Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristina Maria Martins Almeida | -- | 6264 | 2023-08-21 13:01:50 | | | |
| 2 | Peter Tang | Meta information modification | 6264 | 2023-08-22 02:56:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Almeida, C.M.M. Sample Preparation Methods of PhACs in Waters Matrices. Encyclopedia. Available online: https://encyclopedia.pub/entry/48274 (accessed on 08 February 2026).
Almeida CMM. Sample Preparation Methods of PhACs in Waters Matrices. Encyclopedia. Available at: https://encyclopedia.pub/entry/48274. Accessed February 08, 2026.
Almeida, Cristina M. M.. "Sample Preparation Methods of PhACs in Waters Matrices" Encyclopedia, https://encyclopedia.pub/entry/48274 (accessed February 08, 2026).
Almeida, C.M.M. (2023, August 21). Sample Preparation Methods of PhACs in Waters Matrices. In Encyclopedia. https://encyclopedia.pub/entry/48274
Almeida, Cristina M. M.. "Sample Preparation Methods of PhACs in Waters Matrices." Encyclopedia. Web. 21 August, 2023.
Copy Citation
In the environment, pharmaceutical residues are a field of particular interest due to the adverse effects to either human health or aquatic and soil environment. Because of the diversity of these compounds, at least 3000 substances were identified and categorized into 49 different therapeutic classes, and several actions are urgently required at multiple steps, the main ones: (i) occurrence studies of pharmaceutical active compounds (PhACs) in the water cycle; (ii) the analysis of the potential impact of their introduction into the aquatic environment; (iii) the removal/degradation of the pharmaceutical compounds; and, (iv) the development of more sensible and selective analytical methods to their monitorization.
pharmaceutical active compounds
mass spectrometry
chromatographic methods
hyphenated methods
1. Introduction
The problem of water availability and quality is a fundamental issue of the 21st century. Over the past two decades, there has been increasing concern regarding several biologically active environmental contaminants in the aquatic environment. Many of these compounds belong to the group of so-called contaminants of emerging concern (CECs).
The CECs are naturally occurring, manufactured or human-made chemicals, or materials that have now been discovered or suspected in various environmental compartments and whose toxicity or persistence are likely to significantly alter a living being’s metabolism. Such potential CECs should remain “emerging” if the information is scarce in the scientific literature or poorly documented issues regarding the associated potential problems that they could cause [1]. The contamination of environmental compartments, such as surface water, groundwater, and soil with these chemicals shows some potential to pose risks to the environment or human health. Still, they are not yet subjected to regulatory criteria or norms for protecting human health or the environment [1][2].
The CECs include many micropollutants, among them certain pesticides and their degradation/transformation products, industrial chemicals (surfactants and surfactant residues, gasoline additives, brominated flame retardants, plasticizers, and perfluorinated compounds), disinfection by-products, personal care products (PCP), human and veterinary medicines and their metabolites, and nanomaterials, and so on. Some of these compounds also belong to the group of persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) [3][4].
The impact of chronic exposure to these or other contaminants on humans, animals, vegetation, and aquatic species is a matter of concern for the scientific community and regulatory and supervisory entities, since they may not only compromise the functioning and maintenance of ecosystems, which is crucial in promoting the ecosystem services on which humanity depends, but also causing various public health problems [5][6]. Regarding human’s health, special attention has been given to drinking water and the quality and contamination of raw water (surface and groundwater) used for its production. Therefore, the presence of these contaminants is an environmental issue. It has been the backbone for the development of EU’s policy regarding the protection of water resources through the Water Framework Directives, which emphasize the need for well-developed monitoring programs as mechanisms for understanding the occurrence and fate of environmental contaminants, as well as assessing the risks that are associated with long-term exposure to low concentrations of these compounds or combined effects of mixtures. The guidelines will be proposed after the evaluation of these results, if necessary.
Because of the diversity of structures, physico-chemicals and biological properties, special attention has been given to pharmaceutical active compounds (PhACs), because, in contrast to conventional pollutants, they are deliberately designed to have a biological impact, even at low concentrations [7][8][9].
The sources of PhACs, their metabolites, and degradation products include: (i) effluents of pharmaceutical plants; (ii) runoff from agriculture, livestock and aquaculture; (iii) domestic and hospital effluents; and, (iv) municipal wastewater treatment plants (WWTPs). The presence of these target compounds in the environment can have adverse effects on aquatic and terrestrial organisms, and they can occur at any biological hierarchy level, such as cell, organ, organism, population, and ecosystem. These effects can be observed in concentrations in the order of ng/L for certain compounds [10][11].
It is difficult to predict which environmental and public health implications may arise from PhACs in freshwater ecosystems, since the individual concentrations that are usually found in the environment are lower than those that are able to cause direct adverse effects (acute toxicity). However, even trace concentrations have shown that they might have direct toxicity towards individual aquatic organisms [12][13][14][15].
Concerning drinking water, several risk assessments studies indicate that very low concentrations of pharmaceuticals are very unlikely to pose any risks to human health. Still, there are knowledge gaps in assessing the risks that are associated with long term, low-level exposures to pharmaceuticals and possible combined effects of chemical mixtures. The investigation of possible additive or synergistic effects of mixtures would be necessary for an accurate exposure assessment in order to determine whether there are any potential risks to human health, while considering sensitive subpopulations [16][17].
Because of these challenging questions, PhACs in the environment are reported in thousands of publications during the last decades and reviewed by many authors, demonstrating an increasing concern about them [18][19][20][21][22][23][24][25][26][27].
Efforts have been made to improve the knowledge and data available on sources of PhACs, and how pollution occurs to identify targeted and effective control options. The determination of these compounds in the various environmental matrices, such as water (wastewater, surface water, groundwater, and water for human consumption), soils, sediments, and biota, has grown and is subject to increasingly demanding legislative requirements (in the order of µg/L or ng/L, or ng/g for solid matrices).
In water policy, Directive 2013/39/EU refers for the first time to the contamination of water and soil by pharmaceutical waste as an environmental problem [28]. In Europe, to ensure the monitoring of compounds subject to possible hazards, such as emerging pollutants, and to guarantee a quality database to identify/prioritize substances, this directive identifies a set of priority substances in a watch list subject to update. The watch list mechanism was established to require the temporary monitoring of other substances for which evidence suggested a possible risk to or via the environment, in order to inform the selection of additional priority substances. The watch list should contain a maximum of 10 substances or groups of substances, indicating the matrices to be monitored as well as possible methods of analysis that do not involve high costs. Relative to PhACs, this first watch list includes a non-steroidal anti-inflammatory drug (diclofenac) and the hormones 17-β-estradiol (E2) and 17-α-ethinyl estradiol (EE2) to determine the concentration levels that facilitate the application of appropriate measures given the risk that these substances represent. Two other PhACs, carbamazepine and sulfamethoxazole, are also being studied for possible inclusion in this list.
The first watch list was adopted in the Decision 2015/495/EU of 20 March 2015 [29] and it includes seven PhACs: two natural hormones (E2 and estrone (E1)), one synthetic hormone (EE2), one nonsteroidal anti-inflammatory drugs (diclofenac), and three macrolide antibiotics (erythromycin, clarithromycin, and azithromycin). This directive was repealing by Decision 2018/840/EU [30] of 5 June 2018 and the substances mentioned above (EE2, E2, E1, azithromycin, clarithromycin, and erythromycin) were included, together with two other antibiotics, amoxicillin and ciprofloxacin. The Commission removed the diclofenac from the watch list due to the sufficient high-quality monitoring data available for diclofenac [30]. The inclusion of amoxicillin and ciprofloxacin is consistent with the European One Health Action Plan against Antimicrobial Resistance (AMR), which supports the use of the watch list to “improve knowledge of the occurrence and spread of antimicrobials in the environment” [31].
All of the evaluations and decisions were supported by the occurrence studies of these contaminants in the aquatic environment, where the high-quality data on their concentrations (quality of the analytical results) were essential. Besides, the knowledge of their concentration is essential as a starting point to apply more advanced treatments to improve their removal and, consequently, minimize their environmental risk [32].
Under this scenario, analytical chemistry plays an essential part in providing high-quality information by applying two different approaches: (i) target analysis and (ii) screening methods for the determination of non-target or unknown compounds (degradation products). Both of the approaches demand highly sophisticated techniques that enable the implementation of sensitive and selective methods to provide accurate data regarding the identification, confirmation, and quantification of compounds [33].
In this context, the analytical challenges, mainly in quantitative analysis, are focused on developing and validating new materials, strategies, and procedures to quickly meet the requirements for selectivity, sensitivity, speed, and green methods. Because of the constantly updating of international standards, guidelines, and recommendations, constant innovation is mandatory in both the pre-treatment procedures and analytical equipment to obtain reliable, true, and reproducible data [34][35].
Gas chromatography (GC) or liquid chromatography (LC) coupled to mass spectrometry (MS) or tandem mass spectrometry (MS/MS) are advanced methods that can determine target compounds to the nanogram per liter level or lower and they are commonly applied for the detection of PhACs in different water [36][37] and solid matrices, such as sediments, sludges, and biota [38][39][40]. Because of the polar characteristics of PhACs, the liquid chromatography is the most used [41][42][43][44]. Nevertheless, since the late 80s, liquid chromatography-mass spectrometry (LC–MS) has rapidly grown in popularity as a technique for environmental control.
Common mass analyzers applied in target analysis of these compounds are triple quadrupoles (QqQ) [45][46], ion-traps (IT) [38], and quadrupole-linear ion trap (QLIT-MS/MS) [37][47]. On the field of liquid chromatography coupled to tandem mass spectrometry, ultra-performance liquid chromatography (UPLC) has enabled the development of more sensitive, fast, and environmentally friendly methods for pharmaceuticals [10][48][49][50].
Improvements in the identification capability for target compounds have also been achieved by the introduction of high-resolution mass spectrometry (HRMS). The time-of-flight (TOF), quadrupole-TOF (QTOF), and Orbitrap-based mass spectrometry in combination with chromatographic techniques have resulted in a valuable tool, not only for qualitative, but also quantitative, analysis of target compounds [33]. Gómez et al. (2010) have described the advantage of using a QTOF analyzer in the identification, confirmation, and quantification of almost 400 contaminants (mainly pesticides and pharmaceuticals) in surface water and wastewater [51].
The analysis of transformation products has also become an important issue in environmental chemistry, as it has been found that they may be more toxic and/or persistent than the parent compounds, thus representing a higher risk to the environment and human health [52][53][54]. In this area, LC or GC coupled to HRMS has been widely applied in the identification of transformation products, with time-of-flight (TOF), quadrupole-TOF (QTOF), and Orbitrap mass spectrometers increasing in use. These techniques were used in the study of the behavior of PhACs, the identification of their transformation or degradation products in wastewaters, natural waters, and drinking waters [51][55][56].
Owing to the large-scale dilution of these contaminants in water sources (natural waters) or due to the matrix complexity, a preliminary sample preparation technique for concentration or/and clean-up is mandatory, such as liquid-liquid extraction (LLE) [57], solid-phase extraction (SPE) [10][27][58][59][60], solid-phase microextraction (SPME) [27][28], or stir bar sorptive extraction (SBSE) [61]. Currently, pharmaceuticals are usually extracted from water samples by off-line solid-phase extraction [10][46][61] and on-line solid phase extraction [44][62].
Several other methodologies namely microwave assisted extraction (MAE) [63][64], pressurized liquid extraction (PLE) [65], ultrasonic solvent extraction (USE) [66], accelerated solvent extraction (ASE) [67] and QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) [68][69] have been developed as pre-treatment procedures for the determination of PhACs, metabolites, and degradation products in solid samples, such as sediments, sludge or sewage sludge, and biota.
2. Sample Preparation Methods
The main goal of the sample preparation (SP) methods is to transfer the target analytes from the matrix, in a more suitable way, to be analyzed by the selected analytical technique, using, in the case of trace analysis, strategies for the enrichment of the analytes, to gain sensitivity. Usually, several steps are required, involving up to about 80% of the analytical time. Figure 1 shows the main phases of the sample preparation methods. Regarding trace analysis, these steps can represent a significant source of errors that will affect the analytical result. There are two types of errors:(i) negative error, due to the analyte loss; and, (ii) positive error, which results from the addition of analyte to the sample or enrichment, or due to the presence of matrix interferents [70]. Any selected method should be minimized or overcome both errors.
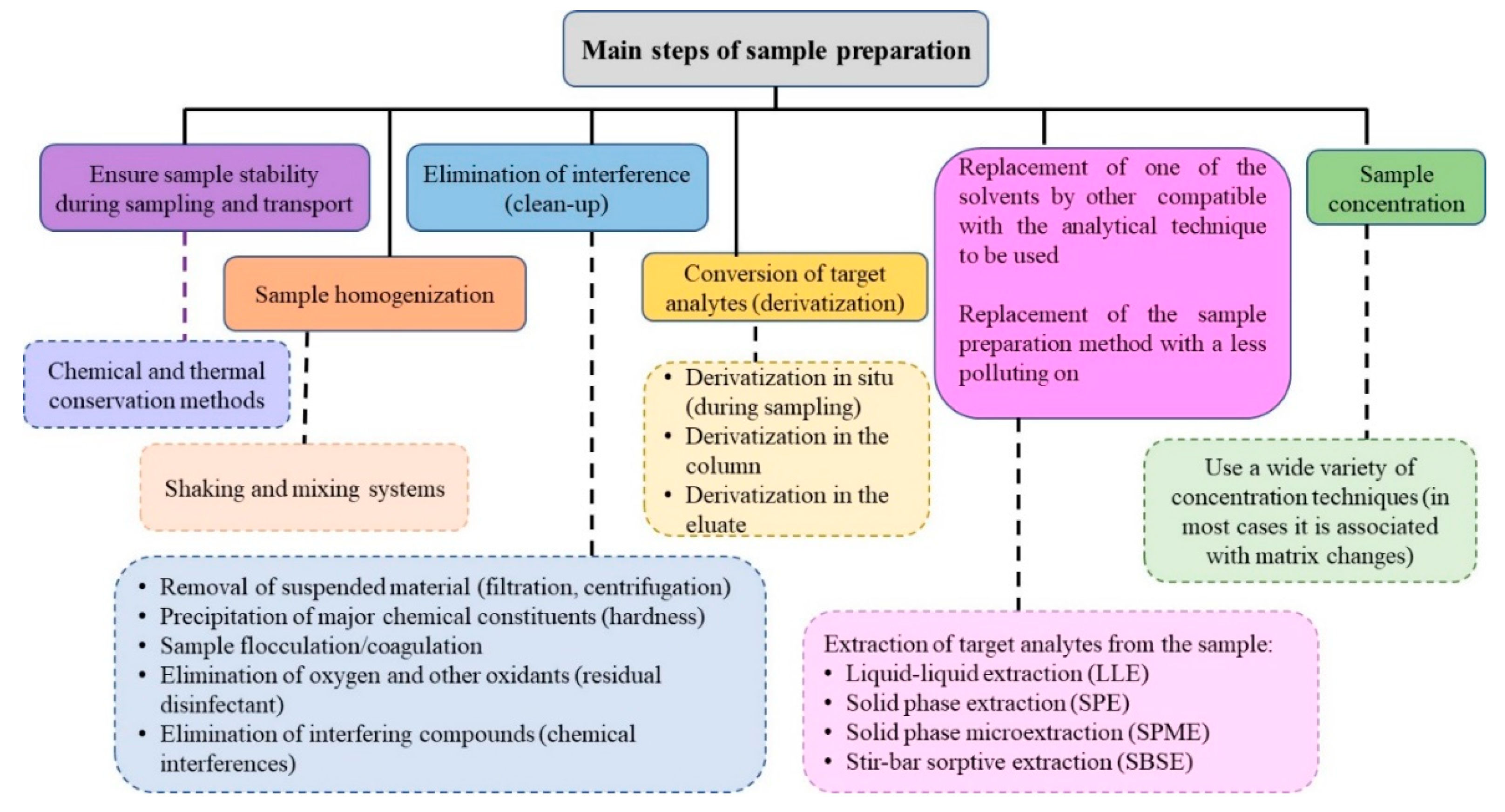
Figure 1. Main steps of sample preparation methods. Legend: Steps (—) and procedures to be implemented (− − −) [70].
The selection of the sample preparation method depends on several factors, namely, the type of matrix, the nature of the target compounds (volatility, polarity, solubility), their concentration in the matrix (mainly in trace or ultra-trace concentrations), the analytical method (gas chromatography, liquid chromatography, and their detectors), costs, ease of automation, and analysis time. Each method should be evaluated relative to their advantages and drawbacks [71].
Sample preparation has been subject to a continuous evolution that is mainly based on two approaches, discovery and development of new materials and improvements in technology, both using the principles of separation science [72].
For decades, liquid-liquid extraction (LLE) was the selected technique in the official methods of the EPA (Environmental Protection Agency) for the pre-concentration of organic compounds in aqueous samples. LLE is based on the partition of organic compounds between the aqueous sample and an immiscible organic solvent. The extraction efficiency depends on the physicochemical properties of analyte (solubility, pKa, log Pow, etc), solvent (polarity), sample, volume of the solvent, number of extractions, water: solvent ratio, as well as other parameters, such as pH and ionic strength of the sample. The solvents, in which the analyte is extracted, may be organic liquids, supercritical fluids, and superheated liquids [73]. Significant progress in LLE was made through the development of liquid–liquid microextraction techniques, such as single liquid microdrops, nanodrops, or picodrops extractions [72]. Other new LLE approaches include single liquid drop extraction with two phases, unsupported liquid membrane extraction with three phases, supported liquid membrane extraction (SLM) [72], microporous membrane liquid–liquid extraction (MMLLE) [74], and membrane-assisted solvent extraction (MASE) [75]. However, the enrichment factor of these techniques is not enough to quantify the organic compounds in trace or ultra-trace concentrations.
In the last two decades, the tendency to extract and analyze the largest number of compounds simultaneously to save time, expenses, and labor has led to the development of simple, fast, and generic sample treatments. Consequently, more “dirty” extracts and smaller recoveries are obtained, an accepted commitment for the analysis of multi-class compounds (multi-resides analysis) with different physical-chemical properties [76]. The selection of SP methods is based on the criteria of both the efficiency and its environmental impact, and SP has undergone considerable movement toward green sample preparation (GSP). In this perspective, new concepts have emerged that are allied to methodologies that use fewer volumes of organic solvents or even free of organic solvents, equally efficient, with less environmental impact, and less risk to public health. Among the various techniques, solid-phase extraction (SPE), solid-phase microextraction (SPME), and stir bar sorption extraction (SBSE) show a good recovery of target analytes (lower threshold limits), high precision, speed, and ease of automation. These techniques combine sample extraction, purification, and enrichment in a unique procedure [77]. Therefore, they are the most used techniques for monitored PhACs belonged to different pharmaceutical classes, both off-line [60][78][79][80] and on-line [44][62][81][82].
Because it is almost impossible to be comprehensive in the coverage of all pre-treatment techniques to the pharmaceutical’s analysis, the attention is focused on most used techniques, namely SPE, DSPE, SBSE and SPME.
2.1. Solid-Phase Extraction
Conventional off-line solid-phase extraction (SPE) on cartridges [83][84] and discs [85] is a well-established technique that is routinely used for the extraction/concentration of several target compounds as well as for removing interfering components from matrix before chromatographic analysis. The technique is very versatile, and it is another criterion for its selection, due to the wide variety of sorbents chemistries available.
The SPE process shows five main stages: adsorbent conditioning, passing the sample, washing the adsorbent, drying the adsorbent, and eluting the analytes. When developing an SPE procedure, it is essential to correctly choose the packing material and the washing and elution solvents, according to the characteristics of the analytes and matrix. The final extract should also be compatible with the analytical technique used [70].
The extracts obtained by SPE technique are cleaner and the recoveries are higher. However, it has some disadvantages, namely: (i) clogging of the adsorbent layer by solid or fatty components present in the sample; (ii) extraction only of analytes dissolved in aqueous solvents; (iii) high variability due to different adsorbent capacity between sorbents batches; and, (iv) the breakthrough or limitation of the extractive capacity of the sorbent material (when he concentration of the analyte(s) is very high) [71][86].
Wastewater samples should be previously filtered and, in some cases, centrifuged in order to remove suspended matter that may clog SPE sorbents [48].
For some target compounds, it may be necessary to adjust the pH of the sample or other chemical corrections. Complexing agents (for example, Na2EDTA or citric acid) are usually added for complexing metal ions, which, otherwise, could bind to some analytes, such as tetracyclines and fluoroquinolones [87][88][89].
The sample pH adjustment can increase the affinity of some analytes for the SPE adsorbent, according to the type of adsorbent and the characteristics of the analytes [89][90][91].
The selection of adsorbent to analyze several PhACs belonging to different therapeutic classes is the critical step in SPE method development, and in most cases, the selected adsorbent is the best compromise, due different physical and chemical properties of the target compounds [10]. Based on the nature of target compounds, a careful choice of the sorbent allows for obtaining high recovery efficiencies and enrichment factors, which typically range between 20 and 1000 [76]. Relative to PhACs in drinking waters and clean waters, the typically factor is 500 and for wastewater the typical factors range between 20 and 100 [92]. These factors are a strong point of this technique.
The adsorbents that are used in SPE can be classified according to the interaction between the adsorbent material and the target compounds. The most common interactions are: (i) hydrophobic, nonpolar or reverse phase interactions; (ii) polar, hydrophilic or normal phase interactions; and, (iii) ionic interactions (cationic or anionic) [71].
There is a wide range of sorbents (packing materials) that can be used in the manufacture of solid phases for SPE. Some of them are of natural origins, such as graphitized carbon black (GCB), diatomaceous earth, alumina, silica, silica-based bonded phase (C18, C8), magnesium silicate (florisil), and cellulose, but others are synthetic polymers, such as the polystyrene-divinylbenzene and poly (divinylbenzene-co-N-vinylpyrrolidone). The last one has a high degree of porosity and, thus, a higher real active surface, allowing for a higher adsorption capacity. This new generation of polymers allows the extraction of a wide variety of analytes regardless of their nature, whether they are acidic, basic, and neutral, whether polar or nonpolar, and they are sometimes called HLB (Hydrophilic-Lipophilic-Balance) [72].
HLB cartridges consist of a hydrophobic component (polystyrene and/or divinylbenzene) and hydrophilic component (methacrylate, N-vinylpyrrolidone, and vinylamidazole). Unlike traditional SPE sorbents, where the reverse phases are made up of silica that can only retain non-polar or moderately polar compounds, this type of polymeric adsorbent can retain a wide range of acidic to basic compounds, with low to high polarity, being very useful in the development of methods that consist of compounds with different physicochemical properties. In addition, silica-based sorbents are unstable over a wide pH range and they contain free silanol groups, which can irreversibly bind to some analytes, such as tetracyclines [93].
The HLB sorbent is the SPE material that is most often used in the extraction of PhACs belonging to different therapeutic classes [10][23][45][48][60][94].
In the analysis of complex matrices, such as wastewater, the extraction/concentration and cleanup process requires passing the sample through more than one type of SPE material. One example of this approach is the use of an anion exchange adsorbent, followed by an HLB adsorbent for the determination of antibiotics, such as fluoroquinolones, sulfonamides, and trimethoprim [95]. Mixed-mode sorbents were developed to simplify this type of analysis. This application combines two or more functional groups into a single cartridge. This combination allows multiple retention interactions between the sorbent and the analytes, improved cleanup, better reproducibility, and recovery, leading to, overall, more sensitive, precise, and accurate analytical methods [72]. An example of this application is a SPE cartridge with a cation exchange resin and an HLB copolymer is the mixed-mode/cationic-exchange (MCX) [96][97], mixed-mode/anion-exchange (MAX) [26], and weak anion-exchange (WAX) [27]. These mixed-mode sorbents were used for reducing interference and matrix effects in the determination of PhACs in waters [90][96][98][99][100][101]. Unfortunately, the sorption capabilities of these cartridges are often limited when compared to the HLB cartridge for large volumes of water or complex environmental matrices [86].
The matrix’s complexity always affects the recovery of the PhACs, regardless of the sorbent used. The relative recoveries always show wide ranges of values due to the diversity of compounds under analysis. This amplitude is higher for methods with a higher number of compounds, and when the number of therapeutic classes is also higher [37][44][51][94][97]. Sorbents with dual polarity (hydrophobic and hydrophilic) are the most versatile, so Oasis HLB is the most used sorbent [45][102][103]. For the analysis of PhACs belonging to the same therapeutic class, usually with similar polarity, more selective cartridges, both polar [26][80] and non-polar [104] are chosen. When mixed-mode sorbents, such as Oasis MAX and WAX, are used, the sample pH is a crucial issue because this parameter can affect the sorption adsorbent capacity to a greater degree. Different ionic forms of the PhACs and the sorbent can be present, depending on the sample pH [105]. Unfortunately, the sorption capabilities of these cartridges are often limited when compared to the HLB cartridge.
Regarding the use of an off-line or on-line SPE, one of the critical issues is the sample size, particularly the relative size of the water sample and injectable sample. This size depends on the expected concentration of PhACs in water samples. The enrichment factor should be high for drinking water with trace concentrations of PhACs. The water sample size is much larger than the injectable sample. Therefore, it is much more useful to opt for off-line SPE in order to obtain lower quantification limits. For wastewater, the enrichment factors can be lower, and the on-line SPE can be an advantageous option. However, coeluting of matrix interferences can be a significative drawback. In addition, on-line SPE coupled to ultra-performance liquid chromatography (UPLC) shows disadvantages because of the elevated back pressure that is generated by the high flow rates used in small particle size columns (<2 µm) (83).
The SPE technique has been applied to the analysis of PhACs that belong to several therapeutical classes in river waters [21][46][106], groundwaters [107], drinking waters [10][108], marine waters, and wastewaters [23][25][27][46][106], both wastewater influent and effluent.
For example, Gilart et al. (108) compared the selectivity and capacity of Oasis HLB, Oasis MAX, Oasis WAX, and a commercially available molecularly imprinted polymer (MIP) specific for non-steroidal anti-inflammatory drugs (NSAIDs) on the extraction of a group of 15 PhACs from wastewater. These four different commercial sorbents were tested to check which is more effective in preconcentrating and selectively extracting acidic PhACs from environmental waters. Although the recoveries that were obtained for the 15 PhACs by Oasis HLB with ultra-pure water and wastewater were similar (71–103%), this sorbent did not enable selective washing of real samples. Regarding the two mixed-mode sorbents, the washing step was able to eliminate all of the basic compounds, in contrast to ultra-pure-water experiments. The recoveries range between 60–100% (Oasis MAX) and 14–105% (Oasis WAX). The recoveries of PhACs in wastewater with MIP were slightly lower (45–102%) than those that were obtained with ultra-pure water, but this sorbent allowed for the selective extraction of acidic analytes from wastewater samples [105].
This technique still has several limitations, most of them being associated with the cartridge packing material, regardless of SPE’s advantages. Consequently, not only sorbents of new forms (fibers, bars) appeared, but, above all, alternative modes of extraction. In this last approach, the dispersive solid phase extraction (DSPE) and the magnetic solid phase extraction (MSPE) stand out.
2.1.1. Dispersive Solid-Phase Extraction
Dispersive SPE (DSPE) is based on the SPE methodology, but the sorbent is directly added to the sample without conditioning the clean-up, being easily carried out by shaking and centrifugation. After that, the sorbent with the analytes is washed with an appropriate solvent to recover analytes [109]. Because the contact surface between the sorbent and sample is higher, the extraction equilibrium is more quickly achieved, reducing the extraction time. The adsorbents do not need to be packed into the SPE cartridges and the problems of column blocking and high pressure often encountered in SPE are overcome. Furthermore, it is a more environmentally friendly method than standard solid-phase extraction, as the amount of sorbent and volume of solvent are lower. However, the crucial centrifugation/filtering step is difficult to automatize and become a critical hindrance in environmental analysis due to the high number of samples [76][110]. A noteworthy improvement of DSPE arises with magnetic solid-phase extraction (MSPE), where the centrifugation step is not necessary, in order to overcome this drawback.
The DSPE was applied to the analysis of antibiotics in mineral waters [111] and wastewaters [109], and natural hormones in river waters [109]. In the last study, a new organic adsorbent was prepared by the electrospinning method with polyacrylonitrile (PAN) and activated carbon. The activated carbon decorated PAN nanofibers showed LOQs that were between 0.53–2.17 µg/L. These sorbents can reusable, with satisfactory recoveries for more than ten uses.
2.1.2. Magnetic Solid-Phase Extraction
Briefly, the magnetic nanoparticles (MNPs) coated or hybridizated with other materials (magnetic sorbent) is added to the sample, vortexed and then separated from the solution under magnetic conditions. After separation, the material (containing the analytes) was dissolved in low amount of a suitable organic solvent to form the extract to be analysed [70][73][86][110].
MNPs have attracted a great deal of interest in the separation of different organic environmental contaminants due to their unique paramagnetic properties, high surface areas, customized surface modifications, and good dispersion in solution, which can affect the sensitivity and selectivity of the method [112]. Additionally, magnetic adsorbents can be readily recycled, which is economical and eco-friendly. The current trends are the development of new magnetic adsorbents with high adsorption capacity and selectivity [110][113].
Several materials have been used for preparation of the surface coatings of MNPs, such as silica, carbon nanomaterials, polymers, surfactants, and ionic liquids [111][114][115][116].
A tremendous increment in progress of MSPE is also associated with the use of carbon nanomaterials. Carbon exists in several allotropic forms, such as fullerenes, carbon nano tubes (CNTs), including single-walled CNTs (SWCNTs) and multi-wall CNTs (MWCNTs), carbon nanohorns, carbon nanocones, carbon nanodisks, carbon nanofibers, nanotube rings, graphene oxide (GO) and graphene (G), and diamonds. However, to date, from the analytical point of view, the applications have mainly been focused on the use of fullerenes, CNTs, and GO/G [117][118][119].
The magnetite (Fe3O4) nanoparticles coated with different materials have been used to extract different classes of pharmaceuticals in surface water. These MNPs were used to extract macrolide antibiotics in surface waters by MSPE [120].
Graphene was easily immobilized on silica-coated magnetite (designated Fe3O4@SiO2/graphene) and it was used as an adsorbent to extract six sulfonamide antibiotics (sulfapyridine, sulfamerazine, sulfameter, sulfachloropyridazine, and sulfadoxine) from surface water and wastewater samples [121].
A novel magnetic polyethyleneimine modified reduced graphene oxide (Fe3O4@PEI-RGO) was used as adsorbents for the extraction of polar non-steroidal anti- drugs (NSAIDs) from tap water, groundwater, and river water. When compared with Fe3O4@PEI and Fe3O4@PEI-GO, the Fe3O4@PEI-RGO showed a higher extraction efficiency for polar NSAIDs [112].
Gemfibrozil was extracted by MSPE using β-cyclodextrin-grafted graphene oxide (GO)/magnetite (Fe3O4) nano-hybrid [122].
Abdolmohammad & Talleb evaluated the use of MNPs composite Fe3O4@(Fe-benzene-1,3,5-tricarboxylic acid) as a sorbent for a higher group of lipid regulators (bezafibrate, clofibric acid, clofibrate, gemfibrozil, and fenofibrate) in water matrices [123].
Ethylenediamine-functionalized magnetic carbon nanotubes (EDA@Mag-CNTs) that were used as sorbent showed a high extraction capacity of the steroid hormone by MSPE procedure [124].
Several other coatings were evaluated as sorbent in MSPE for the extraction of PhACs that belong to different therapeutic classes [125][126].
The metal organic frameworks (MOFs) are another type of sorbents used in the extraction of organic compounds in water matrices. They show several advantageous properties, such as high porosity, tunable structures, ultra-high surface areas, outer-surface functionalization, and high thermal stability. Based on metal ion geometry and bridging ligands, MOFs can show different topologies and dimensionalities: one-dimensional, two-dimension or three-dimension MOFs (1D MOFs, 2D MOFs, and 3D MOFs). The 3D MOFs are highly porous and stable, since coordination bonds spread in three directions [76][110][127].
The analysis of 58 human and veterinary drugs (24 steroid hormones, 22 sulfonamides, and 12 quinolones) in ultra-trace concentrations in river water by MSPE were performed while using three-dimensional interconnected magnetic chemically modified graphene oxide (3D-Mag-CMGO) as adsorbent [128].
Recently, several automated MSPE approaches for the analysis of organic micro-pollutants and antibiotics have been reported [110].
Most of these adsorbent materials are under intensive research, unfortunately they are not commercially available. Thus, its potential use in routine analysis is still limited.
2.1.3. Molecularly Imprinted Polymers
The selectivity of the sorbent can also be achieved through the development of selective extraction phases that were obtained by molecularly imprinted polymers (MIPs), which are synthesized by the polymerization of functional and crosslinking monomers around a template analyte. After the elimination of template by extraction or chemical reaction, the cavities (binding sites) are exposed in the polymeric matrix. These cavities are complementary to the template in size, shape, and position of functional groups of the template analyte and its structurally related analytes [110][129][130].
The MIPs that are associated to SPE are also known in literature as MIP-SPE or MISPE, and they have been applied to the analysis of antidepressants, antibiotics, and beta-blockers [108][130][131]. To date, the potential of MISPE is still poorly explored in the analysis of PhACs in the environmental analysis.
It is difficult to separate MIPs with small particle sizes from aqueous samples when they are applied as the sorbent in SPE or SPME. Therefore, surface molecular imprinting polymerization onto MNPs to form magnetic MIPs (MMIPs) is usually applied to increase the number of imprinting or recognition sites (because of the high surface to volume ratio of MNPs) and mass transfer kinetics of the MIPs, as well as their simple magnetic separation from the sample solution [110].
Some MMIPs have been used for the specific recovery of estrogenic compounds and antibiotics from water matrices [116][132].
This molecular imprinting technology has also been applied in electrochemical MIP sensors [133]. Some of them were constructed on different sensing platforms for the detection of pharmaceuticals in water samples [134].
2.2. Sorbent-Based Microextraction
The sorbent-based microextraction has led to a new generation of techniques with different and improved characteristics. Different solid-phase microextraction (SPME) techniques, including fibers, stir bar sorptive extraction (SBSE), thin-film microextraction (TFME), and automated techniques, such as in-tube solid-phase microextraction (IT-SPME) enhance the capacity of monitorization of a broad number of compounds that belong to different classes of organic contaminants, including CECs in complex water matrices. Some of these procedures are in automated configurations, on-line SPME, and IT-SPME. Although many of these automated techniques have been normally coupled with gas chromatography (GC), there are also some applications with liquid chromatography (LC) [135][136].
A clear advantage of the miniaturized devices is their portability, which significantly facilitates the implementation of on-site sampling and reduces errors that are associated with the sample transport and possible changes during storage [76].
Solid-phase microextraction (SPME) with fibers (usually referred as SPME) and stir-bar sorptive extraction (SBSE) have been the most used in water analysis.
The theory of SPME in both the direct immersion (DI) or in headspace technique (HS) was described in detail by Pawliszyn and coworkers [137]. Since then, many configurations have been successfully implemented, which can be classified into static and dynamic techniques. Static procedures are typically carried out in stirred samples, including fiber SPME, and they constitute the most common format for this technique.
The HS-SPME technique is usually applied in an equilibrated situation with the analytes being distributed between the fiber coating and HS gas present in a sealed vial. In theory, the HS-SPME has several advantages: the fiber does not contact with the sample, the background adsorption and matrix effects are reduced, which also enhances the life expectancy of SPME fiber. The extraction by SPME is influenced by several factors: sample matrix, stirring, temperature, sample volume, the size of the HS vial, the ratio of the HS to aqueous phase, and the position of the coated fiber in the HS, which can all affect the time that is required for the analyte to equilibrate between the HS vial contents and the SPME fiber coating [138][139]. The effects of these factors and advantages of HS-SPME versus DI-SPME have been studied by several authors utilizing many groups of compounds and many environmental, biological, and food samples [140][141][142].
As most SPME coatings are nonpolar, the technique is mostly applied to gas chromatography (thermic desorption) [139]. For the analysis of polar compounds, derivatization is required to enhance the selectivity and sensitivity of the SPME method [143][144].
The derivatization process can occur in the solution, in the coating phase, or in the GC injection port, and it is applicable to both techniques, immersion and headspace. The derivatization and extraction procedure can both be performed on a commercial autosampler that is coupled with GC [86].
The ionic liquids (ILs) and polymeric ionic liquids (PILs) overcome the drawback relative to the extraction of highly polar compounds from water matrices, and it has also quickly become a fast and cost-effective alternative technique to be coupled to LC. For LC analysis, these coatings applied to SPME should be highly robust when exposed to organic desorption solvents and not slough from the support material [145].
Other developments of novel polymeric sorbent materials, including graphene, molecularly imprinted polymers (MIPs), and metal-organic frameworks (MOFs), have also been developed for the extraction/enrichment of organic contaminants specifically for aqueous samples [110][136].
Hybrid materials polymerized from an IL monomer and an organic/inorganic crosslinker have been applied as SPME sorbent coatings for the analysis of several organic contaminants in aqueous samples by HPLC, some of them PhACs [104][146].
However, these novel materials were home-made. Therefore, they are not commercially available, and their use is limited.
Regarding water analysis, the SPME has been applied to the analysis of PhACs that belong to a wide range of therapeutic classes, such as NSAIDs [144][147], antiepileptic [147][148], lipid regulators [143], anti-diabetics [143], beta-blockers, anti-depressives [91], and antibiotics. In this last group, several classes were under study, namely tetracyclines [149], macrolides [150], and sulfonamides [62][151].
The number of PhACs that belong to a different therapeutic class analyzed by SPME techniques is much lower than the SPE technique, because this technique is not adequate for high samples volumes. On the other hand, the fiber’s fragility limits its use in complex matrices, mainly wastewaters. This limitation can be overcome in headspace SMPE, but it is limited to volatile compounds. Regarding PhACs, it implies the derivatization of the polar compounds and their analysis by gas chromatography [144].
The principle of SBSE is equal to SPME, but, due to higher volume of sorbent material, about 50—250 times larger than SPME, the sensitivity of this procedure is higher. After exposure to a sample, the stir bar that is covered with a thick film of polydimethylsiloxane (PDMS) is removed and the sorbed compounds are then either thermally desorbed, and analyzed by GC-MS or desorbed by an organic solvent, for improved selectivity or for interfacing to an LC subsequent system [152][153].
Because of the nonpolar character of sorbent, the SBSE has been mostly applied to the analysis of nonpolar or weakly polar compounds, and derivatization is a mandatory step for the analysis of very polar compounds [153].
The SBSE shows some drawbacks regarding the desorption step in GC applications that limits the automatization of the procedure, because the stir bar cannot be transposed directly in the injection port for thermally desorption. Therefore, it is a necessary specially designed thermal desorption unit, which is only available in sophisticated instrumentation. Furthermore, some target compounds can be lost during the manual transfer of stir bar to the desorption device with a loss of sensitivity gained [154]. Another limitation is the number of commercially available sorptive phases, such as polydimethylsiloxane (PDMS), ethylene glycol (EG)-silicone, carboxen, carbowax-divinylbenzene (CW-DVB), and polyacrylate (PA).
As observed for SPME, a variety of home-made stir bar coating have emerged, such as nanocarbon materials, functional monomers, metal-organic frameworks (MOFs), ionic-liquids (ILs), template imprinted polymers, and inorganic particles [133][155]. Some of them have been applied to the analysis of PhACS in complex water matrices [61][156][157].
Fan et al. [156] developed a novel IL-bonded sol–gel stir bar coating for SBSE of NSAIDs, followed by high-performance liquid chromatography-ultraviolet detection (HPLC-UV).
The stir bar was wrapped by a porous membrane (MPSBSE) to filter out the high molecular weight interferences (humic acid among others). A hydrophobic polytetrafluoroethylene (PTFE) membrane that was impregnated with methanol was employed to protect the C18 coated stir bar. The C18-MPSBSE was used for the direct determination of two common NSAIDs, ketoprofen and naproxen in complex water samples [158].
Molecular imprinted polymers and magnetic carbon nanotubes were combined in a stir bar for the enrichment of cefaclor and cefalexin in water samples [159].
A novel dual-template molecularly imprinted polymer (MIP)-coated stir bar was prepared for the analysis of environmental estrogens in complex samples. This dual template showed two different kinds of specific binding sites and three-dimensional cavities increasing the adsorption capacity of the target compounds [160].
A monolithic and hydrophilic stir bar coating based upon a copolymer of methacrylic acid and divinylbenzene copolymer, which was designed as poly(MAA-co-DVB), was synthesized and use for extraction of polar PhACs (paracetamol, caffeine, antipyrine, propranolol, carbamazepine, naproxen, and diclofenac) from river water and effluent wastewater from a treatment plant (WWTP) [161].
There is still a long way to go to put these materials available on the market, regardless of the selected sample preparation method and the new materials used. Selectivity was one of the first requirements under evaluation. The uniformity of new materials′ size, shape, and capacity are essential for their commercialization. The ease of automation is also a factor to consider, mainly in routine water analysis.
References
- Sauve, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8.
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279.
- Bueno, M.; Ucles, S.; Hernando, M.; Fernandez-Alba, A. Development of a solvent-free method for the simultaneous identification/quantification of drugs of abuse and their metabolites in environmental water by LC-MS/MS. Talanta 2011, 85, 157–166.
- Deblonde, T.; Hartemann, P. Environmental impact of medical prescriptions: Assessing the risks and hazards of persistence, bioaccumulation and toxicity of pharmaceuticals. Public Health 2013, 127, 312–317.
- Grizzetti, B.; Lanzanova, D.; Liquete, C.; Reynaud, A.; Cardoso, A. Assessing water ecosystem services for water resource management. Environ. Sci. Policy 2016, 61, 194–203.
- Grizzetti, B.; Liquete, C.; Antunes, P.; Carvalho, L.; Geamana, N.; Giuca, R.; Leone, M.; McConnell, S.; Preda, E.; Santos, R.; et al. Ecosystem services for water policy: Insights across Europe. Environ. Sci. Policy 2016, 66, 179–190.
- Daughton, C.; Ternes, T. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938.
- Daughton, C. Pharmaceuticals as environmental pollutants: The ramifications for human exposure. In International Encyclopedia of Public Health; Academic Press: Oxford, UK, 2008.
- Kummerer, K. Pharmaceuticals in the environment. Ann. Rev. Environ. Resour. 2010, 35, 57–75.
- Gaffney, V.D.J.; Cardoso, V.V.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Almeida, C.M.M. Analysis of pharmaceutical compounds in waters by SPE-UPLC-ESI-MS/MS. Química Nova 2014, 37, 138–149.
- Kummerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366.
- Fong, P.; Molnar, N. Norfluoxetine induces spawning and parturition in estuarine and freshwater bivalves. Bull. Environ. Contam. Toxicol. 2008, 81, 535–538.
- Franzellitti, S.; Buratti, S.; Valbonesi, P.; Fabbri, E. The mode of action (MOA) approach reveals interactive effects of environmental pharmaceuticals on Mytilus galloprovincialis. Aquat. Toxicol. 2013, 140, 249–256.
- Franzellitti, S.; Buratti, S.; Capolupo, M.; Du, B.; Haddad, S.P.; Chambliss, C.K.; Brooks, B.W.; Fabbri, E. An exploratory investigation of various modes of action and potential adverse outcomes of fluoxetine in marine mussels. Aquat. Toxicol. 2014, 151, 14–26.
- Koutsogiannaki, S.; Franzellitti, S.; Fabbri, E.; Kaloyianni, M. Oxidative stress parameters induced by exposure to either cadmium or 17β-estradiol on Mytilus galloprovincialis hemocytes. The role of signaling molecules. Aquat. Toxicol. 2014, 146, 186–195.
- Gaffney, V.; Mota-Filipe, H.; Pinto, R.; Thiemermann, C.; Loureiro, M.; Cardoso, V.; Benoliel, M.; Almeida, C. Chemical and biochemical characterization and in vivo safety evaluation of pharmaceuticals in drinking water. Environ. Toxicol. Chem. 2016, 35, 2674–2682.
- WHO. Pharmaceuticals in Drinking-Water; WHO Press: Geneva, Switzerland, 2011.
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211.
- Grenni, P.; Ancona, V.; Caracciolo, A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39.
- Patrolecco, L.; Rauseo, J.; Ademollo, N.; Grenni, P.; Cardoni, M.; Levantesi, C.; Luprano, M.; Caracciolo, A. Persistence of the antibiotic sulfamethoxazole in river water alone or in the co-presence of ciprofloxacin. Sci. Total Environ. 2018, 640, 1438–1446.
- Boyd, G.; Palmeri, J.; Zhang, S.; Grimm, D. Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) in stormwater canals and Bayou St. John in New Orleans, Louisiana, USA. Sci. Total Environ. 2004, 333, 137–148.
- Wen, Z.H.; Chen, L.; Meng, X.Z.; Duan, Y.P.; Zhang, Z.S.; Zeng, E.Y. Occurrence and human health risk of wastewater-derived pharmaceuticals in a drinking water source for Shanghai, East China. Sci. Total Environ. 2014, 490, 987–993.
- Salgado, R.; Noronha, J.; Oehmen, A.; Carvalho, G.; Reis, M. Analysis of 65 pharmaceuticals and personal care products in 5 wastewater treatment plants in Portugal using a simplified analytical methodology. Water Sci. Technol. 2010, 62, 2862–2871.
- Salgado, R.; Pereira, V.J.; Carvalho, G.; Soeiro, R.; Gaffney, V.; Almeida, C.; Cardoso, V.V.; Ferreira, E.; Benoliel, M.J.; Ternes, T.A.; et al. Photodegradation kinetics and transformation products of ketoprofen, diclofenac and atenolol in pure water and treated wastewater. J. Hazard. Mater. 2013, 244, 516–527.
- De Jesus Gaffney, V.; Almeida, C.M.M.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Cardoso, V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015, 72, 199–208.
- Pereira, A.; Silva, L.; Meisel, L.; Lino, C.; Pena, A. Environmental impact of pharmaceuticals from Portuguese wastewaters: Geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015, 136, 108–119.
- Sousa, M.; Goncalves, C.; Cunha, E.; Hajslova, J.; Alpendurada, M. Cleanup strategies and advantages in the determination of several therapeutic classes of pharmaceuticals in wastewater samples by SPE-LC-MS/MS. Anal. Bioanal. Chem. 2011, 399, 807–822.
- European Union. Directive 2013/39/EU of the European Parliament and of the Council of 12 august 2013. Off. J. Eur. Union 2013, 226, 13–15.
- European Union. Commission implementing decision (EU) 2015/495 of 20 march 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2015, 78, 40–42.
- European Union. Commission implementing decision (EU) 2018/840 of 5 june 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495. Off. J. Eur. Union 2018, 141, 9–12.
- European Community. Communication from the Commission of the Council and the European Parliament. In A European One Heath Action Plan against Antimicrobial Resistance (AMR); European Community: Brussels, Belgium, 2017.
- Afonso-Olivares, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Occurrence and environmental impact of pharmaceutical residues from conventional and natural wastewater treatment plants in Gran Canaria (Spain). Sci. Total Environ. 2017, 599, 934–943.
- Agüera, A.; Martínez Bueno, M.J.; Fernández-Alba, A.R. New trends in the analytical determination of emerging contaminants and their transformation products in environmental waters. Environ. Sci. Pollut. Res. Int. 2013, 20, 3496–3515.
- Merone, G.M.T.; Locatelli, A.; D’Ovidio, M.; Rosato, C.; de Grazia, E.; Santavenere, U.; Rossi, F.; Savini, S.F. Analytical chemistry in the 21st century: Chalenges, solutions, and future perspectives of complex matrices quantitative analysis in biological/clinical field. Analytica 2020, 1, 44–59.
- Patnaik, P. Handbook of Environmental Analysis: Chemical Pollutants in Air, Water, Soil, and Solid Wastes; CRC Press: Boca Raton, FL, USA, 2010.
- Loos, R.; Carvalho, R.; Antonio, D.; Cornero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487.
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2012, 1248, 104–121.
- Jelic, A.; Petrovic, M.; Barceló, D. Multi-residue method for trace level determination of pharmaceuticals in solid samples using pressurized liquid extraction followed by liquid chromatography/quadropole-linear ion trap mass spectrometry. Talanta 2009, 80, 363–371.
- Chiaia-Hernandez, A.; Krauss, M.; Hollender, J. Screening of lake sediments for emerging contaminants by liquid chromatography atmospheric pressure photoionization and electrospray ionization coupled to high resolution mass spectrometry. Environ. Sci. Technol. 2013, 47, 976–986.
- Barron, L.; Tobin, J.; Paull, B. Multi-residue determination of pharmaceuticals in sludge and sludge enriched soils using pressurized liquid extraction, solid phase extraction and liquid chromatography with tandem mass spectrometry. J. Environ. Monit. 2008, 10, 353–361.
- Hernández, F.; Sancho, J.V.; Ibáñez, M.; Abad, E.; Portolés, T.; Mattioli, L. Current use of high-resolution mass spectrometry in the environmental sciences. Anal. Bioanal. Chem. 2012, 403, 1251–1264.
- Farré, M.; Kantiani, L.; Petrovic, M.; Pérez, S.; Barceló, D. Achievements and future trends in the analysis of emerging organic contaminants in environmental samples by mass spectrometry and bioanalytical techniques. J. Chromatogr. A 2012, 1259, 86–99.
- Petrovic, M.; Farré, M.; de Alda, M.L.; Perez, S.; Postigo, C.; Köck, M.; Radjenovic, J.; Gros, M.; Barcelo, D. Recent trends in the liquid chromatography-mass spectrometry analysis of organic contaminants in environmental samples. J. Chromatogr. A 2010, 1217, 4004–4017.
- López-Serna, R.; Pérez, S.; Ginebreda, A.; Petrović, M.; Barceló, D. Fully automated determination of 74 pharmaceuticals in enctrvironmental and waste waters by online solid phase extraction-liquid chromatography-electrospray-tandem mass speometry. Talanta 2010, 83, 410–424.
- Gracia-Lor, E.; Sancho, J.V.; Hernández, F. Simultaneous determination of acidic, neutral and basic pharmaceuticals in urban wastewater by ultra high-pressure liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 622–632.
- Grabic, R.; Fick, J.; Lindberg, R.H.; Fedorova, G.; Tysklind, M. Multi-residue method for trace level determination of pharmaceuticals in environmental samples using liquid chromatography coupled to triple quadrupole mass spectrometry. Talanta 2012, 100, 183–195.
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Fast liquid chromatography-quadrupole-linear ion trap mass spectrometry for the analysis of pharmaceuticals and hormones in water resources. J. Chromatogr. A 2010, 1217, 4212–4222.
- Gaffney, V.; Cardoso, V.; Cardoso, E.; Teixeira, A.; Martins, J.; Benoliel, M.; Almeida, C. Occurrence and behaviour of pharmaceutical compounds in a Portuguese wastewater treatment plant: Removal efficiency through conventional treatment processes. Environ. Sci. Pollut. Res. 2017, 24, 14717–14734.
- Richardson, S. Environmental mass spectrometry: Emerging contaminants and current issues. Anal. Chem. 2012, 84, 747–778.
- Petrović, M.; Barceló, D. LC-MS for identifying photodegradation products of pharmaceuticals in the environment. Trends Anal. Chem. 2007, 26, 486–493.
- Gómez, M.J.; Gómez-Ramos, M.M.; Malato, O.; Mezcua, M.; Férnandez-Alba, A.R. Rapid automated screening, identification and quantification of organic micro-contaminants and their main transformation products in wastewater and river waters using liquid chromatography-quadrupole-time-of-flight mass spectrometry with an accurate-mass database. J. Chromatogr. A 2010, 1217, 7038–7054.
- Gaffney, V.D.; Cardoso, V.V.; Benoliel, M.J.; Almeida, C.M.M. Chlorination and oxidation of sulfonamides by free chlorine: Identification and behaviour of reaction products by UPLC-MS/MS. J. Environ. Manag. 2016, 166, 466–477.
- Escher, B.I.; Fenner, K. Recent advances in environmental risk assessment of transformation products. Environ. Sci. Technol. 2011, 45, 3835–3847.
- Küster, A.; Alder, A.C.; Escher, B.I.; Duis, K.; Fenner, K.; Garric, J.; Hutchinson, T.H.; Lapen, D.R.; Péry, A.; Römbke, J.; et al. Environmental risk assessment of human pharmaceuticals in the European Union: A case study with the β-blocker atenolol. Integr. Environ. Assess. Manag. 2010, 6, 514–523.
- Xia, B.; Liu, X.; Gu, Y.; Zhang, Z.; Wang, H.; Ding, L.; Zhou, Y. Non-target screening of veterinary drugs using tandem mass spectrometry on smart mass. J. Am. Soc. Mass Spectrom. 2013, 24, 789–793.
- Beccaria, M.; Cabooter, D. Current developments in LC-MS for pharmaceutical analysis. Analyst 2020, 145.
- Chen, F.; Gong, Z.; Kelly, B.C. Rapid analysis of pharmaceuticals and personal care products in fish plasma micro-aliquots using liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2015, 1383, 104–111.
- Ferhi, S.; Bourdat-Deschamps, M.; Daudin, J.; Houot, S.; Nelieu, S. Factors influencing the extraction of pharmaceuticals from sewage sludge and soil: An experimental design approach. Anal. Bioanal. Chem. 2016, 408, 6153–6168.
- Henriques, M.; Cardoso, V.; Rodrigues, A.; Ferreira, E.; Benoliel, M.; Almeida, C. Experimental and statistical validation of several endocrine disrupters by solid-phase extraction, liquid chromatography tandem mass spectrometry. Water Resour. Prot. 2010, 2, 818–829.
- Baranowska, I.; Kowalski, B. An analytical procedure for the determination of different therapeutic drugs in surface waters. Water Sci. Technol. 2009, 60, 449–458.
- Gilart, N.; Miralles, N.; Marce, R.; Borrull, F.; Fontanals, N. Novel coatings for stir bar sorptive extraction to determine pharmaceuticals and personal care products in environmental waters by liquid chromatography and tandem mass spectrometry. Anal. Chim. Acta 2013, 774, 51–60.
- Garcia-Galan, M.; Diaz-Cruz, M.; Barcelo, D. Determination of 19 sulfonamides in environmental water samples by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry (SPE-LC-MS/MS). Talanta 2010, 355–366.
- Akhtar, I.; Javad, S.; Yousaf, Z.; Iqbal, S.; Jabeen, K. Review: Microwave assisted extraction of phytochemicals an efficient and modern approach for botanicals and pharmaceuticals. Pak. J. Pharm. Sci. 2019, 32, 223–230.
- Evans, S.E.; Davies, P.; Lubben, A.; Kasprzyk-Hordern, B. Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction, solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta 2015, 882, 112–126.
- Nunez, M.; Borrull, F.; Pocurull, E.; Fontanals, N. Pressurized liquid extraction followed by liquid chromatography with tandem mass spectrometry to determine pharmaceuticals in mussels. J. Sep. Sci. 2016, 39, 741–747.
- Löffler, D.; Ternes, T.A. Determination of acidic pharmaceuticals, antibiotics and ivermectin in river sediment using liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2003, 1021, 133–144.
- Sun, H.; Ge, X.; Lv, Y.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A 2012, 1237, 1–23.
- Rodrigues, J.; Albino, S.; Silva, S.; Cravo, A.; Cardoso, V.; Benoliel, M.; Almeida, C. Development of a multiresidue method for the determination of 24 pharmaceuticals in clams by QuEChERS and liquid chromatography-triple quadrupole tandem mass spectrometry. Food Anal. Methods 2019, 12, 838–851.
- Núñez, M.; Borrull, F.; Fontanals, N.; Pocurull, E. Determination of pharmaceuticals in bivalves using QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 3841–3849.
- Wasik, A.; Kot-Wasik, A.; Namiesnik, J. New trends in sample preparation techniques for the analysis of the residues of pharmaceuticals in environmental samples. Curr. Anal. Chem. 2016, 12, 280–302.
- Ali, I.; Suhail, M.; Alharbi, O.; Hussain, I. Advances in sample preparation in chromatography for organic environmental pollutants analyses. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 137–160.
- Moldoveanu, S. Solutions and challenges in sample preparation for chromatography. J. Chromatogr. Sci. 2004, 42, 1–14.
- Maciel, E.; de Toffoli, A.; Lancas, F. Recent trends in sorption-based sample preparation and liquid chromatography techniques for food analysis. Electrophoresis 2018, 39, 1582–1596.
- Hyötyläinen, T.; Riekkola, M.L. Sorbent- and liquid-phase microextraction techniques and membrane-assisted extraction in combination with gas chromatographic analysis: A review. Anal. Chim. Acta 2008, 614, 27–37.
- Iparraguirre, A.; Navarro, P.; Rodil, R.; Prieto, A.; Olivares, M.; Etxebarria, N.; Zuloaga, O. Matrix effect during the membrane-assisted solvent extraction coupled to liquid chromatography tandem mass spectrometry for the determination of a variety of endocrine disrupting compounds in wastewater. J. Chromatogr. A 2014, 1356, 163–170.
- Perez-Fernandez, V.; Rocca, L.; Tomai, P.; Fanali, S.; Gentili, A. Recent advancements and future trends in environmental analysis: Sample preparation, liquid chromatography and mass spectrometry. Anal. Chim. Acta 2017, 983, 9–41.
- Tranchida, P.; Maimone, M.; Purcaro, G.; Dugo, P.; Mondello, L. The penetration of green sample-preparation techniques in comprehensive two-dimensional gas chromatography. Trends Anal. Chem. 2015, 71, 74–84.
- Cimetiere, N.; Soutrel, I.; Lemasle, M.; Laplanche, A.; Crocq, A. Standard addition method for the determination of pharmaceutical residues in drinking water by SPE-LC-MS/MS. Environ. Technol. 2013, 34, 3031–3041.
- Gilart, N.; Marcé, R.M.; Fontanals, N.; Borrull, F. A rapid determination of acidic pharmaceuticals in environmental waters by molecularly imprinted solid-phase extraction coupled to tandem mass spectrometry without chromatography. Talanta 2013, 110, 196–201.
- Xu, J.; Sun, H.; Zhang, Y.; Alder, A.C. Occurrence and enantiomer profiles of β-blockers in wastewater and a receiving water body and adjacent soil in Tianjin, China. Sci. Total Environ. 2019, 650, 1122–1130.
- Togola, A.; Baran, N.; Coureau, C. Advantages of online SPE coupled with UPLC/MS/MS for determining the fate of pesticides and pharmaceutical compounds. Anal. Bioanal. Chem. 2014, 406, 1181–1191.
- Huntscha, S.; Singer, H.P.; McArdell, C.S.; Frank, C.E.; Hollender, J. Multiresidue analysis of 88 polar organic micropollutants in ground, surface and wastewater using online mixed-bed multilayer solid-phase extraction coupled to high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1268, 74–83.
- Diaz-Cruz, M.; de Alda, M.; Barcelo, D. Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge. Trends Anal. Chem. 2003, 22, 340–351.
- Richardson, S.; Ternes, T. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2011, 83, 4614–4648.
- Borecka, M.; Białk-Bielińska, A.; Siedlewicz, G.; Kornowska, K.; Kumirska, J.; Stepnowski, P.; Pazdro, K. A new approach for the estimation of expanded uncertainty of results of an analytical method developed for determining antibiotics in seawater using solid-phase extraction disks and liquid chromatography coupled with tandem mass spectrometry technique. J. Chromatogr. A 2013, 1304, 138–146.
- Daniels, K.; Park, M.; Huang, Z.; Jia, A.; Flores, G.; Lee, H.; Snyder, S. A review of extraction methods for the analysis of pharmaceuticals in environmental waters. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2271–2299.
- Kim, S.; Carlson, K. Quantification of human and veterinary antibiotics in water and sediment using SPE/LC/MS/MS. Anal. Bioanal. Chem. 2007, 387, 1301–1315.
- Lindsey, M.E.; Meyer, T.M.; Thurman, E.M. Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry. Anal. Chem. 2001, 73, 4640–4646.
- Stolker, A.A.M.; Nielsing, W.; Hogendoorn, E.A.; Versteegh, J.F.M.; Fuchs, R.; Brinkman, U.A.T. Liquid chromatography with triple-quadrupole or quadrupole-time of flight mass spectrometry for screening and confirmation of residues of pharmaceuticals in water. Anal. Bioanal. Chem. 2004, 378, 955–963.
- Batt, A.L.; Aga, D.S. Simultaneous analysis of multiple classes of antibiotics by ion trap LC/MS/MS for assessing surface water and groundwater contamination. Anal. Chem. 2005, 77.
- Unceta, N.; Sampedro, M.C.; Abu Bakar, N.K.; Gómez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Multi-residue analysis of pharmaceutical compounds in wastewaters by dual solid-phase microextraction coupled to liquid chromatography electrospray ionization ion trap mass spectrometry. J. Chromatogr. A 2010, 1217, 3392–3399.
- López-Serna, R.; Petrović, M.; Barceló, D. Development of a fast instrumental method for the analysis of pharmaceuticals in environmental and wastewaters based on ultra high performance liquid chromatography (UHPLC)-tandem mass spectrometry (MS/MS). Chemosphere 2011, 85, 1390–1399.
- Seifrtová, M.; Nováková, L.; Lino, C.; Pena, A.; Solich, P. An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal. Chim. Acta 2009, 649, 158–179.
- Gros, M.; Petrovic, M.; Barcelo, D. Tracing pharmaceutical residues of different therapeutic classes in environmental Waters by using liquid chromatography/quadrupole-linear ion trap mass spectrometry and automated library searching. Anal. Chem. 2009, 81, 898–912.
- Wong, C.S.; MacLeod, S.L. JEM spotlight: Recent advances in analysis of pharmaceuticals in the aquatic environment. J. Environ. Monit. 2009, 11, 923–936.
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra performance liquid chromatography-positive electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 132–145.
- Robles-Molina, J.; Lara-Ortega, F.J.; Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Multi-residue method for the determination of over 400 priority and emerging pollutants in water and wastewater by solid-phase extraction and liquid chromatography-time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1350, 30–43.
- Kasprzyk-Hordern, B.; Dinsdale, R.; Guwy, A. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380.
- Batt, A.L.; Kostich, M.S.; Lazorchak, J.M. Analysis of ecologically relevant pharmaceuticals in wastewater and surface water using selective solid-phase extraction and UPLC-MS/MS. Anal. Chem. 2008, 80, 5021–5030.
- Ido, A.; Hiromori, Y.; Meng, L.; Usuda, H.; Nagase, H.; Yang, M.; Hu, J.; Nakanishi, T. Occurrence of fibrates and their metabolites in source and drinking water in Shanghai and Zhejiang, China. Sci. Rep. 2017, 7.
- Panditi, V.R.; Batchu, S.R.; Gardinali, P.R. Online solid-phase extraction-liquid chromatography-electrospray-tandem mass spectrometry determination of multiple classes of antibiotics in environmental and treated waters. Anal. Bioanal. Chem. 2013, 405, 5953–5964.
- Ferrer, I.; Thurman, E.M. Analysis of 100 pharmaceuticals and their degradates in water samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2012, 1259, 148–157.
- Hernández, F.; Ibáñez, M.; Gracia-Lor, E.; Sancho, J.V. Retrospective LC-QTOF-MS analysis searching for pharmaceutical metabolites in urban wastewater. J. Sep. Sci. 2011, 34, 3517–3526.
- Hu, J.; Zhang, H.; Chang, H. Improved method for analyzing estrogens in water by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. A 2005, 1070, 221–224.
- Gilart, N.; Marcé, R.M.; Borrull, F.; Fontanals, N. Determination of pharmaceuticals in wastewaters using solid-phase extraction-liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2012, 35, 875–882.
- Ammann, A.A.; Macikova, P.; Groh, K.J.; Schirmer, K.; Suter, M.J. LC-MS/MS determination of potential endocrine disruptors of cortico signalling in rivers and wastewaters. Anal. Bioanal. Chem. 2014, 406, 7653–7665.
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography-tandem mass spectrometry detection. J. Chromatogr. A 2006, 1134, 101–111.
- Rodriguez, E.; Navarro-Villoslada, F.; Benito-Pena, E.; Marazuela, M.; Moreno-Bondi, M. Multiresidue determination of ultratrace levels of fluoroquinolone antimicrobials in drinking and aquaculture water samples by automated online molecularly imprinted solid phase extraction and liquid chromatography. Anal. Chem. 2011, 83, 2046–2055.
- Mogolodi Dimpe, K.; Nomngongo, P.N. Application of activated carbon-decorated polyacrylonitrile nanofibers as an adsorbent in dispersive solid-phase extraction of fluoroquinolones from wastewater. J. Pharm. Anal. 2019, 9, 117–126.
- Wu, A.; Zhao, X.; Wang, J.; Tang, Z.; Zhao, T.; Niu, L.; Yu, W.; Yang, C.; Fang, M.; Lv, H.; et al. Application of solid-phase extraction based on magnetic nanoparticle adsorbents for the analysis of selected persistent organic pollutants in environmental water: A review of recent advances. Crit. Rev. Environ. Sci. Technol. 2020.
- Herrera-Herrera, A.V.; Hernández-Borges, J.; Afonso, M.M.; Palenzuela, J.A.; Rodríguez-Delgado, M. Comparison between magnetic and non magnetic multi-walled carbon nanotubes-dispersive solid-phase extraction combined with ultra-high performance liquid chromatography for the determination of sulfonamide antibiotics in water samples. Talanta 2013, 116, 695–703.
- Li, N.; Chen, J.; Shi, Y. Magnetic polyethyleneimine functionalized reduced graphene oxide as a novel magnetic sorbent for the separation of polar non-steroidal anti-inflammatory drugs in waters. Talanta 2019, 191, 526–534.
- Capriotti, A.; Cavaliere, C.; La Barbera, G.; Montone, C.; Piovesana, S.; Lagana, A. Recent applications of magnetic solid-phase extraction for sample preparation. Chromatographia 2019, 82, 1251–1274.
- Liu, D.; Huang, Z.; Li, M.; Li, X.; Sun, P.; Zhou, L. Construction of magnetic bifunctional beta-cyclodextrin nanocomposites for adsorption and degradation of persistent organic pollutants. Carbohydr. Polym. 2020, 230.
- Liu, D.; Huang, Z.; Li, M.; Sun, P.; Yu, T.; Zhou, L. Novel porous magnetic nanospheres functionalized by beta-cyclodextrin polymer and its application in organic pollutants from aqueous solution. Environ. Pollut. 2019, 250, 639–649.
- Lin, Z.; He, Q.; Wang, L.; Wang, X.; Dong, Q.; Huang, C. Preparation of magnetic multi-functional molecularly imprinted polymer beads for determining environmental estrogens in water samples. J. Hazard. Mater. 2013, 252, 57–63.
- Jiménez-Soto, J.M.; Cárdenas, S.; Valcárcel, M. Evaluation of carbon nanocones/disks as sorbent material for solid-phase extraction. J. Chromatogr. A 2009, 1216, 5626–5633.
- Wen, Y.; Chen, L.; Li, J.; Liu, D.; Chen, L. Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. Trends Anal. Chem. 2014, 59, 26–41.
- Li, X.; Zhu, G.; Luo, Y.; Yuan, B.; Feng, Y. Synthesis and applications of functionalized magnetic materials in sample preparation. Trends Anal. Chem. 2013, 45, 233–247.
- Pérez, R.A.; Albero, B.; Férriz, M.; Tadeo, J.L. Analysis of macrolide antibiotics in water by magnetic solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2017, 146, 79–85.
- Luo, Y.; Shi, Z.; Gao, Q.; Feng, Y. Magnetic retrieval of graphene: Extraction of sulfonamide antibiotics from environmental water samples. J. Chromatogr. A 2011, 1218, 1353–1358.
- Abdolmohammad-Zadeh, H.; Talleb, Z. Magnetic solid phase extraction of gemfibrozil from human serum and pharmaceutical wastewater samples utilizing a beta-cyclodextrin grafted graphene oxide-magnetite nano-hybrid. Talanta 2015, 134, 387–393.
- Pena-Mendez, E.; Mawale, R.; Conde-Gonzalez, J.; Socas-Rodriguez, B.; Havel, J.; Ruiz-Perez, C. Metal organic framework composite, nano-Fe3O4@Fe-(benzene-1,3,5-tricarboxylic acid), for solid phase extraction of blood lipid regulators from water. Talanta 2020, 207.
- Zhao, Y.; Zhang, Y.; Zhan, P.; Chen, X.; Pan, S.; Jin, M. Fast determination of 24 steroid hormones in river water using magnetic dispersive solid phase extraction followed by liquid chromatography-tandem mass spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 1529–1539.
- Aguilar-Arteaga, K.; Rodriguez, J.; Miranda, J.; Medina, J.; Barrado, E. Determination of non-steroidal anti-inflammatory drugs in wastewaters by magnetic matrix solid phase dispersion-HPLC. Talanta 2010, 80, 1152–1157.
- Socas-Rodríguez, B.; Hernández-Borges, J.; Salazar, P.; Martín, M.; Rodríguez-Delgado, M. Core-shell polydopamine magnetic nanoparticles as sorbent in micro-dispersive solid-phase extraction for the determination of estrogenic compounds in water samples prior to high-performance liquid chromatography-mass spectrometry analysis. J. Chromatogr. A 2015, 1397, 1–10.
- Sajid, M.; Nazal, M.K.; Ihsanullah, I. Novel materials for dispersive (micro) solid-phase extraction of polycyclic aromatic hydrocarbons in environmental water samples: A review. Anal. Chim. Acta 2021, 1141, 246–262.
- Chen, X.; Zhao, Y.; Qiu, Q.; Zhu, Y.; Min, J.; Jin, M. A fast and high throughput LC-MS/MS method for the determination of 58 human and veterinary drugs in river water. Anal. Methods 2017, 9, 4228–4233.
- Wackerlig, J.; Schirhagl, R. Applications of molecularly imprinted polymer nanoparticles and their advances toward industrial use: A review. Anal. Chem. 2016, 88, 250–261.
- Demeestere, K.; Petrovic, M.; Gros, M.; Dewulf, J.; Van Langenhove, H.; Barcelo, D. Trace analysis of antidepressants in environmental waters by molecularly imprinted polymer-based solid-phase extraction followed by ultra-performance liquid chromatography coupled to triple quadrupole mass spectrometry. Anal. Bioanal. Chem. 2010, 825–837.
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211.
- Hu, Y.; Wang, C.; Li, X.; Liu, L. Preparation and application of epitope magnetic molecularly imprinted polymers for enrichment of sulfonamide antibiotics in water. Electrophoresis 2017, 38, 2462–2467.
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719.
- Wackerlig, J.; Lieberzeit, P. Molecularly imprinted polymer nanoparticles in chemical sensing—Synthesis, characterisation and application. Sens. Actuators B Chem. 2015, 207, 144–157.
- Ouyang, G. Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 251–290.
- Montesdeoca-Esponda, S.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Analytical Separation Science; Jared, L., Anderson, A.B., Pino, V., Stalcup, A., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 1897–1927.
- Pawliszyn, J. Solid Phase Microextraction, Theory and Practice; Wiley-VCH: New York, NY, USA, 1997.
- Almeida, C.; Boas, L. Analysis of BTEX and other substituted benzenes in water using headspace SPME-GC-FID: Method validation. J. Environ. Monit. 2004, 6, 80–88.
- Simoes, N.G.; Cardoso, V.V.; Ferreira, E.; Benoliel, M.J.; Almeida, C.M.M. Experimental and statistical validation of SPME-GC-MS analysis of phenol and chlorophenols in raw and treated water. Chemosphere 2007, 68, 501–510.
- Castells, P.; Santos, F.; Galceran, M. Solid-phase extraction versus solid-phase microextraction for the determination of chlorinated paraffins in water using gas chromatography—Negative chemical ionisation mass spectrometry. J. Chromatogr. A 2004, 157–162.
- Sarafraz-Yazdi, A.; Amiri, A.; Rounaghi, G.; Eshtiagh-Hosseini, H. Determination of non-steroidal anti-inflammatory drugs in water samples by solid-phase microextraction based sol-gel technique using poly(ethylene glycol) grafted multi-walled carbon nanotubes coated fiber. Anal. Chim. Acta 2012, 720, 134–141.
- López-Serna, R.; Marín-de-Jesús, D.; Irusta-Mata, R.; García-Encina, P.A.; Lebrero, R.; Fdez-Polanco, M.; Muñoz, R. Multiresidue analytical method for pharmaceuticals and personal care products in sewage and sewage sludge by online direct immersion SPME on-fiber derivatization—GCMS. Talanta 2018, 186, 506–512.
- Huang, S.; Zhu, F.; Jiang, R.; Zhou, S.; Zhu, D.; Liu, H.; Ouyang, G. Determination of eight pharmaceuticals in an aqueous sample using automated derivatization solid-phase microextraction combined with gas chromatography-mass spectrometry. Talanta 2015, 136, 198–203.
- Araujo, L.; Wild, J.; Villa, N.; Camargo, N.; Cubillan, D.; Prieto, A. Determination of anti-inflammatory drugs in water samples, by in situ derivatization, solid phase microextraction and gas chromatography-mass spectrometry. Talanta 2008, 75, 111–115.
- Yu, H.; Merib, J.; Anderson, J.L. Crosslinked polymeric ionic liquids as solid-phase microextraction sorbent coatings for high performance liquid chromatography. J. Chromatogr. A 2016, 1438, 10–21.
- Mei, M.; Yu, J.; Huang, X.; Li, H.; Lin, L.; Yuan, D. Monitoring of selected estrogen mimics in complicated samples usingpolymeric ionic liquid-based multiple monolithic fiber solid-phasemicroextraction combined with high-performance liquidchromatography. J. Chromatogr. A 2015, 1385, 12–19.
- Gil García, M.D.; Cañada Cañada, F.; Culzoni, M.J.; Vera-Candioti, L.; Siano, G.G.; Goicoechea, H.C.; Martínez Galera, M. Chemometric tools improving the determination of anti-inflammatory and antiepileptic drugs in river and wastewater by solid-phase microextraction and liquid chromatography diode array detection. J. Chromatogr. A 2009, 1216, 5489–5496.
- Vera-Candioti, L.; Gil García, M.D.; Martínez Galera, M.; Goicoechea, H.C. Chemometric assisted solid-phase microextraction for the determination of anti-inflammatory and antiepileptic drugs in river water by liquid chromatography-diode array detection. J. Chromatogr. A 2008, 1211, 22–32.
- Lock, C.M.; Chen, L.; Volmer, D.A. Rapid analysis of tetracycline antibiotics by combined solid phase microextraction/high performance liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 1744–1754.
- McClure, E.L.; Wong, C.S. Solid phase microextraction of macrolide, trimethoprim, and sulfonamide antibiotics in wastewaters. J. Chromatogr. A 2007, 1169, 53–62.
- Balakrishnan, V.K.; Terry, K.A.; Toito, J. Determination of sulfonamide antibiotics in wastewater: A comparison of solid phase microextraction and solid phase extraction methods. J. Chromatogr. A 2006, 1131, 1–10.
- Sánchez-Rojas, F.; Bosch-Ojeda, C.; Cano-Pavó, J.M. A review of stir bar sorptive extraction. Chromatographia 2009, 69, S69–S84.
- He, M.; Chen, B.; Hu, B. Recent developments in stir bar sorptive extraction. Anal. Bioanal. Chem. 2014, 406, 2001–2026.
- Camino-Sánchez, F.J.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Santos-Fandila, A.; Vílchez, J.L. Stir bar sorptive extraction: Recent applications, limitations and future trends. Talanta 2014, 130, 388–399.
- Hashemi, S.H.; Kaykhaii, M. Nanoparticle coatings for stir bar sorptive extraction, synthesis, characterization and application. Talanta 2021, 221, 121568.
- Fan, W.; Mao, X.; He, M.; Chen, B.; Hu, B. Development of novel sol-gel coatings by chemically bonded ionic liquids for stir bar sorptive extraction--application for the determination of NSAIDS in real samples. Anal. Bioanal. Chem. 2014, 406, 7261–7273.
- Gilart, N.; Marcé, R.M.; Cormack, P.A.; Fontanals, N.; Borrull, F. Development of new polar monolithic coatings for stir bar sorptive extraction. J. Sep. Sci. 2014, 37, 2225–2232.
- Mao, X.; He, M.; Chen, B.; Hu, B. Membrane protected C. J. Chromatogr. A 2016, 1472, 27–34.
- Peng, J.; Liu, D.; Shi, T.; Tian, H.; Hui, X.; He, H. Molecularly imprinted polymers based stir bar sorptive extraction for determination of cefaclor and cefalexin in environmental water. Anal. Bioanal. Chem. 2017, 409, 4157–4166.
- Xu, Z.; Yang, Z.; Liu, Z. Development of dual-templates molecularly imprinted stir bar sorptive extraction and its application for the analysis of environmental estrogens in water and plastic samples. J. Chromatogr. A 2014, 1358, 52–59.
- Bratkowska, D.; Fontanals, N.; Cormack, P.A.; Borrull, F.; Marcé, R.M. Preparation of a polar monolithic stir bar based on methacrylic acid and divinylbenzene for the sorptive extraction of polar pharmaceuticals from complex water samples. J. Chromatogr. A 2012, 1225, 1–7.
More
Information
Subjects:
Water Resources
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
700
Revisions:
2 times
(View History)
Update Date:
22 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




