Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Naoki Oishi | -- | 1538 | 2023-08-22 12:54:19 | | | |
| 2 | Rita Xu | Meta information modification | 1538 | 2023-08-23 03:43:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shimanuki, M.N.; Nishiyama, T.; Hosoya, M.; Wakabayashi, T.; Ozawa, H.; Oishi, N. Temporal Bone Mass Lesions. Encyclopedia. Available online: https://encyclopedia.pub/entry/48321 (accessed on 07 February 2026).
Shimanuki MN, Nishiyama T, Hosoya M, Wakabayashi T, Ozawa H, Oishi N. Temporal Bone Mass Lesions. Encyclopedia. Available at: https://encyclopedia.pub/entry/48321. Accessed February 07, 2026.
Shimanuki, Marie N., Takanori Nishiyama, Makoto Hosoya, Takeshi Wakabayashi, Hiroyuki Ozawa, Naoki Oishi. "Temporal Bone Mass Lesions" Encyclopedia, https://encyclopedia.pub/entry/48321 (accessed February 07, 2026).
Shimanuki, M.N., Nishiyama, T., Hosoya, M., Wakabayashi, T., Ozawa, H., & Oishi, N. (2023, August 22). Temporal Bone Mass Lesions. In Encyclopedia. https://encyclopedia.pub/entry/48321
Shimanuki, Marie N., et al. "Temporal Bone Mass Lesions." Encyclopedia. Web. 22 August, 2023.
Copy Citation
Tumoral lesions of the temporal bone include benign or malignant tumors and congenital or inflammatory lesions. Temporal bone lesions are difficult to approach. Therefore, making a preoperative diagnosis and considering whether the lesions require treatment are necessary; if they require treatment, then the type of treatment requires consideration. These tumors cannot be observed directly and must be diagnosed based on symptoms and imaging findings.
flowchart
imaging
temporal bone tumor
1. Introduction
Tumoral lesions of the temporal bone include benign or malignant tumors and congenital or inflammatory lesions. These tumors cannot be directly observed and must be diagnosed based on symptoms and imaging findings. However, the differentiation of temporal bone lesions is difficult. This is because the temporal bone includes various parts from the inner auditory canal to the middle ear and different types of lesions occur in each part. Furthermore, all tumoral lesions are rare. However, among them, several articles have reported lesions with a relatively high frequency in detail [1][2][3] and diagnostic methods have been established. Although studies have summarized the characteristics of each rare tumor, no cross-sectional classification method has been established. Kim et al. evaluated cases of skull-base surgery for intratemporal tumors during the previous 25 years and found that facial nerve schwannomas, squamous cell carcinomas, glomus tumors, and lower cranial nerve schwannomas were the most common [4]. However, other rare tumors have not been reported in detail.
Campion et al. summarized the differential diagnoses of inflammatory lesions in the temporal bones of children [5]. This suggested that a similar flowchart for tumoral lesions and adult cases would be useful for diagnosis.
The temporal bone includes numerous vital structures such as the cochlea, semicircular canal, facial nerve, internal carotid artery, and jugular vein and forms the lateral part of the skull base; therefore, temporal bone lesions are difficult to approach [4]. Making a preoperative diagnosis and considering whether the lesion requires treatment are necessary; if treatment is required, the type of treatment must be determined. The treatment varies, depending on the type of lesion. For example, cholesterol granulomas require a surgical approach that allows drainage, while cholesteatomas require the total removal of the epithelium and a field of view that allows total removal. Not only surgical approaches but also radiological treatments are useful for some lesions.
2. Along the Facial Nerve
Facial nerve schwannomas, hemangiomas, and the perineural spread of tumors extend along the facial nerve. Facial nerve schwannomas may cause hearing loss and facial nerve paresis. Facial nerve schwannomas exhibit expansile destruction of the surrounding bone on computed tomography (CT) images and a tubular or sausage-link appearance with contrast effects on magnetic resonance imaging (MRI) images [1] (Figure 1). Hemangiomas arise from the perineural venous plexus and occur most often in the region of the geniculate ganglion along the facial nerve [6]. They cause hearing loss and facial nerve paresis. Hemangiomas may show bone spicules within the mass in CT images and MRI typically shows hyperintensity in T1-weighted and T2-weighted images [7]. However, some cases have shown low to isointensity in T1-weighted images, which can make it difficult to distinguish them from facial nerve schwannomas [8]. Moreover, there have been reports of heterogeneous signal intensities and avid enhancements [1]. It is necessary to distinguish between facial nerve schwannomas, hemangiomas, and meningiomas, especially in the geniculate ganglion; however, hemangiomas and meningiomas are differentiated from facial nerve schwannomas by the presence of intertumoral bone spicules [1].
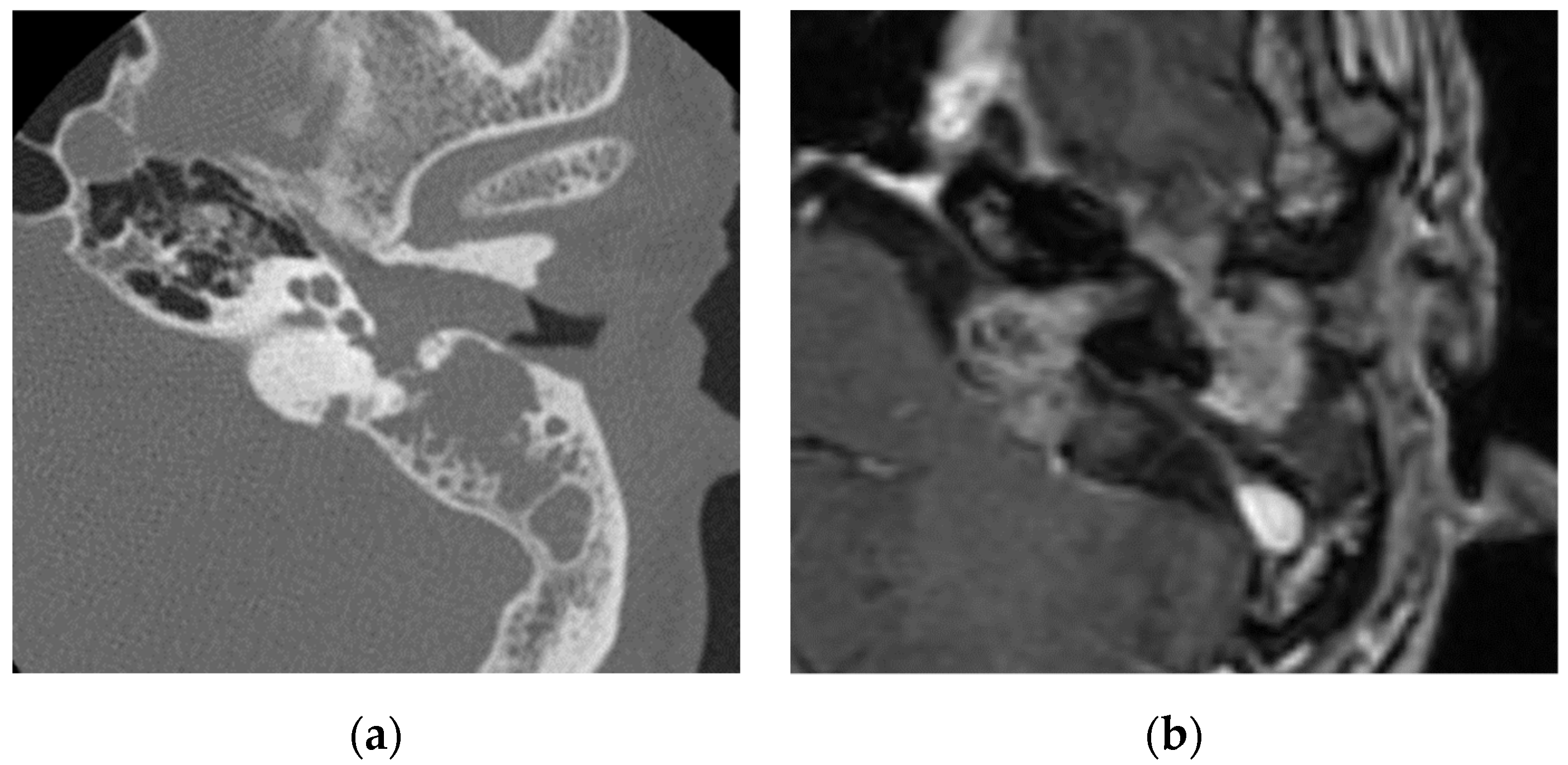
Figure 1. Facial nerve schwannoma: (a) in CT, a soft-tissue shadow is seen in the external auditory canal, middle ear, and mastoid with evidence of bone erosion; (b) in contrasted T1-weighted images, a mass lesion is visualized in the middle ear, internal auditory canal, and cerebellopontine angle along the facial nerve, which shows enhancement.
The perineural spread of a malignant tumor is suspected when the thickening and enhancement of the segmental facial nerve are observed. Parotid-gland cancers such as adenoid cystic carcinomas and mucoepidermoid carcinomas as well as nearby skin malignancies that invade or metastasize secondarily to the parotid gland and hematogenous metastases of breast, tracheal, or prostate cancer can all potentially spread along the facial nerve [2].
A comparison of facial nerve schwannomas and the perineural spread of parotid carcinomas showed that facial nerve palsy is less common; target signs are seen in intraparotid facial nerve schwannomas and parotid carcinomas present with an undefined margin [9].
3. Along the Internal Jugular Vein
Paragangliomas called glomus tumors (glomus jugulare and glomus jugulotympanicum) are most common along the internal jugular vein. Paragangliomas arise from neural crest-derived paraganglia. Paragangliomas extend along the jugular bulb, cranial nerves such as the vagus nerve, the tympanic branch of the glosspharyngeal nerve, and the auricular branch of the vagus nerve. Clinically, dysfunction of cranial nerves IX-XII (in the case of jugular lesions) and pulsatile tinnitus (in the case of tympanic lesions) may occur [6]. Glomus tumors have high vascularity and are homogeneous and significantly enhanced. Bone erosion occurs around the jugular foramen. T2-weighted images show isointensity [2]. Unenhanced T1-weighted and T2-weighted images may show a “salt and pepper” appearance [1] (Figure 2).
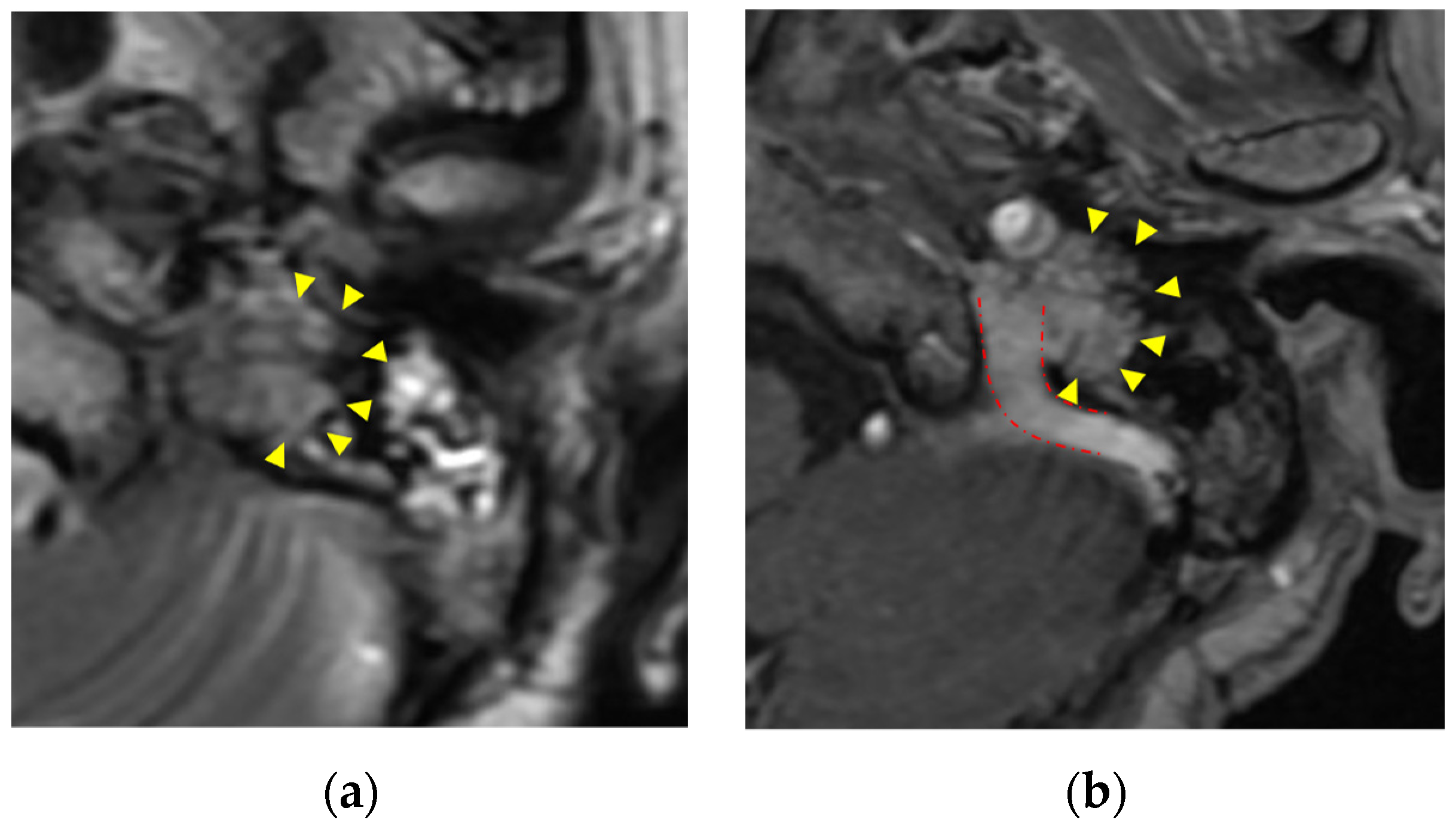
Figure 2. Paraganglioma (glomus jugulare): (a) paraganglioma shows moderate intensity with “salt and pepper” appearance in T2-weighted images (yellow triangle); (b) homogeneous enhancement in enhanced CT T1-weighted images(yellow triangle). Tumor extends along the internal jugular vein (red dashed line).
Additionally, other lesions extending around the jugular foramen have been reported. Schwannomas of the lower cranial nerves extend along the nerves with moderate to high enhancement. Schwannomas of the lower cranial nerves can be differentiated from glomus tumors by the absence of flow voids. Additionally, meningiomas can occur less frequently. In CT images, meningiomas show sclerosis and hyperostosis with local bone destruction [1]. Using MRI, T1-weighted images show hypointensity to the brain parenchyma and T2-weighted images show isointensity that is often homogenously contrast-enhanced. Cystic degeneration is uncommon and a dural tail sign is a characteristic finding of meningiomas [10]. Meningiomas are differentiated from other lesions by an enhanced dural tail and the absence of flow voids.
The hematogenous metastasis of distant tumors, invasion of nasal carcinomas or chondrosarcomas, and perineural invasion of lymphomas, melanomas, or squamous cell carcinomas may also occur. For patients with a history of cancer or the presence of enhancing and erosive lesions, it is necessary to search for primary cancers [6].
4. Around the Endolymphatic Sac
Endolymphatic sac tumors and meningiomas are considered to be around the endolymphatic sac. Most endolymphatic sac tumors are sporadic, although some cases happen with Von Hippel–Lindau disease. Endolymphatic sac tumors are locally invasive. The bone invaded by the tumor shows a moth-eaten lytic appearance with intratumoral bone spicules in CT images. Using MRI, smaller tumors show a heterogeneous signal intensity and a peripheral zone of hyperintensity in T1-weighted images; however, the hyperintensity zone of larger tumors is more scattered in T1-weighted images. Enhancement is strong and heterogeneous [1][2]. Glomus tumors are usually located inferior to the labyrinth, whereas endolymphatic sac tumors are located posterior to the labyrinth. However, when tumors are enlarged, it is difficult to differentiate endolymphatic sac tumors from glomus tumors based on their location. Both tumors have high vascularity, but endolymphatic sac tumors usually spare the jugular foramen and do not have a “salt and pepper” appearance [2]. Meningiomas also occur in this area. Tumors occur in the internal auditory canal and cerebellopontine angle. The petrous apex is sometimes expanded in this area.
5. Internal Auditory Canal/Cerebellopontine Angle
Vestibular schwannomas are the most common, accounting for 60% to 90% of cerebellopontine angle cases. Meningiomas, epidermoids, and nonvestibular posterior fossa schwannomas (trigeminal, facial, and glossopharyngeal nerves) are also common. Additionally, arachnoid cysts, lipomas, dermoids, malignancies (lymphomas, melanomas, and metastases), tumors from the petrous bone (chondrosarcoma), and gliomas have been reported [1].
Patients with vestibular schwannomas experience sensorineural hearing loss, tinnitus, disequilibrium, and decreased speech discrimination. Vestibular schwannomas are isointense or mildly hypointense in T1-weighted images and mildly hyperintense in T2-weighted images with homogeneous contrast. Larger tumors may be heterogeneous and may have cystic components. As described, meningiomas are isointense or slightly hyperintense in T1-weighted images or isointense or hypointense in T2-weighted images and homogeneous. Most vestibular schwannomas grow slowly, from 0.2 mm to a few millimeters per year [1]. Meningiomas seldom expand to the internal auditory canal. The tumor extends along the posterior petrous wall, resulting in an obtuse angle at the bone–tumor interface. In CT images, meningiomas may exhibit calcification, sclerosis, or hyperostosis in the adjacent bone [1]. Epidermoids have hypodensity similar to that of cerebrospinal fluid (CSF) in CT images, thus resembling arachnoid cysts. The smooth remodeling and erosion of petrous bone were observed. Epidermoids show isointensity or mild hyperintensity in T1-weighted images and mild hyperintensity in T2-weighted images. They are characterized by a lack of enhancement and reduced diffusivity in diffusion-weighted images [1]. Dermoids have variable images, depending on the tumor content. MRI shows hyperintensity in T1-weighted images and intense mixing in T2-weighted images. Dermoids often show hyperintensity in diffusion-weighted images [11]. Arachnoid cysts are hyperintense in T2-weighted images with no contrast. Although arachnoid cysts are similar to epidermoids, cholesteatomas, and mucoceles [12], epidermoids and cholesteatomas are hyperintense in diffusion-weighted images and arachnoid cysts are hypointense. Lipomas show fat signals on all sequences such as hyperintensity on nonfat-suppressed T1-weighted images and they may be differentiated from dermoids by a lack of calcification [6].
References
- Juliano, A.F.; Ginat, D.T.; Moonis, G. Imaging review of the temporal bone: Part I. Anatomy and inflammatory and neoplastic processes. Radiology 2013, 269, 17–33.
- De Foer, B.; Kenis, C.; Vercruysse, J.P.; Somers, T.; Pouillon, M.; Offeciers, E.; Casselman, J.W. Imaging of temporal bone tumors. Neuroimaging Clin. N. Am. 2009, 19, 339–366.
- Chapman, P.R.; Shah, R.; Curé, J.K.; Bag, A.K. Petrous apex lesions: Pictorial review. AJR Am. J. Roentgenol. 2011, 196, WS26–WS37.
- Kim, C.S.; Suh, M.W. Skull base surgery for removal of temporal bone tumors. Acta Otolaryngol. Suppl. 2007, 127, 4–14.
- Campion, T.; Taranath, A.; Pinelli, L.; Ugga, L.; Nash, R.; Talenti, G.; Dahmoush, H.; D’Arco, F. Imaging of temporal bone inflammations in children: A pictorial review. Neuroradiology 2019, 61, 959–970.
- Touska, P.; Juliano, A.F. Temporal Bone Tumors: An Imaging Update. Neuroimaging Clin. N. Am. 2019, 29, 145–172.
- Martin, N.; Sterkers, O.; Nahum, H. Haemangioma of the petrous bone: MRI. Neuroradiology 1992, 34, 420–422.
- Friedman, O.; Neff, B.A.; Willcox, T.O.; Kenyon, L.C.; Sataloff, R.T. Temporal bone hemangiomas involving the facial nerve. Otol. Neurotol. 2002, 23, 760–766.
- Lee, D.W.; Byeon, H.K.; Chung, H.P.; Choi, E.C.; Kim, S.H.; Park, Y.M. Diagnosis and surgical outcomes of intraparotid facial nerve schwannoma showing normal facial nerve function. Int. J. Oral Maxillofac. Surg. 2013, 42, 874–879.
- Hou, Z.H.; Huang, D.L.; Han, D.Y.; Dai, P.; Young, W.Y.; Yang, S.M. Surgical treatment of endolymphatic sac tumor. Acta Otolaryngol. 2012, 132, 329–336.
- Onoda, K.; Kawaguchi, A.; Takaya, Y.; Inoue, Y.; Nakazato, I.; Saito, Y.; Ishikawa, H.; Oyama, K.; Oshima, Y.; Saito, K.; et al. A Case of Dermoid Cyst Arising in the Temporal Lobe. NMC Case Rep. J. 2021, 8, 529–534.
- Cristobal, R.; Oghalai, J.S. Peripetrosal arachnoid cysts. Curr. Opin. Otolaryngol. Head. Neck Surg. 2007, 15, 323–329.
More
Information
Subjects:
Otorhinolaryngology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
566
Revisions:
2 times
(View History)
Update Date:
23 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




