Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vimala S K Bharathi | -- | 6666 | 2023-08-22 18:40:17 | | | |
| 2 | Vimala S K Bharathi | + 9 word(s) | 6675 | 2023-08-22 19:03:40 | | | | |
| 3 | Jason Zhu | Meta information modification | 6675 | 2023-08-23 03:25:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bharathi, V.S.K.; Jian, F.; Jayas, D.S. Rusty Grain Beetle. Encyclopedia. Available online: https://encyclopedia.pub/entry/48335 (accessed on 05 March 2026).
Bharathi VSK, Jian F, Jayas DS. Rusty Grain Beetle. Encyclopedia. Available at: https://encyclopedia.pub/entry/48335. Accessed March 05, 2026.
Bharathi, Vimala S. K, Fuji Jian, Digvir S. Jayas. "Rusty Grain Beetle" Encyclopedia, https://encyclopedia.pub/entry/48335 (accessed March 05, 2026).
Bharathi, V.S.K., Jian, F., & Jayas, D.S. (2023, August 22). Rusty Grain Beetle. In Encyclopedia. https://encyclopedia.pub/entry/48335
Bharathi, Vimala S. K, et al. "Rusty Grain Beetle." Encyclopedia. Web. 22 August, 2023.
Copy Citation
Cryptolestes ferrugineus, the rusty grain beetle, is a cosmopolitan pest that has adapted to cool and warm climates due to its unique biology, ecology, and behavior. The rusty grain beetle is a pest of high economic importance; hence, understanding their biology, ecology, and behavior could be useful in designing effective management strategies.
Cryptolestes ferrugineus
rusty grain beetle
stored grain pest
1. Introduction
The rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae), is considered a significant threat to the global food supply chain, causing significant economic losses and food waste. The insect is known for its reddish-brown coloration, adaptability to wide environmental conditions, cosmopolitan nature, unique behavior, and reproductive capabilities. Despite its importance, a comprehensive publication on the biology, ecology, and behavior of C. ferrugineus is currently lacking in the literature. In 1949, Rilett [1] summarized the biology of C. ferrugineus in detail. In 2009, Jian and Jayas [2] provided a detailed review focusing mainly on the movement of the insect. Several aspects of its behavior have been explored by various researchers around the world.
2. Geographical Distribution
The rusty grain beetle has been reported in more than 110 countries (Figure 1) and can be found in almost any country of the world, ranging from humid to dry as well as cool to warm climates, due to its ability to develop in wide environmental conditions and the world trade. The climatic plasticity index of C. ferrugineus is 570 [3], indicating its remarkable ability to adapt to changes in environmental conditions. Among the 195 countries listed in FAO [4], researchers could not find the sources to confirm the presence of C. ferrugineus in 82 countries on different continents, such as Africa (19), Asia (18), Europe (12), North America (12), South America (5), and Oceania (15). Considering the nature of C. ferrugineus, it could possibly be established in most of those countries as well. In countries like Canada with cold winters, the establishment of other stored grain pests was limited. However, C. ferrugineus has been identified as one of the major grain pests in western Canada since the early 1940s. The species has been found in Roman archaeological excavations in England and Israel [5]. Since it is widely distributed in the world, researchers have extensively explored its ecology, behavior, and control techniques.
Even though C. ferrugineus can develop on botanicals such as Pimpinella anisum (L.) (anise), Hibiscus sabdariffa (L.) (roselle), Coriandrum sativum (L.) (coriander), Matricaria chamomilla (L.) (chamomile), Glossostemon bruguieri (Desf.) (mogat), and Origanum majorana (L.) (marjoram), it thrives in stored grain [6]. Cryptolestes ferrugineus mainly infests the following stored products: wheat, maize, barley, sorghum, oats, flour, groundnuts, beans, cassava, rice, sunflower seeds, palm kernels, cacao beans, and cotton seeds. They are found in farms, maltings, mills, warehouses, storage bins, and other storage structures [7]. At their optimum temperature (33 °C) [3], Cryptolestes ferrugineus can rapidly multiply and damage the grain, leaving the hollow grain kernels as leftovers.
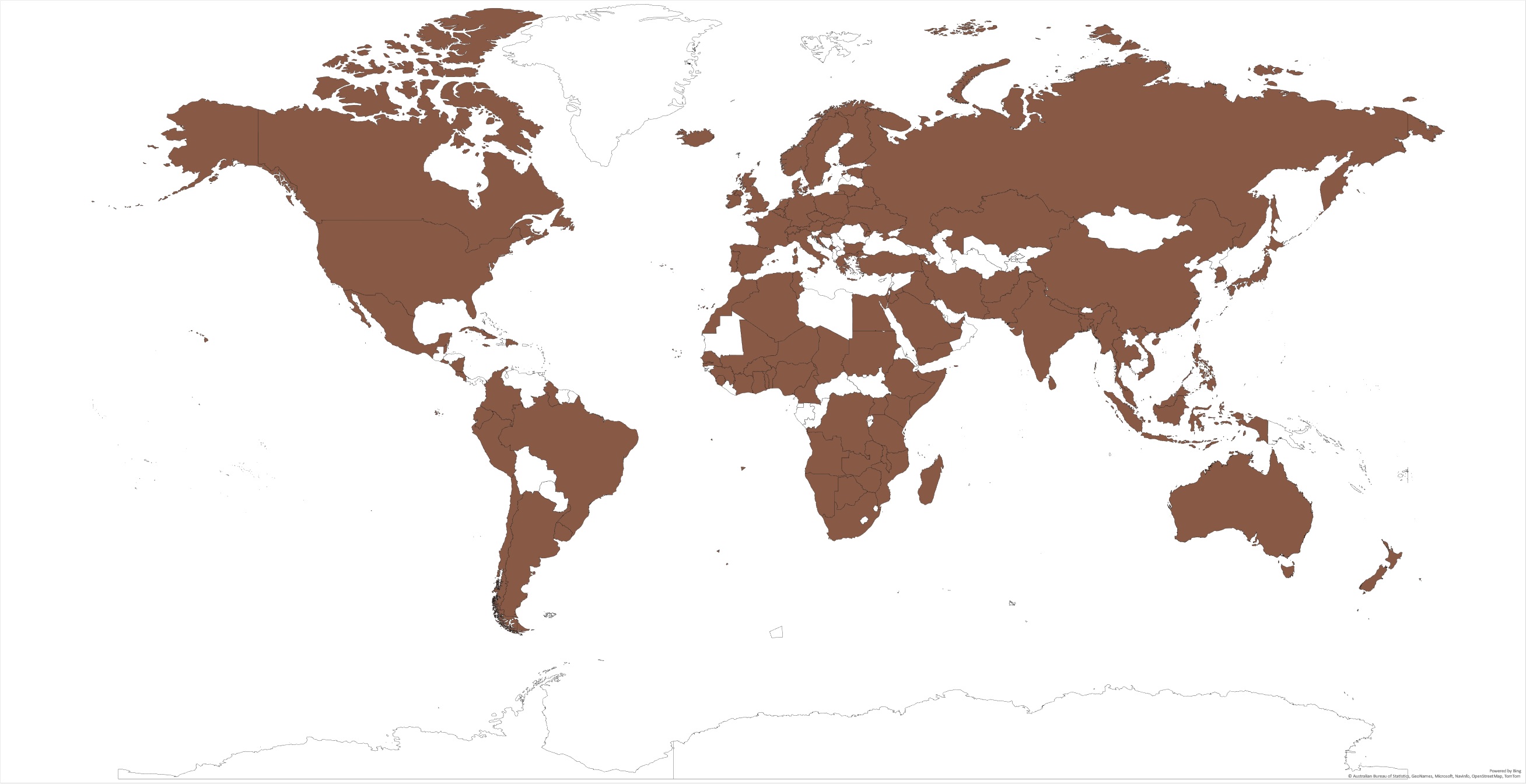
Figure 1. Countries where Cryptolestes ferrugineus have been identified.
3. Taxonomic Hierarchy, Identification, and Synonyms
The rusty grain beetle, also known as the rust-red grain beetle or flat grain beetle, was initially described by James Francis Stephens in 1831 under the name Cucujus ferrugineus. Cryptolestes was listed as a subgenus under the genus Laemophloeus, and the insect was referred to as Laemophloeus ferrugineus (Stephens) by Leng, whereas Casey claimed that Cryptolestes could be referred to as an individual genus due to its distinct nature, which was agreed upon by other researchers such as Sheppard [1]. Available synonyms are Cucujus monilicornis (Stephens, 1831), L. concolor (Smith, 1851), L. obsoletus (Smith, 1851), L. carinulatus (Wollaston, 1877), L. emgei (Reitter, 1887), and L. alluaudi (Grouvelle, 1906) [8]. In the mid-20th century, researchers often used L. ferrugineus (Stephens). Currently, Cryptolestes ferrugineus (Stephens) is widely used.
The order, suborder, infraorder, superfamily, family, genus, and species of rusty grain beetle are Coleoptera, Polyphaga, Cucujiformia, Cucujoidea, Laemophloeidae, Cryptolestes, and Cryptolestes ferrugineus, respectively [9]. There are about 50 species in the genus Cryptolestes Ganglbauer, 1899, but only nine are considered pests of stored products: C. capensis Waltl, 1834; C. cornutus Thomas and Zimmerman, 1989; C. divaricatus Grouvelle, 1898; C. ferrugineus; C. klapperichi Lefkovitch, 1962; C. pusillus Schönherr, 1817; C. pusilloides Steel and Howe, 1952; C. turcicus Grouvelle, 1876; and C. ugandae Steel and Howe, 1952. Differentiation of C. ferrugineus from other Cryptolestes spp. could be performed by identifying the morphological differences in those species. Different Cryptolestes species could also be differentiated by examining their genitalia. For instance, the accessory sclerite in male C. ferrugineus is intricately connected to the two robustly sclerotized lobes located at the posterior end of the aedeagus, whereas other species like C. capensis exhibit relatively weaker sclerotization of these lobes. The sclerotization of the posterior lobes of the aedeagus in C. ugandae, while not as pronounced as in C. ferrugineus, is still visible [10]. Moreover, several researchers [11][12][13] proposed the identification of different species of Cryptolestes (C. ferrugineus, C. pusillus, C. turcicus, C. pusilloides, and C. capensis) based on the mitochondrial cytochrome c oxidase subunit I (COI) barcode region.
4. Biology and Development
4.1. Life Stages
Cryptolestes ferrugineus is holometabolous, which implies they undergo complete metamorphosis and consist of four life stages, namely egg, larva, pupa, and adult.
-
Egg
The female Cryptolestes ferrugineus deposits eggs in small gaps in the grain kernels (under the outer layer of the seed coat), between the grain kernels, in small crevices or fractures in any structures, or in debris with the help of their substitutional ovipositor. Those caudal segments are generally retracted in the abdomen. During oviposition, those segments protrude out to facilitate the placement of the egg at a suitable location. The styli aid in the suitable orientation of the egg. Each female could lay about 200 to 500 eggs [14]. The eggs appear to be white and moderately translucent, with length and width in the range of 0.68 to 0.81 mm and 0.20 to 0.30 mm, respectively. The eggshell, after hatching, has a distinct iridescence [1].
- b.
-
Larva
Once the egg is ready to hatch, the larva breaks the eggshell (termed ‘chorion’) through a series of movements. The larva continuously produces those movements until its head emerges from the egg. Then, the larva crawls out of the eggshell with the help of its legs and a series of to-and-fro movements. Then, the larva starts its exploration of food. The larva mainly feeds on the germ portion of the wheat but also feeds on the endosperm during germ scarcity. The amount of food consumed depends on the environmental conditions. Under suitable conditions, the larva stays inside a kernel of wheat and forms a burrow through the consumption of wheat germ. It ejects the fecal material and molted exuviae through the opening created by the female adult during oviposition or by the larvae to enter the wheat germ [1]. The size of the larva ranges from 1 to 4 mm [14]. The average length of the larval stage varies under varying physical, environmental, and ecological stresses. For instance, the average length of C. ferrugineus larval stages was 56, 50, 36, and 21.8 days (d) in white flour, bran, wheat without germ, and wheat with germ, respectively [1].
There are four instars for C. ferrugineus larvae, which implies that they molt four times and become pupae after the fourth molting. The first, second, third, and fourth instar larval stages last about three to four, two to five, two to five, and five to eight days, respectively, at suitable conditions. The first instar larva is white in color, whereas the fourth instar larva becomes light tan in color. At the end of the abdomen of the fourth-instar larvae, caudal hooks are present, which aid in the backward movement of the larvae. The mouth parts of larvae and adults are similar. On evaluating the bioenergetics of C. ferrugineus, Campbell and Sinha [15] reported that the immature stages assimilated about 66% to 79% of the food consumed. They also reported that during development, the proportion of assimilated energy converted into tissue growth/biomass ranged from 3% (early first-instar larva) to 23% (older larva).
Before entering the next stage of development, the fourth-instar larva enters the burrow of the wheat and seals the burrow using debris and excrement through silken threads. Sometimes, they also pupate in other locations, such as crevices or the space between grain kernels. Two papillae, which are slightly and distinctly noticeable in the third and fourth instars, respectively, were reported to be responsible for the silk thread formation [1]. Compared with other Cryptolestes species such as C. turcicus, which can produce tough silk strong enough to produce a cocoon, C. ferrugineus forms fragile silk, which can only hold debris, bran, and excrement in place [16].
- c.
-
Pupa
Initially, the pupa is white, and over time, it turns into a light tan color with a triangular shape to some extent. The eyes of the pupae are dark brown in color [1]. The pupal stage lasts about three to six days at 32 °C and 75% relative humidity (RH).
- d.
-
Adult
The adult that emerged from the pupa is light tan in color, which turns into a rusty brown color in one or two days. Immediately after emergence, the membranous pair of wings are stretched for a short duration, after which they fold beneath the elytra. The length of the adult is in the range of 1.70 to 2.34 mm, and the antennal length ranges from 0.70 to 1.14 mm [17]. A day or two after emergence, the adults start mating, the oviposition begins, and the cycle continues. The mean life span of adults ranges from 12 to 32 weeks (wk) depending on the density, feed, and sex ratio [18]. White and Bell [18] reported that the isolated virgin adults have a greater life span than the mated adults. The female:male sex ratio of C. ferrugineus adults was reported to be 1:0.64 [1] and 1:0.69 [19] on wheat and 1.1:0.8 on dates [20]. The longevity of female C. ferrugineus is longer than that of males [21]. Vendl et al. [22] studied the tarsal and inter-claw adhesive structures of C. ferrugineus using a scanning electron microscope and reported the following observations: (1) the length-to-width ratio of tarsi is about 9.5; (2) the first tarsomere is short and small (almost the same shape as the next tarsomere); (3) the last tarsomere is the longest among other tarsomeres; (4) the ventral side of the tarsomeres and the pre-tarsi do not have any adhesive structures; (5) a pair of apical setae on the unguitractor is present; (6) the lateral margin of the terminal tarsomere contains two pairs of setae, whereas the medial part of the ventral side of the margin is trapezoidal. The absence of adhesive structures in the tarsomeres is responsible for this species’ inability to climb inclined and smooth surfaces. The researchers compared the claw shapes of C. ferrugineus and Oryzaephilus surinamensis (L.) and reported that both species had similar claw shapes; however, the claws of C. ferrugineus were comparatively sharper and shorter (with a radius of 1.17 µm) than those of O. surinamensis (with a radius of 1.63 µm). This implies that C. ferrugineus has adapted its morphology to move over rough surfaces with smaller irregularities.
4.2. Sexual Dimorphism
Male and female C. ferrugineus can be differentiated by observing their genitalia. The tarsi of female C. ferrugineus are all five-segmented (with tarsal formula 5-5-5), whereas those of males are four- and five-segmented (with tarsal formula 5-5-4). On the other hand, in females, the styli are present on the ninth abdominal segment, whereas they are absent in males. The male C. ferrugineus has a larger head and a wider thorax than the female [1]. A sex difference is observed in the mandibles. Precisely, the male mandible has a toothlike projection on the lateral ventral side near the base, while the female does not have the projection [1][23]. Chambers et al. [24] reported that the sexual differences of C. ferrugineus could also be identified based on the electroantennogram responses of the adults and showed greater electroantennogram amplitude in females than males towards the synthetic samples of the macrocyclic lactones containing aggregation pheromones.
4.3. Effects of Various Environmental Parameters on the Biology of Cryptolestes ferrugineus
The development time, oviposition rate, and life span of the adults depend on various environmental, physical, and ecological factors such as temperature, RH, availability of food, type of food, pesticide exposure, presence of predators or parasitoids, and genetics. For instance, during the first 30 d of adult life at 30 °C and 70% RH, the average oviposition rate of C. ferrugineus females is 7.5 and 5.6 eggs/d in flour and wheat kernels (moisture content 16 to 18%) consisting of 3% (weight basis) flour, respectively [25]. At most temperatures, temperature has the highest relative influence on insect development, followed by moisture and diet; near the optimal temperature, moisture and diet have a stronger effect on larval development than temperature [26].
4.3.1. Temperature
Al-Salihi and Al-Azawi [20] reported that the duration is 3.2 d for eggs with a hatching rate of 96.8%, 70.3 d for larvae, 3.6 d for the pre-pupal stage, 6.1 d for the pupal stage, and 186 d for adults at 30 °C and 70% RH. One female can lay 558 eggs. The developmental temperature and RH range from 20 to 40 °C and 40 to 95%, respectively [3]. The optimal temperature and RH are 33 °C and 70 to 80%, respectively [3], whereas the preferred temperature (the temperature towards which the insects move) is 30 to 36.5 °C [27]. Cryptolestes ferrugineus can also develop at temperatures ranging from 20 to 42.5 °C [28]. The intrinsic rates of natural increase of C. ferrugineus were the highest at 35 °C and 90% RH and the lowest at 20 °C and 70% RH [29]. At 42.5 °C, larval and pupal mortality were 98% [29]. Cryptolestes ferrugineus is one of the most cold-tolerant species, with the adult being the most cold-hardy stage [30]. Cryptolestes ferrugineus adults, after being acclimated to temperatures of 18, 10, and 5 °C for one week at each temperature, took about 58 d at −10 °C to reach 95% mortality, whereas C. turcicus and C. pusillus reached 95% mortality at 39 and 11 d, respectively [31]. At temperatures below 23 °C, the rate of reproduction decreases; at temperatures below 21 °C, the insects cannot fly [13]. On the other hand, Cox and Dolder [32] reported that the minimum temperature for C. ferrugineus flight was 20 °C, whereas in laboratory-cultured strains for a period of over 20 years, one insect was reported to fly at 17.5 °C. Cryptolestes ferrugineus does not lay eggs below 17.5 °C [29]. Under suitable environmental conditions (at 32 °C and 75% RH), the eggs hatch in three to four days [1]. Eggs do not hatch below 15 °C [33]. Ashby [34] reported that the rate of respiration and development of C. ferrugineus proportionally increased with the rise in temperature, in the range of 21 to 33 °C. The development rate of C. ferrugineus eggs is linearly related to temperature (T) (Egg development rate, D = 0.0169–0.258 T) [33].
Temperature is one of the main factors that influences the population dynamics of C. ferrugineus [35]. An extensive review of the application of temperature to control stored product insects is available [36]. At −10 °C, the LT50 (lethal time for 50% of a population) values for egg, young larva, old larva, pupa, and adult were reported to be 8, 4, 16, 11, and 91 h, respectively [31].
Acclimation
Acclimation is one of the important parameters that determines the cold tolerance levels of insects, in addition to influencing their behavior, survival, growth, and multiplication. In cold-acclimated C. ferrugineus, trehalose and amino acids including proline, asparagine, valine, lysine, leucine, isoleucine, alanine, phenyl alanine, glutamic acid, and aspartic acid, as well as phosphoethanolamine (a phospholipid precursor), were higher than in unacclimated C. ferrugineus [37]. Furthermore, the acclimation increased the mean fresh weights of C. ferrugineus [38]. The acclimation temperature was found to affect the behavior of C. ferrugineus more than the exposure time [39]. When acclimated to low temperatures (15 to 5 °C) for some time, most stored-product insects were found to increase their cold tolerance by 2 to 10 times [36]. The acclimated C. ferrugineus was reported to be more cold-hardy than the non-acclimated ones [40]. Precisely, C. ferrugineus acclimated at 15, 10, and 5 °C consecutively for two weeks at each temperature had LT50 and LT90 (lethal time for 90% tested individuals) of 24 and 42 d, respectively, at −10 °C, whereas unacclimated C. ferrugineus had LT50 and LT90 of 1.4 and 2.7 d, respectively, at the same temperature [37]. Smith [30] reported that the LT50 values of acclimated adults increased by 9 and 56 times, respectively, at −6 and −12 °C compared with the unacclimated adults. In addition, the supercooling points of C. ferrugineus adults were −16.5, −20, and −21 °C for unacclimated, acclimated at 15 °C, and acclimated at 15 °C followed by acclimation at 4 °C, respectively. The mean survival times of C. ferrugineus directly transferred to 9 °C from warmer temperatures (30 or 32 °C) were 4.3 weeks, whereas the mean survival times of those acclimated (about 4.5 °C/week) to 9 °C were 7.6 weeks [41]. Burks and Hagstrum [42] examined the rapid cold hardening ability of five different species (C. ferrugineus, O. surinamensis, Rhyzopertha dominica (Fabricius), Sitophilus oryzae (L.), and Tribolium castaneum (Herbst)) and reported that C. ferrugineus is more capable of rapid cold hardening than other tested species.
4.3.2. Moisture Content
Damper grains facilitate more convenient feeding than dry grains for C. ferrugineus. The development of C. ferrugineus is limited when the moisture content of the grain or RH is below 12% or 40%, respectively [14]. The intrinsic rates of natural increase of C. ferrugineus were almost the same at 70 and 90% RH, whereas they were the lowest at 40% RH [29]. Similarly, Evans [41] reported shorter insect survival at 45% RH than at 70% RH. Throne [43] studied the progeny of C. ferrugineus at different moisture contents (11.3, 12.4, and 14.8% at 43, 56, and 75% RH, respectively) and reported that the number of offspring produced on damaged grain increased linearly with moisture content. Similarly, Throne and Culik [44] reported that the corn maintained at 75% RH showed higher progeny production and lower development time for C. ferrugineus when compared with those at 43% RH. Bishop [21] reported that the egg production and longevity of C. ferrugineus increased with an increase in RH. However, compared with C. minutus and C. turcicus, C. ferrugineus was less sensitive to 40% RH at 32.2 °C [21]. Kawamoto et al. [33] studied the mortality and development of C. ferrugineus eggs at different RH (50, 60, 70, 80, and 90%) and reported that RH does not affect the mortality and development of eggs. With an increase in temperature from 25 to 35 °C, the effect of RH on C. ferrugineus rate of oviposition was reported to be more pronounced [29]. Cryptolestes ferrugineus adults preferred to lay eggs in damper grain (18% moisture content) to drier grain (14% moisture content) [45]. They preferred the drier region (70% RH) to the moister region (85% RH) in the absence of grain, whereas within a grain bulk, adults accumulated in the pockets of damp grain [45].
4.3.3. Diet
Although C. ferrugineus primarily feeds on germ and is considered a secondary pest, it is capable of infesting grain kernels with broken seed coats that are present in a sound grain mass [3]. The type and quality of food greatly influence the survival, growth, and multiplication of C. ferrugineus. However, certain studies found contradictory results regarding the suitability of diets for C. ferrugineus. Larvae of C. ferrugineus have better survival and faster development in a wheat kernel with a germ than those without a germ or on bran or white flour [1]. Moreover, the oviposition rate on whole-wheat flour was greater than wheat kernel at all tested densities (4, 16, and 64 pairs per vial), except for one pair per vial [46]. Tuff and Telford [47] reported that C. ferrugineus was not able to invade sound kernels, whereas it could infest seeds with damaged grain coats. Similarly, Throne and Culik [44] reported higher progeny production and decreased development duration on cracked corn compared with undamaged kernels. However, the level of cracking on the corn did not significantly affect the survival of the immature stages of C. ferrugineus [48]. Shufran et al. [49] performed a laboratory experiment on the host suitability of pecan and wheat for various stored-product insects and reported that C. ferrugineus were observed to produce more immatures on unsorted pecan, cracked pecan, and nutmeats than on in-shell pecan; however, only fewer adults were observed on different types of pecans than wheat. This implies that pecans lack certain dietary requirements for C. ferrugineus. White and Loschiavo [50] reported that the slower developmental time and higher larval mortality of C. ferrugineus on oats compared with wheat were due to the nutritional insufficiency and unpalatability of oats. Even though C. ferrugineus can survive on hemp seed and its dockage, it does not flourish [51]. Also, C. ferrugineus prefers wheat kernels as compared with canola and rapeseed [52]. Durum Kyle, Coulter, and Medora are suitable wheat varieties for the oviposition and development of C. ferrugineus [50]. Jagadeesan et al. [53] evaluated the suitability of nineteen grain-based diets on the number of live adult progeny developed and concluded that diets containing (a) barley flour, (b) rolled oats and cracked sorghum, (c) wheat flour and barley flour, and (d) cracked sorghum alone resulted in higher progeny production of laboratory strains, whereas diets containing (a) rolled oats and cracked sorghum, (b) wheat flour and barley flour, and (c) barley flour alone were suitable for field-collected strains. They also reported that diets containing cracked sorghum were better than those containing cracked maize or wheat. The reason might be that the laboratory strain used was cultured in a diet containing rolled oats, cracked sorghum, and yeast for five generations prior to the experiment; furthermore, the insects were collected from stored sorghum. They hypothesized that the literature published on the successful culturing of C. ferrugineus on corn [44][48] could have been collected from stored maize. The diet also influences the cold tolerance of C. ferrugineus. For instance, the LT50 of C. ferrugineus adults at −10 °C in grain, flour, and Brewer’s yeast and flour alone were 104, 79, and 42 h, respectively, and the supercooling points were −20.6, −22.9, and −19.4 °C, respectively [31].
Cryptolestes ferrugineus feeds on certain fungal species as supplementary or alternative food sources. Nevertheless, in the absence of grain, C. ferrugineus feeds mainly on fungi. Sinha [54] reported that C. ferrugineus completed its development on 10 species of fungi (Absidia orchidis (Vuill.) Hagem, Alternaria tenuis sensu Wiltshire, Curvularia tetramera (McKinney) Boedijn, Fusarium moniliforme Sheld, Helminthosporium sativum P., K., and B., Mucor sphaerosporus Hagem, Nigrospora sphaerica (Sacc.) Mason, Penicillium cyclopium Westl., Stemphylium botryosum Wallr, and Trichothecium roseum Lk., among 23 species tested. The shortest and longest developmental periods were about 22 and 34 d, respectively, on T. roseum and F. moniliforme. Similarly, Loschiavo and Sinha [55] studied the oviposition, feeding, and aggregation of C. ferrugineus in the presence of different species of seed-borne fungi and revealed that N. sphaerica, M. sphaerosporus, Hormodendrum cladosporiodes (Fres.) Sacc., and C. tetramera were the most suitable fungi for oviposition and feeding. The differences in responses of C. ferrugineus were observed for different species from the same genus. For instance, P. terrestre was not suitable for oviposition and feeding, whereas P. cyclopium and P. funiculosum were moderately suitable. On the other hand, C. ferrugineus was observed to feed moderately on Aspergillus flavus and did not lay eggs; however, they were observed to lay a few eggs and feed slightly on A. fumigatus. Aggregation of C. ferrugineus was observed on grain kernels containing mycelia and spores of N. sphaerica [55].
Overall, C. ferrugineus can feed on more than 65 commodities, including but not limited to wheat, paddy, sorghum, barley, flax, black pepper, cocoa bean, coffee bean, cassava root, palm kernel, peanut, chili, hemp, sunflower seed, oat, bamboo leaf (dried), bark, animal feed, beam cake, wheat flour, wheat product, barley (pearl), yam, rice, cashew, raisin, date, fennel seed, fig, broad bran, cassava root flour, chili pod (dried), peanut product, soybean paste, and vegetable (preserved) [56], but do not actively multiply on products such as wood, fiber, and textile.
4.3.4. Insect Density
Crowding plays a significant role in the population dynamics of C. ferrugineus since crowding can encourage fighting and cannibalism, which results in high egg damage and high mortality [35]. At 30 °C and 70% RH, the number of eggs produced per female per day was 6.4 and 1.5 when 1 and 64 pairs of adults, respectively, were present in a vial containing 0.5 g flour, whereas in a 1 g wheat kernel, the number of eggs produced per female per day was 5.6 and 0.75 in the presence of 1 and 64 pairs of adults, respectively [46]. Development times (egg to adult) on 0.5 g flour were 24 and 87.1 d, with an initial larval count of 1 and 32, respectively, per vial. Smith [46] also found that the mortality of the insects increased with density. White and Bell [18] reported that the amount of energy outflow and the physical injury during copulation affect the survival of insects at different densities and at different sex ratios. Studies on the population dynamics of C. ferrugineus revealed that the population dynamics of the species are influenced by patch size and temperature [35][57]. Moreover, they also reported that the total insect number and kernel infestation percentage were positively correlated. All these studies concluded that density affects the oviposition, development, and mortality of C. ferrugineus.
5. Ecology and Behavior
5.1. Refuge-Seeking Behavior
Some of the review articles covered the refuge-seeking behavior of stored grain insects [58][59]. Refuge-seeking behavior is the ability of the stored grain pests to hide in the structural cracks and crevices of the storage structure, which contain grain residues. The refuge provides food and shelter to the insects, in addition to protecting them from insecticide treatments. The hidden insects emerge and reinfest nearby grain when the conditions are favorable. Even in the absence of food, C. ferrugineus was reported to be attracted to the refuge, possibly for the physical contact around their bodies. This could also be the reason for their occurrence near the container boundary during laboratory experiments [60].
On analyzing the samples obtained from structural cracks and surfaces from 34 empty storage structures in the Prairie provinces of Canada (Manitoba, Saskatchewan, and Alberta), C. ferrugineus was identified in 36% of the sampled structures [61]. The effects of different temperatures, refuge contents, food availability, and different strains on the refuge-seeking behavior of C. ferrugineus have been evaluated by Cox et al. [62] and Cox and Parish [60]. Cox et al. [62] observed the refuge-seeking behavior of different strains of C. ferrugineus at different temperatures (15, 20, 25, and 30 °C) and reported that about 45% and 20–30% of the insects were found to remain inside the refuge at the end of 2 wk for C. ferrugineus strains that were reared in the laboratory for over 25 years (yr) and those obtained from grain stores and mills in the UK, respectively. Moreover, they also observed that the refuge-seeking behavior of different strains of C. ferrugineus varied with varying temperatures. The refuge-seeking behavior of C. ferrugineus females was greater than that of males, and that of adults 0–3 wk old was greater than that of 10–12 wk and 16–18 wk old adults [63]. This was because the refuge would have attracted females for oviposition and younger adults since oviposition is greater in younger adults than older ones [29].
5.2. Flight Activity
The flight activity of insects determines their ability to infest the stored grains in different bins. The level of infestation inside a grain bin varies with the number of insects immigrating into the bin. The flight activity of C. ferrugineus depends on external factors such as air temperature, wind direction, wind speed, and day length [64][65]. During a flight activity study of C. ferrugineus in southern New South Wales, Australia, Holloway et al. [65] observed no flight activity during the winter months (June, July, and August). Cryptolestes ferrugineus, captured on glue boards installed in and around the warehouses of Kansas and Nebraska, U.S., reached a peak in early September and declined through early November [66]. Hagstrum [67] studied the immigration of insects in 34 bins with varying capacities (36 to 238 t) containing hard red winter wheat on 12 farms from July to December 1998 in Kansas, U.S., and found the immigration of C. ferrugineus in all the 34 bins. The drop in immigrated insect count was reported when the ambient temperature dropped below 20 °C. Thus, C. ferrugineus shows seasonal variation in flight activity and immigration. This is because the minimum temperature for their flight initiation is 20 °C [32]. Hagstrum [68] observed the distribution of C. ferrugineus on three farms in Kansas, U.S., and reported that most of the C. ferrugineus infestation occurred after the grain was loaded into the bin. In addition, the number of insect counts decreased in the top layers. Hagstrum [68] concluded that C. ferrugineus adults fly to the top of the bin and then distribute it to other parts of the grain inside the bin.
5.3. Mating Behavior
Male and female adults of C. ferrugineus start mating within one or two days after they emerge. When a male identifies a potential female, the male adult turns and follows the female. Boukouvala et al. [23] performed an experiment to evaluate the lateralization of males during courtship and mating and reported that most (41%) C. ferrugineus males showed a left-biased approach (turning 180° to their left) towards females, whereas 34%, 14%, and 11% approached females from the right side, back side, and front side, respectively. Moreover, they also revealed that the left-biased males showed shorter durations of mate recognition and chasing as well as lower copulation attempt durations, with higher successful mating attempts compared with the right-biased males. The male follows the female by nudging the tip of the female’s abdomen with the male’s head. Once the female stops, the male strokes the female elytra with its antenna. The male continues its efforts to succeed by crawling on the back of the female and turning. Only the flickering of the female’s antenna was reported during the process. Once the male and female are coupled, the first copulation was observed to last for 105 min, followed by separation for 20 min. Then, the second and third copulations were observed for 35 and 95 min, respectively. During coition, the male and female are firmly attached since the aedeagus is inserted deeply into the female’s genital tract [1].
5.4. Chemical Ecology
Pheromones
Pheromones are chemical substances produced by insects that affect the behavior of other individuals of the same or other species. Cryptolestes ferrugineus males produce pheromones, namely (E, E)-4,8-dimethyl-4,8-decadien-10-olide (ferrulactone I) and (3Z,11S)-3-dodecen-11-olide (ferrulactone II) [69]. The pheromones are produced in the alimentary canal and/or the Malpighian tubules. Researchers have shown the possibility of isolating the aggregation pheromones (ferrulactone I and II) from C. ferrugineus [70]. The naturally produced ratio of ferrulactone I to ferrulactone II by C. ferrugineus was 1.6:1.0 [71]. Adults of mixed sex and age responded to the odor of mixed-sex adults, frass, pentane extracts of frass, and Porapak Q-captured volatiles from adults or frass [69]. Moreover, they also observed responses from both sexes to the volatiles in males. Similarly, Currie et al. [72] reported that in the absence of air currents and food for feeding, male and female C. ferrugineus were attracted to a single male in an apparatus of 10 cm length; when grain was present, a single male was not enough to attract C. ferrugineus. However, a significant number of females were attracted to 50 males.
According to Oehlschlager et al. [73], the aggregation pheromones produced by C. ferrugineus can act alone as well as synergistically. Moreover, the C. ferrugineus species is not cross-attracted to the pheromones produced by other species such as Oryzaephilus mercator, O. surinamensis, C. turcicus, and C. pusillus (Schonherr) [73]. Chambers et al. [24] analyzed the electroantennogram (EAG) responses of the males and females of C. ferrugineus and reported that females produced EAG with higher amplitude. Thus, the greater response of females to the pheromones produced by males implies the importance of pheromones in mate identification and courtship. While determining the flight activity of C. ferrugineus in farms in south-eastern Australia, Holloway et al. [65] reported that more females were trapped in traps with pheromone (female:male ratio of 3:1), whereas in passive trapping, the female:male ratio caught was 1:1. Similarly, during a seasonal flight activity study at grain storage sites in South Carolina, U.S., Throne and Cline [74] observed more C. ferrugineus females at all the tested sites. These results further confirm the higher attraction of females towards the pheromone.
5.5. Heat Production
Heat production of 4 wk old adults and second, third, and fourth instar larvae was in the range of 0.72 to 21.47 µW/insect and 0.37 to 17.53 µW/larvae, respectively, at the tested temperatures (15, 20, 25, 30, and 35 °C) and moisture contents (12, 15, and 18% wet basis) [75][76]. The maximum rate of heat production was observed in adults over the age of 4 wk. Cofie-Agblor et al. [75][76] also found heat production of adults: (1) varied with insect density; (2) exponentially increased with increase in temperature from 15 to 35 °C; (3) increased with increase in moisture constant; however, the rate of increase from 15 to 18% was lower than that from 12 to 15%; and (4) increased with increase in level of wheat breakage; however, the rate of increase from 10 to 20% breakage was lower than that from 0 to 10%.
Cryptolestes ferrugineus multiplication is associated with grain heating; however, at low density (less than five adults/kg), they cannot initiate heating [77]. Smith [78] added water to increase the moisture content of wheat stored in a metal granary and reported that the increase in moisture content led to the heating of the grain as well as a rapid increase in the C. ferrugineus population. Thus, the high multiplication of C. ferrugineus is a consequence of grain heating and not the cause.
5.6. Movement and Distribution Inside Grain
The movement of insects inside grain can be random (non-directional) or biased (non-random or directional). Under uniform environmental conditions, insects tend to wander inside the grain randomly and reach biologically suitable locations. On the other hand, individual insects move in a non-random direction in search of food, refuge, a mating partner, to escape predators, or due to non-uniform environmental conditions in stored grain structures [59]. The biased movement is also influenced by pheromones, host stimuli, the presence of other organisms such as parasitoids and predators, and other physical stimuli such as temperature, moisture content, gas concentration, dockage, foreign materials, light, and radiation [79]. The tendency of the insects to move towards a particular environment could be due to (a) behavioral response to physical stimuli, (b) physical response, wherein the rate of their metabolic activities changes at different environments, and (c) survival response, wherein the insects avoid extreme temperatures unsuitable for their survival, growth, and multiplication [79]. More detailed information on the factors influencing the movement and distribution of C. ferrugineus [2] and other stored-product insects [80] is available elsewhere. From those review articles, it can be noted that C. ferrugineus detects cues and resources and tends to move towards an environment for their growth and multiplication. For instance, Cryptolestes ferrugineus adults prefer warmer grain to their surrounding cooler grain in the absence of other factors, and the adults could detect the temperature gradient in less than 1 h [81][82]. They detected a temperature difference of 1 °C in 24 h in a tested cylinder of diameter 56 cm and height 9 cm [81]. Cryptolestes ferrugineus prefer damp and mold-infected grain rather than dry grain since damp grain is soft and easy to oviposit into, and they could feed on the mold itself [45]. While determining the spatial and temporal distributions of C. ferrugineus adults inside a grain bin containing 1.5 t of wheat, Jian et al. [83] reported that the level of aggregation decreased with an increase in insect density. This is because at lower density, aggregation would increase their possibility of meeting mating partners. White et al. [84] reported that C. ferrugineus adults prefer to move towards elevated carbon dioxide levels, whereas prolonged exposure to higher levels of carbon dioxide is lethal to the insects.
The one- and two-dimensional (D) movements of C. ferrugineus have been extensively studied at various environmental conditions, such as temperature, moisture contents, and their gradients, in the laboratory [27][82][85][86][87]. Recently, Bharathi et al. [88] developed an experimental setup consisting of 343 metal-based mesh cubes arranged inside a wooden box to study the movement of insects in three dimensions. The researchers concluded that the 3D movement and distribution patterns were similar to those in 1D and 2D [88][89]. However, the 3D movement of C. ferrugineus was observed only in uniform environmental conditions. Bharathi et al. [90] observed the movement and distribution of C. ferrugineus inside a grain bin filled with 300 t of wheat for 26 months in Winnipeg, Canada. They reported that C. ferrugineus inside the grain bin followed a movement and distribution pattern similar to those reported in the laboratory experiments under similar environmental conditions. The activity of C. ferrugineus inside a 300 t wheat grain bin was reported to reduce near the boundary when the temperature dropped during the winter (especially when the temperature dropped below 2.5 °C) and resume when the temperature increased above 4.5 °C [90][91].
Briefly, temperature gradients and moisture differences are the predominant factors that influence the movement and distribution of C. ferrugineus, whereas the presence of mold, type of food, dockage, intergranular grain spaces, and ventilation are trivial factors that influence the movement and distribution of C. ferrugineus adults [2]. Based on limited research, the carbon dioxide gradient also seems to be a significant factor [84], but more research is required on the movement of insects under carbon dioxide gradients. Thus, the behavior of C. ferrugineus is the result of exploration for a location that is biologically suitable and physically comfortable for their survival, growth, and multiplication.
6. Interaction with Other Organisms
6.1. Interspecific Interaction
The interaction of C. ferrugineus with the following stored grain insects in the laboratory has been studied: C. turcicus, C. pusillus, T. castaneum, Lasioderma serriocorne, and S. oryzae. Few studies explored the interspecific interaction of different stored-product insect species in field conditions. For instance, Nansen et al. [92] sampled wheat from 129 grain silos in Kansas in 1999–2001, analyzed the densities of R. dominica, C. ferrugineus, and T. castaneum, and reported the intra- and inter-specific interactions of the insects. The researchers observed that the presence of C. ferrugineus reduced the density of both R. dominica and T. castaneum. However, the populations of T. castaenum and R. dominica did not influence the insect count of C. ferrugineus. These studies imply that the interspecific interaction of species depends on various factors such as the availability of food, cannibalistic behavior, environmental conditions, whether the insect is a primary or secondary feeder, and its predative nature.
6.2. Nature Enemies
Wasps such as Habrobracon hebetor (Say) (Hymenoptera: Braconidae), Cephalonomia waterstoni (Gahan) (Hymenoptera: Bethylidae), Brachymeria sp., Anisopteromalus calandrae (Howard) (Hymenoptera: Pteromalidae), Lariophagus distinguendus (Förster) (Hymenoptera: Pteromalidae), Holepyris sylvanidis (Brèthes) (Hymenoptera: Bethylidae), and Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae), have been identified as parasitoids on stored grain pests and are used as biological control agents [56][93][94][95]. Among these, the most common parasitoid of C. ferrugineus is C. waterstoni [56]. Adult females of C. waterstoni paralyze, feed, and oviposit on their hosts. Cryptolestes ferrugineus larvae paralyzed by C. waterstoni cannot advance into the next developmental stage, and as a result, those larvae are available as oviposition sites for a minimum of 2 wk [96]. Cephalonomia waterstoni has been identified as one of the best biological control agents because it effectively follows the kairomonal trail inside the grain [97], has the ability to feed on all larval instars, has a generation time half that of the host, and is extremely host-specific [96]. Flinn and Hagstrum [98] developed a model to predict the phenology of C. waterstoni and C. ferrugineus in relation to grain temperature and reported that the effect of the parasitoid on the host is the highest when released during the first production of fourth-instar larvae. The combined application of an insecticide and a parasitic wasp could result in effective control of C. ferrugineus.
Mites such as Acarophenax lacunatus (Cross and Krantz) [99] and Cheyletus eruditus (Schrank) [100] have been identified as biological control agents of C. ferrugineus. Acarophenax lacunatus was observed to prey on the eggs and reduce the larval population of C. ferrugineus. Thus, these parasitic mites were able to successfully reduce the instantaneous rate of C. ferrugineus increase [101].
Entomopathogenic fungi such as Beauveria varroae, B. bassiana, and Purpureocillium lilacinum were found to be associated with C. ferrugineus in wheat and maize samples in Central and South Anatolia in Turkey [102]. Similarly, Wakil et al. [103] explored the naturally occurring entomopathogenic fungi infecting stored grain insects in Punjab, Pakistan, and reported fungal species such as Alternaria alternata, A. solani, Aspergillus flavus, A. fumigatus, A. parasiticus, A. niger, Bipolaris oryzae, C. lunata, Fusarium oxysporium, Helminthosporium oryzae, P. capsulatum, P. chrysogenum, Phomopsis sp., and Rhizopus stolonifers. Among the insect species tested, C. ferrugineus (0.1% occurrence) was less affected by entomopathogenic fungi than Tribolium castaneum (Herbst) (0.3% occurrence) and Sitophilus oryzae (L.) (0.2% occurrence). However, C. ferrugineus eggs are resistant to B. bassiana infection [104]. In 1988, Isabelle I. Tavares identified an undescribed fungal parasite species in the genus Dimeromyces (Ascomycetes: Laboulbeniales) on the last visible abdominal segment near the ovipositor base of C. ferrugineus [105]. Lord et al. [106] reported that Nosema oryzaephili microsporidia at 106 spores/g of diet resulted in about 99% infection of C. ferrugineus larvae after three weeks of exposure.
Ünal and Koçak [107] reported the association of endosymbionts such as Wolbachia, Rickettsia, and Spiroplasma with C. ferrugineus. Mattesia oryzaephili and M. dispora were reported to be two of the pathogens that infect C. ferrugineus. Lord [108] reported that at a dose rate of 105 oocysts/g of diet, the mortality and infection rates of the fourth instar of C. ferrugineus were higher with M. oryzaepili than with M. dispora. Thus, the presence of these two Mattesia species could lead to a decline in C. ferrugineus populations.
References
- Rilett, R.O. The biology of Laemophloeus ferrugineus (Steph.). Can. J. Res. 1949, 27, 112–148.
- Jian, F.; Jayas, D.S. Detecting and responding to resource and stimulus during the movements of Cryptolestes ferrugineus adults. Food Bioproc. Technol. 2009, 2, 45–56.
- Sinha, R.N.; Watters, F.L. Insect Pests of Flour Mills, Grain Elevators, and Feed Mills and Their Control; Agriculture Canada Publication: Ottawa, ON, Canada, 1985.
- FAO. Country Profiles. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/countryprofiles/en/ (accessed on 9 May 2023).
- Kislev, M.; Simchoni, O. Hygiene and insect damage of crops and foods at Masada. Masada VIII. In The Yigael Yadin Excavations 1963–1965; Israel Exploration Society: Jerusalem, Israel, 2007.
- Abdelghany, A.Y.; Awadalla, S.S.; Abdel-Baky, N.F.; El-Syrafi, H.A.; Fields, P.G. Stored-product insects in botanical warehouses. J. Stored Prod. Res. 2010, 46, 93–97.
- Howe, R.W.; Lefkovitch, L.P. The distribution of the storage species of Cryptolestes (Col., Cucujidae). Bull. Entomol. Res. 1957, 48, 795–809.
- Halstead, D.G.H. Keys for the identification of beetles associated with stored products-II. Laemophloeidae, Passandridae and Silvanidae. J. Stored Prod. Res. 1993, 29, 99–197.
- Integrated Taxonomic Information System-Report. Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=114309#null (accessed on 8 June 2023).
- Banks, H.J. Identification of stored product Cryptolestes spp. (Coleoptera: Cucujidae): A rapid technique for preparation of suitable mounts. J. Aust. Ent. Soc. 1979, 18, 217–222.
- Wang, Y.J.; Li, Z.H.; Zhang, S.F.; Varadínová, Z.; Jiang, F.; Kučerová, Z.; Stejskal, V.; Opit, G.; Cao, Y.; Li, F.J. DNA barcoding of five common stored product pest species of genus Cryptolestes (Coleoptera: Laemophloeidae). Bull. Entomol. Res. 2014, 104, 486–493.
- Varadínová, Z.; Wang, Y.J.; Kučerová, Z.; Stejskal, V.; Opit, G.; Cao, Y.; Li, F.J.; Li, Z.H. COI barcode based species-specific primers for identification of five species of stored-product pests from genus Cryptolestes (Coleoptera: Laemophloeidae). Bull. Entomol. Res. 2015, 105, 202–209.
- Chen, D.; Zhang, T.; Aulicky, R.; Stejskal, V.; Ren, Y.; Cao, Y.; Hawthorne, D.; Li, Z. Real-time PCR for identification of five species of Cryptolestes based on COI barcode region. J. Stored Prod. Res. 2020, 87, 101623.
- Canadian Grain Commission, 2021. Rusty Grain Beetle. Available online: https://grainscanada.gc.ca/en/grain-quality/manage/identify-an-insect/primary-insect-pests/rusty-grain-beetle.html (accessed on 12 August 2021).
- Campbell, A.; Sinha, R.N. Bioenergetics of granivorous beetles, Cryptolestes ferrugineus and Rhyzopertha dominica (Coleoptera: Cucujidae and Bostrichidae). Can. J. Zool. 1978, 56, 624–633.
- Lefkovitch, L.P.; Milnes, R.H. Interaction of two species of Cryptolestes (Coleoptera, Cucujidae). Bull. Entomol. Res. 1963, 54, 107–112.
- Reid, J.A. The relative sizes of different parts in beetle of the genus Laemophloeus (Coleopt.: Cucujidae). In Proceedings of the Royal Entomological Society of London. Series A, General Entomology, Oxford, UK, March 1942; Blackwell Publishing Ltd.: London, UK, 1942; pp. 19–26.
- White, N.D.G.; Bell, R.J. Effects of mating status, sex ratio, and population density on longevity and offspring production of Cryptolestes ferrugineus (Stephens) (Coloeptera: Cucujidae). Exp. Gerontol. 1993, 28, 617–631.
- Hulasare, R.B.; White, N.D.G.; Jayas, D.S. Effect of suboptimal temperatures and sublethal CO2 levels on multiplication of Tribolium castaneum (Coleoptera: Tenebrionidae), alone or competing with Cryptolestes ferrugineus (Coleoptera: Cucujidae). J. Stored Prod. Res. 2005, 41, 187–197.
- Al-Salihi, M.A.; Al-Azawi, A.F. The life history of the rusty grain beetle Cryptolestes ferrugineus (steph.) (Coleoptera: Cucujidae) on Sayer dates in Iraq. Date Palm J. 1985, 4, 69–76.
- Bishop, G.U.Y.W. The comparative bionomics of American Cryptolestes (Coleoptera-Cucujidae) that infest stored grain. Ann. Entomol. Soc. Am. 1959, 52, 657–665.
- Vendl, T.; Stejskal, V.; Aulicky, R. Comparative tarsal morphology of two secondary stored product beetle pests, Oryzaephilus surinamensis (L.) and Cryptolestes ferrugineus (Stephens), that vary in their climbing ability on smooth surfaces. J. Stored Prod. Res. 2019, 82, 116–122.
- Boukouvala, M.C.; Kavallieratos, N.G.; Canale, A.; Benelli, G. Functional asymmetries routing the mating behavior of the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae). Insects 2022, 13, 699.
- Chambers, J.; Morgan, C.P.; White, P.R.; Mori, K.; Finnegan, D.E.; Pinniger, D.B. Rust-red grain beetle, Cryptolestes ferrugineus, and flat grain beetle, Cryptolestes pusillus: Antennal and behavioral responses to synthetic components of their aggregation pheromones. J. Chem. Ecol. 1990, 16, 3353–3372.
- Smith, L.B. Observations on the oviposition rate of the rusty grain beetle, Cryptolestes ferrugineus (Steph.) (Coleoptera: Cucujidae). Ann. Entomol. Soc. Am. 1962, 55, 77–82.
- Hagstrum, D.W.; Milliken, G.A. Quantitative analysis of temperature, moisture, and diet factors affecting insect development. Ann. Entomol. Soc. Am. 1988, 81, 539–546.
- Jian, F.; Jayas, D.S.; White, N.D.G.; Muir, W.E. Temperature and geotaxis preference by Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) adults in response to 5 C/m temperature gradients at optimum and hot temperatures in stored wheat and their mortality at high temperature. Environ. Entomol. 2002, 31, 816–826.
- White, N.D.G.; Demianyk, C.J.; Kawamoto, H.; Sinha, R.N. Population growth of Cryptolestes ferrugineus and C. pusillus (Coleoptera: Cucujidae) alone, or in competition in stored wheat or maize at different temperatures. Bull. Entomol. Res. 1995, 85, 425–429.
- Smith, L.B. The intrinsic rate of natural increase of Cryptolestes ferrugineus (Stephens) (Coleoptera, Cucujidae). J. Stored Prod. Res. 1965, 1, 35–49.
- Smith, L.B. Effects of cold acclimation on supercooling and survival of the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae), at sub-zero temperatures. Can. J. Zool. 1970, 48, 853–858.
- Ganesan, L.; Fields, P.G.; Jayas, D.S.; Jian, F. Effects of developmental stage, cold acclimation and diet on the cold tolerance of three species of Cryptolestes (Coleoptera: Laemophloeidae). J. Stored Prod. Res. 2021, 91, 101773.
- Cox, P.D.; Dolder, H.S. A simple flight chamber to determine flight activity in small insects. J. Stored Prod. Res. 1995, 31, 311–316.
- Kawamoto, H.; Sinha, R.N.; Muir, W.E. Effects of temperature and relative humidity on egg development of Cryptolestes ferrugineus compared with C. pusillus (Coleoptera: Cucujidae). Appl. Entomol. Zool. 1990, 25, 35–41.
- Ashby, K.R. The population dynamics of Cryptolestes ferrugineus (Stephens) (Col., Cucujidae) in flour and on Manitoba wheat. Bull. Entomol. Res. 1961, 52, 363–379.
- Jian, F.; Jayas, D.S.; Fields, P.G.; White, N.D.G.; Zhang, H.; Tang, P. Demography of rusty grain beetle in stored bulk wheat: Part I. Population dynamics at different temperatures and grain bulk sizes. Environ. Entomol. 2018, 47, 244–255.
- Fields, P.G. The control of stored-product insects and mites with extreme temperatures. J. Stored Prod. Res. 1992, 28, 89–118.
- Fields, P.G.; Fleurat-Lessard, F.; Lavenseau, L.; Febvay, G.; Peypelut, L.; Bonnot, G. The effect of cold acclimation and deacclimation on cold tolerance, trehalose and free amino acid levels in Sitophilus granarius and Cryptolestes ferrugineus (Coleoptera). J. Insect Physiol. 1998, 44, 955–965.
- Evans, D.E. Thermal acclimation in several species of stored-grain beetles. Aust. J. Zool. 1981, 29, 483–492.
- Jian, F.; Jayas, D.S.; White, N.D.G. Effects of temperature acclimation and age on movement of Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) adults in response to temperature gradients. Can. Entomol. 2005, 137, 71–82.
- Fields, P.G. Reduction of cold tolerance of stored-product insects by ice-nucleating-active bacteria. Environ. Entomol. 1993, 22, 470–476.
- Evans, D.E. The influence of relative humidity and thermal acclimation on the survival of adult grain beetles in cooled grain. J. Stored Prod. Res. 1983, 19, 173–180.
- Burks, C.S.; Hagstrum, D.W. Rapid cold hardening capacity in five species of Coleopteran pests of stored grain. J. Stored Prod. Res. 1999, 35, 65–75.
- Throne, J.E. Effects of moisture content and initial insect density on ability of rusty grain beetles (Coleoptera: Cucujidae) to infest whole corn. J. Entomol. Sci. 1990, 25, 25–29.
- Throne, J.E.; Culik, M.P. Progency production and duration of development of rusty grain beetles, Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae), on cracked and whole corn. J. Entomol. Sci. 1989, 24, 150–155.
- Surtees, G. Laboratory studies on dispersion behaviour of adult beetles in grain. XII.—The effect of isolated pockets of damp and mouldy wheat on Cryptolestes ferrugineus (Steph.) (Coleoptera, Cucujidae). Bull. Entomol. Res. 1965, 55, 673–680.
- Smith, L.B. Effect of crowding on oviposition, development, and mortality of Cryptolestes ferrugineus (Stephens) (Coleoptera, Cucujidae). J. Stored Prod. Res. 1966, 2, 91–104.
- Tuff, D.W.; Telford, H.S. Wheat fracturing as affecting infestation by Cryptolestes ferrugineus. J. Econ. Entomol. 1964, 57, 513–516.
- Throne, J.E. Survival of immature rusty grain beetles (Coleoptera: Cucujidae) on various particle sizes of cracked corn. J. Entomol. Sci. 1992, 27, 65–70.
- Shufran, A.A.; Mulder, P.G.; Payton, M.E.; Shufran, K.A. Determining host suitability of pecan for stored-product insects. J. Econ. Entomol. 2013, 106, 1071–1074.
- White, N.D.G.; Loschiavo, S.R. Oviposition and larval development of the red flour beetle and the rusty grain beetle on ground and ball-milled kernels of various cereal cultivars. Can. J. Plant Sci. 1988, 68, 617–626.
- Hamilton, K.; White, N.D.G.; Jian, F.; Fields, P.G. Hemp (Cannabis sativa) seed for reproduction of stored-product insects. J. Stored Prod. Res. 2021, 92, 101787.
- Loschiavo, S.R.; Lamb, R.J. Food preferences, survival, and development of four stored-product pests (Coleoptera) on rapeseed and canola (Brassica spp.). Can. Entomol. 1985, 117, 575–580.
- Jagadeesan, R.; Nayak, M.K.; Dawson, K.; Byrne, V.; Collins, P.J. Dietary media for mass rearing of rusty grain beetle, Cryptolestes ferrugineus (Stephens) and flat grain beetle, Cryptolestes pusillus (Schonherr) (Coleoptera: Cucujidae). J. Stored Prod. Res. 2013, 55, 68–72.
- Sinha, R.N. Development of Cryptolestes ferrugineus (Stephens) and Oryzaephilus mercator (Fauvel) on seed-borne fungi. Entomol. Exp. Appl. 1965, 8, 309–313.
- Loschiavo, S.R.; Sinha, R.N. Feeding, oviposition, and aggregation by the rusty grain beetle, Cryptolestes ferrugineus (Coleoptera: Cucujidae) on seed-borne fungi. Ann. Entomol. Soc. Am. 1966, 59, 578–585.
- Hagstrum, D.W.; Subramanyam, B. Stored-Product Insect Resource; AACC International: Minnesota, MN, USA, 2009.
- Jian, F.; Jayas, D.S.; Fields, P.G.; White, N.D.G. Demography of rusty grain beetle in stored bulk wheat: Part II. Mathematical modeling to characterize and predict population dynamics. Environ. Entomol. 2018, 47, 256–263.
- Cox, P.D.; Collins, L.E. Factors affecting the behaviour of beetle pests in stored grain, with particular reference to the development of lures. J. Stored Prod. Res. 2002, 38, 95–115.
- Jian, F.; Jayas, D.S.; White, N.D.G. Optimal environmental search and scattered orientations during movement of adult rusty grain beetles, Cryptolestes ferrugineus (Stephens), in grain bulks–suggested movement and distribution patterns. J. Stored Prod. Res. 2009, 45, 177–183.
- Cox, P.D.; Parish, W.E. Effects on refuge content and food availability on refuge-seeking behaviour in Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae). J. Stored Prod. Res. 1991, 27, 135–139.
- Liscombe, E.A.R.; Watters, F.L. Insect and mite infestations in empty granaries in the prairie provinces. Can. Entomol. 1962, 94, 433–441.
- Cox, P.D.; Parish, W.E.; Beirne, M.A. Variations in the refuge-seeking behaviour of four strains of Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae) at different temperatures. J. Stored Prod. Res. 1989, 25, 239–242.
- Cox, P.D.; Parish, W.E.; Ledson, M. Factors affecting the refuge-seeking behaviour of Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae). J. Stored Prod. Res. 1990, 26, 169–174.
- Nansen, C.; Bonjour, E.L.; Gates, M.W.; Phillips, T.W.; Cuperus, G.W.; Payton, M.E. Model of Cryptolestes ferrugineus flight activity outside commercial steel grain bins in central Oklahoma. Environ. Entomol. 2004, 33, 426–434.
- Holloway, J.C.; Mayer, D.G.; Daglish, G.J. Flight activity of Cryptolestes ferrugineus in southern New South Wales, Australia. J. Pest Sci. 2018, 91, 1353–1362.
- Toews, M.D.; Campbell, J.F.; Arthur, F.H.; Ramaswamy, S.B. Outdoor flight activity and immigration of Rhyzopertha dominica into seed wheat warehouses. Entomol. Exp. Appl. 2006, 121, 73–85.
- Hagstrum, D.W. Immigration of insects into bins storing newly harvested wheat on 12 Kansas farms. J. Stored Prod. Res. 2001, 37, 221–229.
- Hagstrum, D.W. Infestation by Cryptolestes ferrugineus (Coleoptera: Cucujidae) of newly harvested wheat stored on three Kansas farms. J. Econ. Entomol. 1989, 82, 655–659.
- Borden, J.H.; Dolinski, M.G.; Chong, L.; Verigin, V.; Pierce, H.D.; Oehlschlager, A.C. Aggregation pheromone in the rusty grain beetle, Cryptolestes ferrugineus (Coleoptera: Cucujidae). Can. Entomol. 1979, 111, 681–688.
- Wong, J.W.; Verigin, V.; Oehlschlager, A.C.; Borden, J.H.; Pierce, J.H.D.; Pierce, A.M.; Chong, L. Isolation and identification of two macrolide pheromones from the frass of Cryptolestes ferrugineus (Coleoptera: Cucujidae). J. Chem. Ecol. 1983, 9, 451–474.
- Lindgren, B.S.; Borden, J.H.; Pierce, A.M.; Pierce, H.D.; Oehlschlager, A.C.; Wong, J.W. A potential method for simultaneous, semiochemical-based monitoring of Cryptolestes ferrugineus and Tribolium castaneum (Coleoptera: Cucujidae and Tenebrionidae). J. Stored Prod. Res. 1985, 21, 83–87.
- Currie, S.; Bharathi, V.S.K.; Jian, F.; Fields, P.G.; Jayas, D.S. Attractiveness of male and female adults of Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) to conspecifics with and without grain. Environ. Entomol. 2020, 49, 1282–1289.
- Oehlschlager, A.C.; King, G.G.S.; Pierce, H.D.; Pierce, A.M.; Slessor, K.N.; Millar, J.G.; Borden, J.H. Chirality of macrolide pheromones of grain beetles in the genera Oryzaephilus and Cryptolestes and its implications for species specificity. J. Chem. Ecol. 1987, 13, 1543–1554.
- Throne, J.E.; Cline, L.D. Seasonal flight activity and seasonal abundance of selected stored-product Coleoptera around grain storages in South Carolina. J. Agric. Entomol. 1994, 11, 321–338.
- Cofie-Agblor, R.; Muir, W.E.; Sinha, R.N.; Fields, P.G. Heat production by adult Cryptolestes ferrugineus (Stephens) of different ages and densities. Postharvest Biol. Technol. 1996, 7, 371–380.
- Cofie-Agblor, R.; Muir, W.E.; Zhang, Q.; Sinha, R.N. Heat of respiration of Cryptolestes ferrugineus (Stephens) adults and larvae in stored wheat. Can. Agric. Eng. 1996, 38, 37–44.
- Smith, L.B. Ecology of stored grain in the Canadian prairies: I. The distribution and size of a low density population of Cryptolestes ferrugineus (Coleoptera: Cucujidae). Can. Entomol. 1978, 110, 1281–1292.
- Smith, L.B. The relationship between wet grain, Cryptolestes ferrugineus (Coleoptera: Cucujidae) populations, and heating in wheat stored in granaries. Can. Entomol. 1983, 115, 1383–1394.
- Jian, F. Computer Modelling of Temperature and Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) Adult Distribution in Grain Bins. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2003.
- Anukiruthika, T.; Jian, F.; Jayas, D.S. Movement and behavioral response of stored product insects under stored grain environments—A review. J. Stored Prod. Res. 2021, 90, 101752.
- Flinn, P.W.; Hagstrum, D.W. Distribution of Cryptolestes ferrugineus (Coleoptera: Cucujidae) in response to temperature gradients in stored wheat. J. Stored Prod. Res. 1998, 34, 107–112.
- Jian, F.; Jayas, D.S.; White, N.D.G. Movement of adult Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) in wheat: Response to temperature gradients and gravity. Environ. Entomol. 2004, 33, 1003–1013.
- Jian, F.; Larson, R.; Jayas, D.S.; White, N.D.G. Three-dimensional spatial distribution of adults of Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) in stored wheat under different temperatures, moisture contents, and adult densities. J. Stored Prod. Res. 2011, 47, 293–305.
- White, N.D.G.; Sinha, R.N.; Jayas, D.S.; Muir, W.E. Movement of Cryptolestes ferrugineus (Coleoptera: Cucujidae) through carbon dioxide gradients in stored wheat. J. Econ. Entomol. 1993, 86, 1846–1851.
- Jian, F.; Jayas, D.S.; White, N.D.G. Movement and distribution of adult rusty grain beetle, Cryptolestes ferrugineus (Coleoptera: Laemophloeidae), in stored wheat in response to different temperature gradients and insect densities. J. Econ. Entomol. 2004, 97, 1148–1158.
- Jian, F.; Jayas, D.S.; White, N.D.G. Movement and distribution of adult Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) in stored wheat in response to temperature gradients, dockage, and moisture differences. J. Stored Prod. Res. 2005, 41, 401–422.
- Jian, F.; Jayas, D.S.; White, N.D.G.; Smith, E.A. Two-dimensional diffusion of Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae) populations in stored wheat under constant environmental conditions. J. Stored Prod. Res. 2007, 43, 342–348.
- Bharathi, V.S.K.; Jian, F.; Jayas, D.S.; Morrison, J. Three-dimensional movement and distribution of Cryptolestes ferrugineus (Coleoptera: Laemophloeidae) adults in stored wheat under constant temperatures and moisture contents. Environ. Entomol. 2022, 51, 11–21.
- Bharathi, V.S.K.; Jayas, D.S.; Jian, F. Effects of insect density, movement period, and temperature on three-dimensional movement and distribution of adult Cryptolestes ferrugineus (Coleoptera: Laemophloeidae). J. Insect Sci. 2022, 22, 3.
- Bharathi, V.S.K.; Jayas, D.S.; Jian, F. Study on 300 t of wheat stored in corrugated steel bin for two years in Canada. Part II–Movement and distribution of Cryptolestes ferrugineus (Stephens) and Tribolium castaneum (Herbst). J. Stored Prod. Res. 2023, 100, 102062.
- Bharathi, V.S.K.; Jian, F.; Jayas, D.S. Study on 300 t of wheat stored in corrugated steel bin for two years in Canada. Part I–Temperature and moisture profiles of the grain. J. Stored Prod. Res. 2023, 100, 102057.
- Nansen, C.; Flinn, P.; Hagstrum, D.; Toews, M.D.; Meikle, W.G. Interspecific associations among stored-grain beetles. J. Stored Prod. Res. 2009, 45, 254–260.
- Stejskal, V.; Kosina, P.; Kanyomeka, L. Arthropod pests and their natural enemies in stored crops in northern Namibia. J. Pest Sci. 2006, 79, 51–55.
- Hou, X.; Fields, P.; Flinn, P.; Perez-Mendoza, J.; Baker, J. Control of stored-product beetles with combinations of protein-rich pea flour and parasitoids. Environ. Entomol. 2004, 33, 671–680.
- Steidle, J.L.M.; Schöller, M. Fecundity and ability of the parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae) to find larvae of the granary weevil Sitophilus granarius (Coleoptera: Curculionidae) in bulk grain. J. Stored Prod. Res. 2002, 38, 43–53.
- Flinn, P.W. Temperature-dependent functional response of the parasitoid Cephalonomia waterstoni (Gahan) (Hymenoptera: Bethylidae) attacking rusty grain beetle larvae (Coleoptera: Cucujidae). Environ. Entomol. 1991, 20, 872–876.
- Howard, R.W.; Flinn, P.W. Larval trails of Cryptolestes ferrugineus (Coleoptera: Cucujidae) as kairomonal host-finding cues for the parasitoid Cephalonomia waterstoni (Hymenoptera: Bethylidae). Ann. Entomol. Soc. Am. 1990, 83, 239–245.
- Flinn, P.W.; Hagstrum, D.W. Simulation model of Cephalonomia waterstoni (Hymenoptera: Bethylidae) parasitizing the rusty grain beetle (Coleoptera: Cucujidae). Environ. Entomol. 1995, 24, 1608–1615.
- Oliveira, C.R.F.; Faroni, L.R.A.; Guedes, R.N.C.; Gonçalves, J.R.; Garcia, F.M. Biology of Acarophenax lacunatus (Cross & Krantz) (Prostigmata: Acarophenacidae) on Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) and Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae). Neotrop. Entomol. 2007, 36, 459–464.
- Barker, P.S. Bionomics of Cheyletus eruditus (Schrank) (Acarina: Cheyletidae), a predator of Lepidoglyphus destructor (Schrank) (Acarina: Glycyphagidae), at three constant temperatures. Can. J. Zool. 1991, 69, 2321–2325.
- Oliveira, C.R.F.; Faroni, L.R.D.A.; Guedes, R.N.C.; Pallini, A. Parasitism by the mite Acarophenax lacunatus on beetle pests of stored products. Biocontrol 2003, 48, 503–513.
- Er, M.K.; Tunaz, H.; Ücük, C.; Bariş, C.; Işikber, A.A. Occurrence of entomopathogenic fungi on insect pests of stored wheat and maize in Central and South Anatolia in Turkey. Turk. Entomoloji Derg. 2016, 40, 249–263.
- Wakil, W.; Ghazanfar, M.U.; Yasin, M. Naturally occurring entomopathogenic fungi infecting stored grain insect species in Punjab, Pakistan. J. Insect Sci. 2014, 14, 182.
- Lord, J.C. Beauveria bassiana infection of eggs of stored-product beetles. Entomol. Res. 2009, 39, 155–157.
- Throne, J.E. A fungal parasite (Ascomycetes: Laboulbeniales) of Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae). J. Stored Prod. Res. 1989, 25, 115–116.
- Lord, J.C.; Vossbrinck, C.R.; Wilson, J.D. Occurrence of Nosema oryzaephili in Cryptolestes ferrugineus and transfer to the genus Paranosema. J. Invertebr. Pathol. 2010, 105, 112–115.
- Ünal, H.; Koçak, E. Endosymbiont microorganisms in rusty grain beetle Cryptolestes ferrugineus (L.) populations. Turk. J. Agric. Food Sci. Technol. 2019, 7, 93–96.
- Lord, J.C. Mattesia oryzaephili (Neogregarinorida: Lipotrophidae), a pathogen of stored-grain insects: Virulence, host range and comparison with Mattesia dispora. Biocontrol Sci. Technol. 2003, 13, 589–598.
More
Information
Subjects:
Ecology; Entomology; Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
23 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





