, E.; Andi, R.; Acosta, S.; Landa-Acuña, D.; Elena, M.; Salvatierra, S.; Reynaldo, J.; Quiroz, R. Omics Insights into Quorum Sensing and Biofilm Formation. In Omics for Environmental Engineering and Microbiology Systems; CRC Press: Boca Raton, FL, USA, 2022; pp. 143–157.
- Barbosa, A.; Miranda, S.; Azevedo, N.F.; Cerqueira, L.; Azevedo, A.S. Imaging biofilms using fluorescence in situ hybridization: Seeing is believing. Front. Cell. Infect. Microbiol. 2023, 13, 1195803.
- Kai, K.; Furuyabu, K.; Tani, A.; Hayashi, H. Production of the quorum-sensing molecules N-acylhomoserine lactones by endobacteria associated with Mortierella alpina A-178. ChemBioChem 2012, 13, 1776–1784.
- Li, J.; Liu, R.; Chen, Y.; Liu, S.; Chen, C.; Liu, T.; Yang, S.; Zhuang, Y.; Yang, R.; Cui, Y.; et al. Computer-Aided Rational Engineering of Signal Sensitivity of Quorum Sensing Protein LuxR in a Whole-Cell Biosensor. Front. Mol. Biosci. 2021, 8, 729350.
- Keleştemur, S.; Avci, E.; Çulha, M. Raman and surface-enhanced Raman scattering for biofilm characterization. Chemosensors 2018, 6, 5.
- Manifold, B.; Fu, D. Quantitative Stimulated Raman Scattering Microscopy: Promises and Pitfalls. Annu. Rev. Anal. Chem. 2022, 15, 269–289.
- Senni, K.; Gueniche, F.; Changotade, S.; Septier, D.; Sinquin, C.; Ratiskol, J.; Lutomski, D.; Godeau, G.; Guezennec, J.; Colliec-Jouault, S. Unusual glycosaminoglycans from a deep sea hydrothermal bacterium improve fibrillar collagen structuring and fibroblast activities in engineered connective tissues. Mar. Drugs 2013, 11, 1351–1369.
- Montgomery, K.; Charlesworth, J.C.; LeBard, R.; Visscher, P.T.; Burns, B.P. Quorum sensing in extreme environments. Life 2013, 3, 131–148.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
24 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




