
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mary Garvey | -- | 3373 | 2023-08-22 13:49:44 | | | |
| 2 | Peter Tang | Meta information modification | 3373 | 2023-08-23 02:52:38 | | |
Video Upload Options
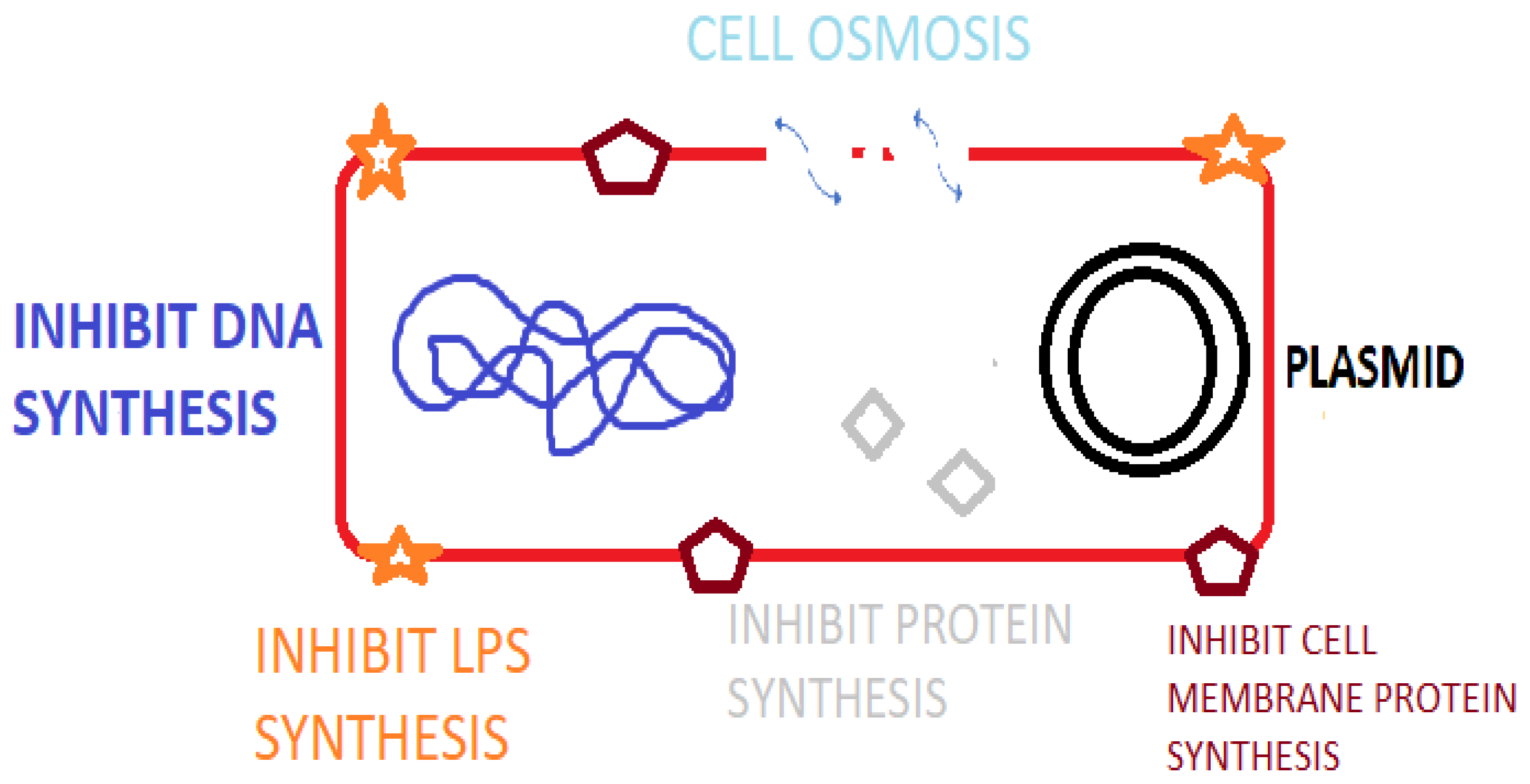
The antimicrobial resistance crisis is an ongoing major threat to public health safety. Low- and middle-income countries are particularly susceptible to higher fatality rates and the economic impact of antimicrobial resistance (AMR). As an increasing number of pathogens emerge with multi- and pan-drug resistance to last-resort antibiotics, there is an urgent need to provide alternative antibacterial options to mitigate disease transmission, morbidity, and mortality. As identified by the World Health Organization (WHO), critically important pathogens such as Klebsiella and Pseudomonas species are becoming resistant to last-resort antibiotics including colistin while being frequently isolated from clinical cases of infection. Antimicrobial peptides are potent amino acid sequences produced by many life forms from prokaryotic, fungal, plant, to animal species. These peptides have many advantages, including their multi-hit mode of action, potency, and rapid onset of action with low levels of resistance being evident. These innate defense mechanisms also have an immune-stimulating action among other activities in vivo, thus making them ideal therapeutic options. Large-scale production and formulation issues (pharmacokinetics, pharmacodynamics), high cost, and protease instability hinder their mass production and limit their clinical application.
1. Introduction
|
Family |
AMP |
Action |
Additional Info |
|---|---|---|---|
|
Defensin |
Alpha- and beta-defensins Human neutrophil peptides (HNPs) [25] Human enteric defensins |
Directly kill phagocytosed microbes. Can enhance the production of inflammatory cytokines, e.g., interleukin-1 [25] |
Produced by neutrophils, lymphocytes, and epithelial cells of the skin and mucous membranes [1]. Stored in the azurophilic granules of human neutrophils. Secreted by paneth cells in the small intestinal |
|
Cathelicidins |
Bactenecins Human cathelicidin LL-37 [9] |
Immunomodulatory activity, impacts quorum sensing mechanisms in P. aeruginosa biofilm formation [25], apoptosis induction, inflammasome activation, and phagocytosis [13] LL-37 inhibits Aspergillus fumigatus infection and reduces inflammation [9], LL37 analogue peptides (AC-1, AC-2, LL37-1, and D) effetive against C. albicans yeasts [3] |
The bactenecin ChBac3.4 appears more active against bacterial membranes and tumor cells compared to normal cells [26] LL-37 effective against various Gram-positive and Gram-negative bacteria, and AMR species [9] |
|
Hepcidins |
Human liver-expressed antimicrobial peptide (LEAP) Fish hepcidins HAMP1 and HAMP2 |
Antibacterial Iron regulatory mode of action [19] |
Type II acute phase protein Results in a reduction in ferroportin expression |
|
Histone- histidine-rich peptides |
Histatins—cationic peptides secreted into human saliva by salivary glands |
Antifungal action, antibacterial [27] |
High biocompatibility, effective against azole-resistant fungi [27] |
|
Piscidins |
Present mainly in the tissues of gills, muscle, head kidney, skin, and intestine [15] |
Potent and broad-spectrum [15], antibacterial, antifungal, and antiviral properties |
Efficacy toward MDR MRSA, vancomycin-resistant Enterococci, piscidin has antitumor activity against cancer-derived cell lines [15] |
HNPs—human neutrophil peptides, AMR—antimicrobial resistance, MDR—multidrug resistant, LEAP—human liver-expressed AMP, MRSA—methicillin-resistant S. aureus.
2. Clinical Bacterial Infectious Disease
3. Issues Preventing the Application of AMPs as Broad-Spectrum Antimicrobials
3.1. Future Direction of AMP Production and Formulation to Overcome Current Issues
3.2. Pharmacokinetics and Pharmacodynamic Considerations
References
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499.
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767.
- Pinilla, G.; Coronado, Y.T.; Chaves, G.; Muñoz, L.; Navarrete, J.; Salazar, L.M.; Taborda, C.P.; Muñoz, J.E. In Vitro Antifungal Activity of LL-37 Analogue Peptides against Candida spp. J. Fungi 2022, 7, 1173.
- Decker, A.P.; Mechesso, A.F.; Wang, G. Expanding the Landscape of Amino Acid-Rich Antimicrobial Peptides: Definition, Deployment in Nature, Implications for Peptide Design and Therapeutic Potential. Int. J. Mol. Sci. 2022, 23, 12874.
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48.
- Huang, H.W. Daptomycin, its membrane-active mechanism vs. that of other antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183395.
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575.
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16.
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979.
- Tan, L.T.H.; Chan, K.G.; Pusparajah, P.; Lee, W.L.; Chuah, L.H.; Khan, T.M.; Goh, B.H. Targeting Membrane Lipid a Potential Cancer Cure? Front. Pharmacol. 2017, 8, 12.
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, Potent Antimicrobial Peptides and the Fight against Multi Drug Resistant Species: Resistance Is Futile? Antibiotics 2020, 9, 32.
- Rončević, T.; Puizina, J.; Tossi, A. Antimicrobial Peptides as Anti-Infective Agents in Pre-Post-Antibiotic Era? Int. J. Mol. Sci. 2019, 20, 5713.
- Fasina, Y.O.; Obanla, T.; Dosu, G.; Muzquiz, S. Significance of Endogenous Antimicrobial Peptides on the Health of Food Animals. Front. Vet. Sci. 2021, 8, 585266.
- Assoni, L.; Milani, B.; Carvalho, M.R.; Nepomuceno, L.N.; Waz, N.T.; Guerra, M.E.S.; Converso, T.R.; Darrieux, M. Resistance Mechanisms to Antimicrobial Peptides in Gram-Positive Bacteria. Front. Microbiol. 2020, 11, 593215.
- Zaccone, G.; Capillo, G.; Fernandes, J.M.O.; Kiron, V.; Lauriano, E.R.; Alesci, A.; Lo Cascio, P.M.C.; Kuciel, M.; Zuwala, K.; Icardo, J.M.; et al. Expression of the Antimicrobial Peptide Piscidin 1 and Neuropeptides in Fish Gill and Skin: A Potential Participation in Neuro-Immune Interaction. Mar. Drugs 2022, 20, 145.
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970.
- Zhang, F.; Cui, X.; Fu, Y.; Zhang, J.; Zhou, Y.; Sun, Y. Antimicrobial activity and mechanism of the human milk-sourced peptide Casein201. Biochem. Biophys. Res. Commun. 2017, 485, 698–704.
- Dutta, P.; Sahu, R.K.; Dey, T.; Lahkar, M.D.; Manna, P.; Kalita, J. Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem. Biol. Interact. 2019, 313, 108824.
- Athira, P.P.; Anooja, V.V.; Anju, M.V.; Neelima, S.; Archana, K.; Muhammed Musthafa, S.; Antony, S.P.; Bright Singh, I.S.; Philip, R. A hepatic antimicrobial peptide, hepcidin from Indian major carp, Catla catla: Molecular identification and functional characterization. J. Genet. Eng. Biotechnol. 2022, 20, 49.
- Koukoulas, K.; Lygoura, V.; Kartalidis, P.; Gatselis, N.K.; Petinaki, E.; Dalekos, G.N.; Simos, G. Hepcidin as a Sensitive and Treatment-Responsive Acute-Phase Marker in Patients with Bacteremia: A Pilot Study. Diagnostics 2022, 12, 1404.
- Dicks, L.; Dreyer, L.; Smith, C.; van Staden, A.D. A Review: The Fate of Bacteriocins in the Human Gastro-Intestinal Tract: Do They Cross the Gut-Blood Barrier? Front. Microbiol. 2018, 9, 2297.
- Lbehiry, A.; Marzouk, E.; Abalkhail, A.; El-Garawany, Y.; Anagreyyah, S.; Alnafea, Y.; Almuzaini, A.M.; Alwarhi, W.; Rawway, M.; Draz, A. The Development of Technology to Prevent, Diagnose, and Manage Antimicrobial Resistance in Healthcare-Associated Infections. Vaccines 2022, 8, 2100.
- Garvey, M. Bacteriophages and Food Production: Biocontrol and Bio-Preservation Options for Food Safety. Antibiotics 2022, 11, 1324.
- Wang, G.; Mechesso, A.F. Realistic and critical review of the state of systemic antimicrobial peptides. ADMET DMPK 2022, 10, 91–105.
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095.
- Kopeikin, P.M.; Zharkova, M.S.; Kolobov, A.A.; Smirnova, M.P.; Sukhareva, M.S.; Umnyakova, E.S.; Kokryakov, V.N.; Orlov, D.S.; Milman, B.L.; Balandin, S.V.; et al. Caprine Bactenecins as Promising Tools for Developing New Antimicrobial and Antitumor Drugs. Front. Cell. Infect. Microbiol. 2020, 10, 552905.
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. Off. Publ. Saudi Pharm. Soc. 2017, 25, 25–31.
- Rabaan, A.A.; Eljaaly, K.; Alhumaid, S.; Albayat, H.; Al-Adsani, W.; Sabour, A.A.; Alshiekheid, M.A.; Al-Jishi, J.M.; Khamis, F.; Alwarthan, S.; et al. An Overview on Phenotypic and Genotypic Characterisation of Carbapenem-Resistant Enterobacterales. Medicina 2022, 58, 1675.
- Calbo, E.; Boix-Palop, L.; Garau, J. Clinical and economic impact of bacterial resistance: An approach to infection control and antimicrobial stewardship solutions. Curr. Opin. Infect. Dis. 2020, 33, 458–463.
- Jeck, J.; Wingen-Heimann, S.M.; Jakobs, F.; Franz, J.; Baltin, C.T.; Kron, A.; Böll, B.; Kochanek, M.; Cornely, O.A.; Kron, F. Last Resort Antibiotics Costs and Reimbursement Analysis of Real-Life ICU Patients with Pneumonia Caused by Multidrug-Resistant Gram-Negative Bacteria in Germany. Healthcare 2022, 10, 2546.
- Ahmed, N.; Khalid, H.; Mushtaq, M.; Basha, S.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Al Mutair, A.; Alhumaid, S.; Al Alawi, Z.; et al. The Molecular Characterization of Virulence Determinants and Antibiotic Resistance Patterns in Human Bacterial Uropathogens. Antibiotics 2022, 11, 516.
- Li, C.; Wang, J.; Wang, Q.; Liu, B.; Dang, H.; Li, J.; Hou, D. Predictive Value of a Quick Pitt Bacteremia Score for Prognosis of Patients with Bloodstream Infection Secondary to Urinary Tract Infection: A Retrospective Cohort Study. Infect. Drug Resist. 2022, 15, 4381–4391.
- Mark, D.G.; Hung, Y.Y.; Salim, Z. Third-generation cephalosporin resistance and associated discordant antibiotic treatment in emergency department febrile urinary tract infections. Ann. Emerg. Med. 2021, 78, 357–369.
- Nordmann, P.; Poirel, L. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin. Infect. Dis. 2019, 69 (Suppl. S7), S521–S528.
- Yusof, N.Y.; Norazzman, N.I.I.; Hakim, S.N.W.A.; Azlan, M.M.; Anthony, A.A.; Mustafa, F.H.; Ahmed, N.; Rabaan, A.A.; Almuthree, S.A.; Alawfi, A.; et al. Prevalence of Mutated Colistin-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 414.
- GBD. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019 GBD 2019 Antimicrobial Resistance Collaborators. Lancet 2022, 400, 2221–2248.
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85.
- Lei, M.; Jayaraman, A.; Van Deventer, J.A.; Lee, K. Annual Review of Biomedical Engineering Engineering Selectively Targeting Antimicrobial Peptides. Annu. Rev. Biomed. Eng. 2021, 23, 339–357.
- Shen, X.; Zhang, Y.; Mao, Q.; Huang, Z.; Yan, T.; Lin, T.; Chen, W.; Wang, Y.; Cai, X.; Liang, Y. Peptide–Polymer Conjugates: A Promising Therapeutic Solution for Drug-Resistant Bacteria. Int. J. Polym. Sci. 2022, 2022, 7610951.
- Pereira, A.G.; Jaramillo, M.L.; Remor, A.P.; Latini, A.; Davico, C.E.; da Silva, M.L.; Müller, Y.M.R.; Ammar, D. Low-concentration exposure to glyphosate-based herbicide modulates the complexes of the mitochondrial respiratory chain and induces mitochondrial hyperpolarization in the Danio rerio brain. Chemosphere 2018, 209, 353–362.
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11.
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 12840.
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in vivo Models. Front. Microbiol. 2021, 12, 630695.
- Wibowo, D.; Zhao, C.X. Recent achievements and perspectives for large-scale recombinant production of antimicrobial peptides. Appl. Microbiol. Biotechnol. 2018, 103, 659–671.
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880.
- Sinha, R.; Shukla, P. Antimicrobial Peptides: Recent Insights on Biotechnological Interventions and Future Perspectives. Protein Pept. Lett. 2019, 26, 79–87.
- Cao, J.; de la Fuente-Nunez, C.; Ou, R.W.; Torres, M.D.; Pande, S.G.; Sinskey, A.J.; Lu, T.K. Yeast-based synthetic biology platform for antimicrobial peptide production. ACS Synth. Biol. 2018, 7, 896–902.
- Shanmugaraj, B.; Bulaon, C.J.I.; Malla, A.; Phoolcharoen, W. Biotechnological Insights on the Expression and Production of Antimicrobial Peptides in Plants. Molecules 2021, 26, 4032.
- Garvey, M. Non-Mammalian Eukaryotic Expression Systems Yeast and Fungi in the Production of Biologics. J. Fungi 2022, 8, 1179.
- Kim, D.S.; Kim, S.W.; Song, J.M.; Kim, S.Y.; Kwon, K.C. A new prokaryotic expression vector for the expression of antimicrobial peptide abaecin using SUMO fusion tag. BMC Biotechnol. 2019, 19, 13.
- Käßer, L.; Rotter, M.; Coletta, L.; Salzig, D.; Czermak, P. Process intensification for the continuous production of an antimicrobial peptide in stably-transformed Sf-9 insect cells. Sci. Rep. 2022, 12, 1086.
- Sabino, Y.N.V.; Fochat, R.C.; Lima, J.C.F.; Ribeiro, M.T.; Arcuri, P.B.; Carneiro, J.; Machado, M.A.; de Lima Reis, D.R.; Machado, A.B.F.; Hungaro, H.M.; et al. Antibacterial activity and lantibiotic post-translational modification genes in Streptococcus spp. isolated from ruminal fluid. Ann. Microbiol. 2019, 69, 131–138.
- Hoelscher, M.P.; Forner, J.; Calderone, S.; Krämer, C.; Taylor, Z.; Loiacono, F.V.; Agrawal, S.; Karcher, D.; Moratti, F.; Kroop, X.; et al. Expression strategies for the efficient synthesis of antimicrobial peptides in plastids. Nat. Commun. 2022, 13, 5856.
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical modifications to increase the therapeutic potential of antimicrobial peptides. Peptides 2021, 146, 170666.
- Rasines Mazo, A.; Allison-Logan, S.; Karimi, F.; Chan, N.J.A.; Qiu, W.; Duan, W.; Qiao, G.G. Ring opening polymerization of α-amino acids: Advances in synthesis, architecture and applications of polypeptides and their hybrids. Chem. Soc. Rev. 2020, 49, 4737–4834.
- Fang, Z.; Wusgal, C.H.; Liang, L. Natural Biodegradable Medical Polymers. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 321–350.
- Available online: https://www.europeanpharmaceuticalreview.com/news/178380/promising-first-in-human-study-results-for-orally-inhaled-antibiotic-macrocycle-compound/ (accessed on 2 April 2023).
- Barreto-Santamaria, A.; Patarroyo, M.E.; Curtidor, H. Designing and optimizing new antimicrobial peptides: All targets are not the same. Crit. Rev. Clin. Lab. Sci. 2019, 56, 351–373.
- Dong, N.; Wang, C.; Zhang, T.; Zhang, L.; Xue, C.; Feng, X.; Bi, C.; Shan, A. Bioactivity and Bactericidal Mechanism of Histidine-Rich β-Hairpin Peptide Against Gram-Negative Bacteria. Int. J. Mol. Sci. 2019, 20, 3954.




