1. Immunotherapy during the Immediate Perioperative Period : Feasibility and Effectiveness
In general, very few clinical studies have evaluated or are currently evaluating the safety and efficacy of immunotherapeutic agents during the immediate perioperative period (IPP) (see
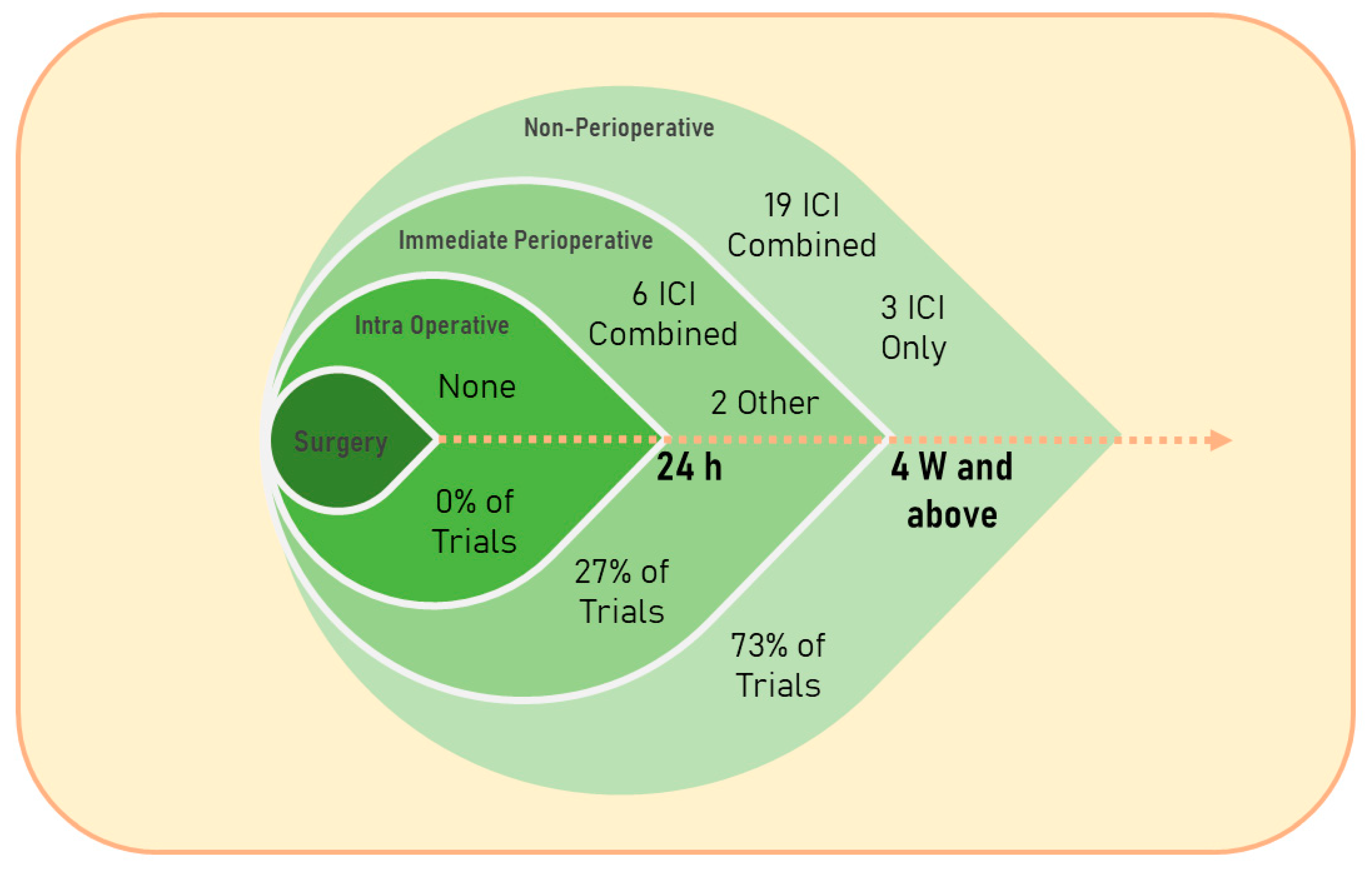
Figure 1)
[1][2][3][4][5], whereas the assessment of non-IPP administration of these agents is becoming acknowledged and established as an effective approach in numerous types of cancer
[6]. For example, researchers searched for the keywords: “cancer” and “perioperative immunotherapy” on clinicaltrials.gov (starting from 1 January 2018) and found 57 interventional studies in which almost half of the trials did not describe the specific timing of the treatment (48%); out of the trials which described the specific timing of treatment, the majority were non-IPP therapies (73%), and the great majority assessed immune checkpoint blockade (93%). The majority of the published literature on immunotherapy, and specifically perioperative immunotherapy in cancer patients, is currently focused on immune checkpoint inhibitors (ICIs). The rapid evolution and expansion of clinical trials assessing numerous regimens of ICIs, as monotherapy or in conjunction with other treatments, at different time points relative to surgery, and in various cancer types and cancer patient populations, position ICIs as a suitable approach for discussing the various considerations for the use of immunotherapy during the IPP. Accordingly, the following sections discuss several types of immunotherapies that are currently being tested during the IPP, and address ICIs in more detail.
Figure 1. Timing relative to surgery and type of the treatment given to cancer patients in clinical trials found on search results of the keywords “cancer” and “perioperative immunotherapy” in the past five years (since 1 January 2018) on
https://clinicaltrials.gov/ (accessed on 14 April 2023). 24 h, 24 hours; 4 W and above, 4 weeks and above; ICI, immune checkpoint inhibition; Combined, ICI therapy combined with other types of interventions.
1.1. Anti-Stress-Inflammatory Approach—The Inhibition of β-Adrenergic and Cyclooxygenase 2 Signaling
As others and researchers have indicated in preclinical studies, excess β-adrenergic signaling and prostaglandins synthesis were each shown to disrupt antimetastatic immune surveillance and cell-mediated immunity, promote metastasis, and worsen survival outcomes
[7][8]. In the past two decades, researchcers have established a novel approach that targets these deleterious responses during the IPP, employing the β-adrenergic receptor antagonist, propranolol, and the prostaglandin synthesis inhibitor, etodolac. The combined treatment was found safe during the IPP
[9][10] and effective in attenuating immunosuppression and metastatic disease. Specifically, researchers assessed the safety of these drugs, alone and in combination, on wound healing, anastomosis strength, and abdominal muscle tensile strength, and reported no deleterious effect
[9][10]. Additionally, the combined propranolol and etodolac treatment was employed by researchers during the IPP in several tumor models, showing enhanced antimetastatic immunity and decreased metastatic burden and/or improved survival rates in all models
[11][12][13][14]. Importantly, the simultaneous administration of propranolol and etodolac was more effective than each drug alone, and often the only effective intervention to improve resistance to postoperative metastasis
[11][12][15].
Consequently, researchers employed this combined approach in the clinical setting, conducting two clinical trials in breast
[16][17] and in colorectal cancer patients
[18][19]. Propranolol and etodolac or placebo control were administered to the patients for up to twenty consecutive days, starting five days prior to tumor resection. Notably, such perioperative use of the drug combination exhibited a promising safety profile, with no adverse events and complication rates relative to the placebo-treated patients
[16][17], including anticipated and manageable treatment-related adverse events, such as reduced blood pressure or heart rate
[16][18]. Additionally, transcriptomic analysis of the excised breast and colorectal tumors revealed that compared to the placebo control group, propranolol and etodolac (i) reduced markers for epithelial-to-mesenchymal transition; (ii) decreased the activity of several transcription factors which are associated with inflammation and/or poor prognosis, including GATA1, GATA2, EGR3, STAT3, IRF1, IFR2, and CREB; and (iii) improved the composition of tumor-infiltrating leukocytes, including reduced monocytes and increased NK and B cell presence
[16][18]. Importantly, the combined treatment reduced the plasma levels of prometastatic and proinflammatory proteins, including the plasma levels of IL-6 and CRP
[16][18], even before surgery. Most importantly, researchers have now completed a 5-year follow-up in the colorectal cancer cohort, and reported a statistically significant improvement in disease-free survival, in both intent-to-treat and protocol-compliant patients
[19]. However, this randomized clinical trial was planned as a biomarker study and did not have the statistical power to assess long-term cancer outcomes. Thus, researchers are now conducting larger and multicenter studies to further test the clinical long-term effectiveness of this novel approach, to advance our understanding of mediating mechanisms of the treatment, and to possibly introduce this prophylactic antimetastatic treatment as a clinical routine, alone or in conjunction with other therapy types.
1.2. Oncolytic Virotherapy
Oncolytic virotherapy (OVT) is a form of immunotherapy that employs a wide range of tumor-selective viruses, each of which target cancerous tissues
[20]. OVT is designed to fight cancer by direct oncolysis and indirectly by stimulating a potent antitumor immune response
[21]. Although still under investigation, OVT was shown to enhance antitumor immunity through the release of (i) tumor-associated antigens, (ii) pathogen-associated molecular patterns, and danger-associated molecular patterns, as well as (iii) cytokines (e.g., IFNγ, IL-12, and type-I IFNs)
[22]. These effects enable oncolytic viruses to eliminate local residual tumor cells and distant micrometastases, as well as to impede angiogenesis, qualifying OVT as a neoadjuvant therapy
[23][24].
According to Thomas and Bartee, there has been a recent surge in trials using neoadjuvant virotherapy, with four trials utilizing OVTs up to a month before surgery, and two trials ending treatment days before surgery, during the IPP
[25]. Although factors determining the time of treatment proximity to surgery in these trials are not stated, a rationale for both distal and proximal intervals can be made. Evidence indicates that antitumor immunity is maintained long after the virus is cleared, allowing for extended intervals that can mitigate potential surgery-related risks. On the other hand, one study showed that OVT given three days before surgery led to a robust immune activation, potentially counteracting surgery-related immune suppression without evident complications
[25]. T-VEC (Imlygic
®, Amgen, CA, USA), a genetically modified herpes simplex virus, is the first and only FDA-approved OVT. It was recently studied in an open-label phase-II trial as a neoadjuvant therapy in stage IIIB–IVM1a melanoma
[26]. Patients were assigned to (i) immediate surgery or (ii) intratumoral T-VEC treatment ending 1–4 weeks before surgery. The T-VEC arm demonstrated a significant 25% reduction in the risk of disease recurrence, with improved 2-year and 3-year relapse-free survival rates compared to the surgery-only group (29.5% vs. 16.5% and 28.1% vs. 16.9%)
[26]. Another study has demonstrated how a single i.v. infusion of reovirus in a small cohort of patients with recurrent high-grade gliomas, 3–17 days before surgery, increased cytotoxic T cell tumor infiltration, upregulated IFN gene expression, and increased the PD-1/PD-L1 axis in tumors via an IFN-mediated mechanism, all of which were shown to potentially enhance the sensitivity of glioma cells to ICI treatment. Efficacy data, however, could not be determined due to the limited cohort size
[27].
To summarize, OVTs demonstrate a generally well-tolerated safety profile, with the majority of adverse events being of low grade, and fewer high-grade adverse events compared to chemotherapy
[28]. As OVT is still emerging and being developed, a qualitative comparison with other treatment modalities is not available, nor is the assessment of the significance of time interval to surgery. However, neoadjuvant OVT in the IPP continues to show encouraging results, particularly when used in combination with other immunotherapies
[25].
1.3. Cytokine Therapies
Cytokines are defined as small proteins that are secreted from cells and are pivotal to cellular communication and interactions. Among other functions, these proteins are involved in controlling inflammation and are key components of an effective antitumor immune response
[29]. Several cytokines have been studied clinically during the IPP as monotherapy or in conjunction with other therapies.
IFNα was the first cytokine-based therapy approved by the FDA in 1986 for patients with hairy-cell leukemia. Clinical studies evaluating IFNα administration during the IPP have found mixed results. For example, a combined treatment of IFNα and continuous arterial infusion of 5FU chemotherapy 2–3 weeks postoperatively increased the 1-year overall survival (OS) of hepatocellular carcinoma patients from 30% in patients who underwent surgery alone to 100% in patients who received the combined treatment
[30]. Additionally, IFNα administration during the IPP reduced the postoperative suppression of NK cell cytotoxicity
[31] and decreased the circulating levels of VEGF and the number of regulatory T cells, all of which are related to a better prognosis
[32]. However, a single dose of IFNα administered immediately following the transurethral resection of superficial bladder cancer did not improve OS compared to surgery alone
[33].
Another promising cytokine in preclinical research, which has advanced to the clinical setting, is IL-2. IL-2 is a powerful regulator of immune responses
[34] and is known to control the behavior of various leukocytes, especially T cells, promoting either antimetastatic or prometastatic effects in a dose-dependent manner
[35]. Treatment with recombinant IL-2 has been studied since the 1990s, but has only been implemented during the IPP in very few studies. Nevertheless, these studies revealed encouraging results. For example, IL-2 therapy given twice daily for three consecutive days starting 5 days prior to surgery was shown to improve 5-year disease-free survival and OS in patients with pancreatic cancer
[1] and reduced the frequency of disease progression in patients with colorectal cancer
[2], compared with placebo. Moreover, IL-2 administered twice daily for five days until the day before surgery has demonstrated similar results
[36]. Notably, the PANCEP-1 trial, evaluating peri- and postoperative treatment with histamine dihydrochloride (HDC) and low-dose IL-2 in patients with primary resectable pancreatic cancer, has recently been initiated. In this trial, patients receive HDC and IL-2 twice daily for three weeks, starting two weeks preoperatively up until the surgical resection, and continuing with the remaining week at three days postoperatively. The primary hypothesis for this trial is that the combined HDC and IL-2 treatment will reduce inflammation, counter surgery-related immunosuppression, and consequently reduce metastatic disease (NCT05810792).
Last, a combined treatment of nivolumab (anti-PD-1) seven days prior to surgery and VDX (a controlled IL-12 gene therapy) three hours before and fourteen days after surgery in patients with resectable recurrent glioblastoma has shown a great increase in serum IL-12 and tumor IFN-gamma postoperatively, leading to an ongoing phase-2 trial
[4]. Nivolumab in conjunction with VDX treatment given during the IPP was manageable with regard to immune-related adverse events (irAEs), as they were reported to be dose-dependent, easily predicted, and reversible
[4].
The safety profiles of these strategies were shown to be tolerable, with almost no surgery delays in neoadjuvant approaches and no increase in short-term or long-term surgical complications
[2][36][37].
1.4. Monoclonal Antibodies
A common immunotherapeutic approach employs monoclonal antibodies (mAbs), which specifically bind to targets on tumor cells or stroma cells in the tumor microenvironment. Their binding can induce tumor lysis through different mechanisms, including a ligand or receptor blockade and the activation of host immunity
[38][39]. Such mAbs can also be conjugated to a cytotoxic compound, such as chemotherapeutic agents, and some mAbs can simultaneously bind more than one target (known as bispecific antibodies)
[38][39]. Common mAbs therapies in solid malignancies are usually given in cycles that can last weeks or even months. Although recent clinical research highlights the potential beneficial effects of neoadjuvant (preoperative) treatments, for example with anti-HER2 or anti-EGFR antibodies in BC
[40], these are rarely administered during the IPP, with the neoadjuvant treatment regimen ending 3 weeks or more before surgery, and adjuvant therapy starting months after it
[41][42][43]. Avoidance of mAbs administration during the IPP is attributed to potential adverse effects that the systemic delivery of mAbs may cause, even though mAbs immunotherapy is considered less toxic than traditional chemotherapy
[44]. Immunotherapy with mAbs is associated with several toxicities, including hepatic, cardiac, pulmonary, renal, neurotoxicity, cytokine release syndrome, and infusion reactions
[38][44][45].
Despite limited clinical research, preclinical studies show promise in the systemic delivery of mAbs during the IPP. Preclinical studies in models of BC have found that neoadjuvant anti-CD25 mAbs therapy targeting regulatory T cells given 3 days before surgery was significantly more beneficial in extending survival outcomes than if given 3 days after surgery
[46]. The same group also found that ICI delivery was beneficial in prolonging survival outcomes when given for short duration starting 4–5 days before surgery, but not if the treatment was terminated more than 8 days before surgery
[47]. Extending therapy duration after surgery did not increase survival benefit, whereas it did induce more treatment-related adverse events
[47]. Such preclinical studies pinpoint the importance of treatment duration and its timing relative to surgery. Additionally, in the setting of anti-HER2 mAbs therapy in BC patients, although pilot clinical trials assessing the delivery of neoadjuvant anti-HER2 mAbs therapy up to 7 days before surgery found it to be safe nearly 20 years ago
[48], not many clinical trials employing such immunotherapy during the IPP have been conducted. A recent phase-II clinical trial evaluated targeted anti-HER2 therapy given at 11 and 3 days before surgery and approximately 2 weeks after surgery, and found that combining two drugs (lapatinib, a tyrosine kinase inhibitor, and trastuzumab, an anti-HER2 mAb) was more beneficial in reducing the proliferation marker Ki67 in the primary tumor within each patient (relative to presurgical biopsy samples), and also improved overall survival
[3]. Of note, recent strategies are being developed for the intratumoral delivery of mAbs, in the form of mAbs gene delivery systems, through viral or nonviral vectors. Such strategies are expected to reduce unwanted adverse events which are observed during the systemic delivery of mAbs, as well as to affect distal metastases (either directly or indirectly), and these are currently being evaluated in both preclinical and clinical trials
[49].
1.5. Immune Checkpoint Inhibitors: Treatment Evolvement and Usage within the Immediate Perioperative Period
ICIs have revolutionized cancer treatment, improving long-term cancer outcomes
[50]. While ICIs are primarily administered in advanced and/or metastatic cancers, their perioperative use has recently gained considerable interest. Numerous studies have investigated the efficacy and safety of non-IPP use of ICIs in different cancer types, but almost none have evaluated ICIs during the IPP due to their confirmed and hypothesized adverse effects on the success of surgery and patients’ safety
[51]. Such adverse effects are commonly characterized and graded for severity by employing the Common Terminology Criteria for Adverse Events (CTCAE) introduced by the US National Cancer Institute
[52]. The CTCAE grades irAEs on an ascending symptom severity scale: grade 1—asymptomatic/mild, grade 2—moderate, grade 3—severe, grade 4—life-threatening, and grade 5—death
[52]. A systemic review and meta-analysis that assessed the general safety of ICIs in 36 phase-II and phase-III clinical trials estimated the pooled incidence rate of irAEs of any grade (1–5) to be between 66.4% and 86.8%, out of which grade-3–4 irAEs accounted for merely 14.1% and 28.6% (depending on the ICI type)
[53], and grade 5 accounted for approximately 1% or less
[54]. Although the incidence rate of ICI-therapy-related irAEs is relatively high, most studies employing these agents as monotherapy, dual therapy, or in conjunction with conventional therapy, describe a well-tolerated or manageable safety profile, especially when ICIs are administered for short durations in a limited number of cycles
[55][56].
Treatment using ICIs was designed based on several key clinical trials in advanced and/or metastatic cancer patients, in multiple cycles and over prolonged treatment periods
[56][57][58]. This strategy was adopted as the prevalent dogma, with the aim of eradicating MRD to prevent or postpone the development of metastatic disease
[56], achieving FDA approvals for numerous indications
[56]. While adjuvant therapy has been shown to mend pathologic responses in a significant portion of patients, some patients do not respond to treatment and some patients require an early cessation of treatment due to toxicities or disease hyperprogression
[55]. Importantly, some studies assessed the long-term outcomes of patients who achieved pathological complete response (pCR) and ended the treatment early due to the manifestation of irAEs
[59] or due to achieving pCR
[60]. Unexpectedly, some patients who experienced a shorter treatment course continued to exhibit a durable response and had similar long-term benefits compared to patients who completed the entire treatment course, consequently raising questions about the necessity of a prolonged treatment for all patients, which is known to induce more irAEs
[55][60]. Thus, further studies are warranted to assess the pathological responses and long-term benefits of shorter treatment courses of ICIs.
Studies that evaluate ICIs’ efficacy in patients with early-stage cancer, which often involves surgical procedures in temporal proximity to the given therapy, have only gained momentum in recent years. A similar trend in other types of antimalignant therapy (such as chemo- and radiotherapy) has also occurred. The limited efficacy of adjuvant chemo- and radiotherapies in a significant proportion of cancer patients has led to the development of preoperative (neoadjuvant) treatment strategies. While adjuvant therapy commonly allows for longer treatment periods and enables a shorter diagnosis-to-surgery interval, neoadjuvant approaches have demonstrated significant advantages in several cancer types
[61][62][63][64], including the following: (i) an increase in complete resection rates by the reduction in tumor load, (ii) the real-time evaluation of patient response to the treatment, before definitive surgery; and (iii) an increase in neoantigen presentation prior to surgery
[65]. Additionally, there are indications that postoperative immunosuppression may hinder the efficacy of adjuvant immunotherapies. Accordingly, neoadjuvant approaches of ICIs have now been approved and are currently being studied in over 400 clinical studies (according to
https://clinicaltrials.gov/ (accessed on 2 April 2023)). In these clinical studies, neoadjuvant ICIs are administered for a short period (up to 12 weeks) in a limited number of cycles. Encouraging results have already been demonstrated in several key studies. Notably, the CheckMate 816 trial evaluated the non-IPP neoadjuvant use of nivolumab and chemotherapy (ending four to six weeks before surgery), compared to chemotherapy alone, in resectable non-small-cell lung cancer (NSCLC) patients. The study demonstrated a significant improvement in median event-free survival (EFS) and a significantly higher pCR in the nivolumab and chemotherapy group (an EFS of 31.6 months vs. 20.8 months and a pCR of 24% vs. 2.2%). The treatment also maintained a satisfactory safety profile, as patients of both groups exhibited similar rates of adverse events and treatment-related adverse events
[66]. Similarly, the NEOSTAR trial investigated the neoadjuvant Ipilimumab (anti-CTLA-4) vs. neoadjuvant nivolumab + ipilimumab in operable NSCLC patients, primarily assessing major pathological response (MPR). Although survival endpoints were not met, the results showed a significant increase in MPR, pCR, and in tumor-infiltrating nonregulatory T cells in the nivolumab + ipilimumab group. Toxicities were described as manageable, and grade >3 irAEs were reported in 13% of patients in the nivolumab + ipilimumab group vs. 10% in the Ipilimumab group
[67]. These studies, like the great majority of clinical studies which evaluate the neoadjuvant use of ICIs, end the treatment before the IPP.
Preclinical studies assessing the timing of ICIs as adjuvant and neoadjuvant therapies have demonstrated the superior efficacy of neoadjuvant treatment in some cancer types, and especially when administered during the IPP. Liu et al. conducted a seminal study (which was briefly discussed above) in which they evaluated the timing relative to tumor resection and the duration of therapy of different ICI combinations in two models of murine breast cancer. Their results indicated that two doses of neoadjuvant anti-PD-1 + anti-CD137 therapy, starting 4–5 days prior to tumor resection, were advantageous over earlier neoadjuvant (10 days) or adjuvant therapies in expanding tumor-specific CD8+ T cells and prolonging survival rates. Nevertheless, when the treatment was initiated two days before surgery, the improvement in long-term survival was jeopardized, suggesting an optimal preoperative window of opportunity during the IPP. Interestingly, adding an adjuvant therapy component to the neoadjuvant therapy did not improve survival rates, but increased the irAE incidence rate
[46][47], which again testifies to the importance of determining not only the treatment timing relative to surgery but also the treatment duration.
Researchers were able to locate only two publications of completed clinical studies that evaluated neoadjuvant ICI therapy during the IPP, and both are small, single-arm, phase-I studies that assessed biomarkers and/or safety profiles. Nevertheless, these studies show a satisfactory safety profile and encouraging findings at the biomarker level. Specifically, Chiocca et al. used a combined treatment of nivolumab administered seven days prior to surgery, and every two weeks following it, and VDX (controlled IL-12 gene therapy) given three hours before and fourteen days after surgery, in resectable recurrent glioblastoma patients. In this research, which was also reviewed briefly above, 21 patients were enrolled in three dose escalating cohorts to establish safe doses and biomarker response to the combined treatment. More than 60% of the patients exhibited grade-2 cytokine release syndrome, and nine patients exhibited nivolumab-related grade-3-and-above irAEs. Overall, treatment-related toxicities were considered tolerable and manageable as they were dose-dependent and easily predicted and reversed. This manageable safety profile and a postoperative increase in serum IL-12 and tumor IFN gamma levels led to an ongoing phase-II trial
[4].
In the second trial, Huang and colleagues studied a single dose of neoadjuvant pembrolizumab (anti-PD-1), within three weeks preceding surgery, in stage IIIB/C or IV melanoma. Twenty-nine patients were enrolled to evaluate whether tumor immune reinvigoration would be evident within three weeks post-treatment (at surgery), and whether this response was correlated with long-term cancer outcomes. All patients were prescribed adjuvant therapy with pembrolizumab. The study met the a priori safety requirement of less than 30% grade >3 toxicities; there were no unexpected treatment-related irEAs, no unexpected delays in surgery or adjuvant therapy, and no surgical complications. Additionally, 27/29 tumor tissues were available for analysis, 8/27 achieved major or complete pathological response and are disease-free to this day, and the median 1-year survival rate of all patients was 63%. Furthermore, an analysis of tumor-specific T cell subsets in the blood at 7 and 21 days post-treatment, and in the tumor following excision (within 21 days post-treatment), indicated an expansion of circulating tumor-specific T cell subsets as early as 7 days post-treatment, and in the excised tumor tissues. These findings demonstrate the quick immunological response following anti-PD-1 therapy, which was also associated with improved long-term cancer outcomes within each subject
[68].
Overall, ICI therapy in cancer patients is progressing rapidly; novel avenues of research are being explored, including an initial focus on the IPP, leading to an improvement in patients’ responses to therapy and a consequent improvement in long-term outcomes. Few clinical trials are currently exploring ICI therapy during the IPP (Table 1), which constitute a very small portion of ICI research, and clearly, more trials are warranted to elucidate the feasibility and efficacy of ICI therapy during the IPP.
Table 1. Summary of clinical studies employing immune checkpoint inhibitors.
| Phase |
Cancer Type |
Patient N |
Treatment |
Timing |
Citation |
| I |
Glioblastoma |
21 |
ICI + GT |
IPP |
[4] |
| III |
Melanoma |
676 |
ICI |
Non-IPP |
[57] |
| III |
Melanoma |
418 |
ICI + CT |
Non-IPP |
[58] |
| I/II |
Melanoma, NSCLC |
1260 |
ICI |
Non-IPP |
[59] |
| Ib |
Metastatic melanoma |
655 |
ICI |
Non-IPP |
[60] |
| III |
Bladder |
317 |
CT |
Non-IPP |
[63] |
| III |
Esophageal |
368 |
CT + RT |
Non-IPP |
[64] |
| III |
NSCLC |
505 |
ICI + CT |
Non-IPP |
[66] |
| II |
NSCLC |
44 |
ICI |
Non-IPP |
[67] |
| I |
Melanoma |
27 |
ICI |
IPP |
[68] |
2. Immunotherapy during the Immediate Perioperative Period: Limitations
The application of immunotherapies during the IPP presents new challenges to perioperative physicians, surgeons, and oncologists. The use of immunomodulatory agents during this period may (i) increase the incidence of irAEs during the IPP, (ii) hinder the success of surgery (e.g., impaired wound healing), and (iii) lessen the efficacy of immunotherapy due to stress- and/or surgery-induced immunosuppression. Some of these challenges may necessitate the use of immunosuppressive drugs, may delay surgery, and potentially involve life-threatening complications
[51].
For example, the use of immunomodulatory agents during the IPP, in conjunction with undetected surgery-related infection, may lead to an excessive inflammatory response, potentially resulting in SIRS (systemic inflammatory response syndrome) and organ failure
[69][70]. Additionally, high-dose IL-2 treatment, but not short-course low doses
[1][2], may induce a variety of adverse effects, including vascular leak syndrome, hypertension, renal failure, myocarditis, and thrombocytopenia, some of which may warrant a postponement of surgery and other supportive treatment
[71][72]. Furthermore, treatment with IL-2 may induce an expansion of immunosuppressive regulatory T cell populations, which may hinder antitumor responses
[71][72]. Similarly, ICIs may induce a range of systemic effects, depending on drug type, which can involve many organ systems, most commonly the skin, gastrointestinal, and endocrine gland systems, while uncommon and severe effects also involve pulmonary, cardiac, and neurologic systems
[73][74]. The predominant method to treat the emergences of therapy-induced severe irAEs is the cessation of therapy and administration of immunosuppressants (e.g., glucocorticoids), both of which may hinder the efficacy of the anticancer therapy.
While most irAEs induced by the immunotherapies discussed in this research were deemed tolerable and manageable
[55][56], the constraints mentioned above, among others, warrant the development of standardized guidelines for the IPP use of immunotherapeutic agents. For the time being, intricate considerations of timing, duration, dosage, and drug combination, in addition to the utilization of existing toxicity management guidelines (e.g., SITC ICI toxicity management
[75]) and assessment of patient- and organ-specific risk factors, should be considered for a clinical evaluation of immunotherapy during the IPP.
3. Conclusions
Immunotherapy has advanced tremendously during the past two decades, with an improvement in administration strategies and the development of new therapeutic approaches. Nevertheless, to avoid contraindications to surgery, the IPP remains relatively unexplored for antimetastatic immune interventions in cancer patients. The existing, yet limited, preclinical and clinical data demonstrate the safety and beneficial effects of immunotherapy during the IPP, yet further research is warranted, especially regarding the translation of preclinical findings into the clinical setting.
To minimize the gap between preclinical findings and their translational impact to the clinical setting, several key concerns and experimental settings should be taken into consideration. First, according to a recent review, fewer than 25% of studies that employed preclinical cancer models, studied processes related to metastasis, and out of these studies, fewer than 6% included a resection of a primary tumor, as in the clinical setting
[76]. Thus, an optimal design of a translational preclinical study should mimic the clinically relevant surgical setting in which immunotherapy is administered. Second, studies that utilize immune-deprived mice (to allow the transplantation of human cancer cells) should also be conducted in immune-competent mice, especially in the context of immunotherapy, possibly alongside humanized models
[77][78]. Third, preclinical studies that assess the impact of immunotherapy during the IPP on long-term cancer outcomes should account for several aspects that may jeopardize therapy potency, including presurgical stress
[79], ambient temperature
[80], treatment duration and timing relative to surgery
[47], and drug release platform (e.g., systemic vs. local and injection vs. suspended release)
[81]. Hopefully, these considerations will promote bench-to-bedside transitions of preclinical studies.
Using immunotherapy during the IPP, especially as a short-course neoadjuvant immunostimulatory application, may (i) circumvent cancer-treatment-induced immunosuppression (following chemotherapy, surgery, and sometimes radiotherapy), (ii) promote a rapid and nondelayed immunological response toward MRD, and (iii) avoid the deleterious effects of surgery on pre-existing metastases while exerting minimal toxicities. Additionally, current biomarker strategies to assess patients’ response to different types of immunotherapies, including the tumor neoantigen landscape, mutational burden, PD-L1 expression, and tumor-infiltrating lymphocyte landscape
[82][83][84] in tumor and/or liquid biopsies
[85], may enable accurate patient selection and improve the efficacy and safety of immunotherapy during the IPP. While pCR is considered a keystone in assessing clinical response to immunotherapy, it was shown, to some extent, to be inaccurate in predicting long-term clinical outcomes
[86][87], in part due to neglecting partial responses such as MPR
[87]. Thus, researchers suggest that future clinical studies should utilize multiple indications of clinical responses to achieve an accurate prediction of long-term clinical benefits alongside the routine approaches (e.g., pCR)
[82][88][89][90][91].
Importantly, perioperative factors which may hinder the efficacy of immunotherapies, such as stress and inflammatory responses
[92] and hypothermia
[93][94], should also be considered. As such factors may vary individually, a patient-personalized approach is warranted. For example, patients who exhibit effective immune responses may benefit more from perioperative immunotherapy, and patients who experience high perioperative stress and immunosuppressive responses may benefit more from immunotherapy when combined with stress management (e.g., inhibition of adrenergic signaling and prostaglandin synthesis), as researchers have previously shown in preclinical studies
[14][79]. Thus, future clinical studies assessing immunotherapy during the IPP may adopt biomarker-driven patient selection in combination with assessments of individual immune responsiveness and susceptibility to stress, while avoiding surgery- and hospitalization-related adverse effects.
Moreover, researchers believe that immunotherapeutic strategies (e.g., anti-stress-inflammatory approaches, ICIs, cytokines, TLR agonists, or oncolytic viruses) should be administered for a short and limited duration, and end or start as temporally close as possible to the surgery, considering the treatment efficacy and patient’s safety. While some immunotherapies may be administered up to a day prior to surgery (e.g., IL-2)
[36] or on the surgery day itself (e.g., inhibition of adrenergic signaling and prostaglandin synthesis)
[19], some were shown in preclinical studies (as elaborated above) to be most effective and exhibit minimal toxicities when administered at a specific time point during the IPP (e.g., anti-PD-1 + anti-CD137)
[46]. Thus, future clinical studies assessing immunotherapy use during the IPP, and specifically ICIs, should address the optimal timing of therapy during this critical timeframe.
Therefore, researchers suggest a combined approach to be tested in patients with early-stage resectable cancers, in which immunostimulatory treatment, such as ICI therapy, will be used for a limited duration (e.g., two cycles of two weeks), ending one to three weeks preoperatively. Additionally, complementing the latter, the inhibition of adrenergic signaling and prostaglandin synthesis during the week before and after surgical resection—in order to attenuate stress and inflammation, respectively—may be used to mitigate perioperative immunosuppression and the direct stimulation of MRD. Potentially, combinations of these drugs, when given in a relative temporal proximity to the surgery and for a short duration, may improve the efficacy of the immunostimulatory agent and may promote high response rates in patients while exerting minimal toxicities. The utilization of such therapy combinations during the IPP may constitute a novel treatment regimen to improve the long-term outcomes of cancer patients.