Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Verónica Fernández-Alvarez | -- | 3397 | 2023-08-19 17:17:49 | | | |
| 2 | Jason Zhu | -2 word(s) | 3395 | 2023-08-21 03:55:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fernández-Alvarez, V.; Linares-Sánchez, M.; Suárez, C.; López, F.; Guntinas-Lichius, O.; Mäkitie, A.A.; Bradley, P.J.; Ferlito, A. Biomarkers of Invasive Imaging for Carotid Plaque Vulnerability. Encyclopedia. Available online: https://encyclopedia.pub/entry/48242 (accessed on 08 February 2026).
Fernández-Alvarez V, Linares-Sánchez M, Suárez C, López F, Guntinas-Lichius O, Mäkitie AA, et al. Biomarkers of Invasive Imaging for Carotid Plaque Vulnerability. Encyclopedia. Available at: https://encyclopedia.pub/entry/48242. Accessed February 08, 2026.
Fernández-Alvarez, Verónica, Miriam Linares-Sánchez, Carlos Suárez, Fernando López, Orlando Guntinas-Lichius, Antti A. Mäkitie, Patrick J. Bradley, Alfio Ferlito. "Biomarkers of Invasive Imaging for Carotid Plaque Vulnerability" Encyclopedia, https://encyclopedia.pub/entry/48242 (accessed February 08, 2026).
Fernández-Alvarez, V., Linares-Sánchez, M., Suárez, C., López, F., Guntinas-Lichius, O., Mäkitie, A.A., Bradley, P.J., & Ferlito, A. (2023, August 19). Biomarkers of Invasive Imaging for Carotid Plaque Vulnerability. In Encyclopedia. https://encyclopedia.pub/entry/48242
Fernández-Alvarez, Verónica, et al. "Biomarkers of Invasive Imaging for Carotid Plaque Vulnerability." Encyclopedia. Web. 19 August, 2023.
Copy Citation
Carotid artery disease has traditionally been assessed based on the degree of luminal narrowing. However, this approach, which solely relies on carotid stenosis, is being questioned with regard to modern risk stratification approaches. Guidelines have introduced the concept of the “vulnerable plaque,” emphasizing specific features such as thin fibrous caps, large lipid cores, intraplaque hemorrhage, plaque rupture, macrophage infiltration, and neovascularization.
carotid artery disease
stroke
vulnerable plaque
optical coherence tomography
1. Introduction
Traditionally, the clinical assessment of carotid artery stenosis has been based on the degree of luminal narrowing, which has been considered the most reliable predictor of intervention according to international guidelines. The 2017 European Society of Vascular Surgery (ESVS) guidelines [1] and the 2017 European Society of Cardiology (ESC) guidelines [2] were the first to propose new criteria for identifying patients at a higher risk of stroke under best medical treatment (BMT), in whom carotid endarterectomy (CEA) or carotid artery stenting (CAS) might be targeted. Criteria include silent infarction on computed tomography (CT)/magnetic resonance imaging (MRI), 20% stenosis progression, large plaque area or large juxtaluminal black area (JBA) on computerized ultrasound plaque analysis, plaque echolucency, intraplaque hemorrhage (IPH) on MRI, impaired cerebral vascular reserve (CVR), and at least one spontaneous microembolic signal (MES) during 1 h of transcranial Doppler (TCD) monitoring [1][2].
Risk stratification based solely on carotid stenosis has become completely outdated. In fact, in the recently published 2023 ESVS guidelines, patients with only a single risk factor of carotid stenosis greater than 80% are no longer considered to be at high risk of stroke [3]. This stance has been partly influenced by a significant portion of the cohort studies published 20 to 30 years ago but also because of a meta-analysis published by Kamtchum Tatuene and the Asymptomatic Carotid Stenosis and Risk of Stroke Study (ACSRS) group. This demonstrated that increasing stenosis severity was an important predictor for late ipsilateral stroke, but only in the presence of concurrent risk factors [4].
These advancements in the knowledge of the natural progression of atherosclerosis has enabled the identification of distinct characteristics of carotid plaques that are linked to an elevated risk of stroke, consequently introducing the concept of the “vulnerable plaque”, referred to by some as the “unstable plaque” [5][6]. Several structural features have been identified as potential markers of vulnerability, including thin fibrous caps, large lipid cores, IPH, plaque rupture, high macrophage counts, and IPH. Inflammation may also play a role in plaque development and progression. These characteristics reflect an unstable plaque prone to rupture, leading to thrombotic events and subsequent ischemic strokes [7].
Several non-invasive imaging modalities, including US, CT, high-resolution MRI, and nuclear imaging techniques, have been used to identify these plaque characteristics with the aim of achieving an accurate risk stratification and providing guidance for clinical decision-making.
2. Carotid Intravascular Ultrasound (IVUS)
Yock et al. introduced IVUS in 1988 as the pioneering intravascular imaging device specifically designed for coronary applications [8]. Since then, the application of IVUS in carotid artery disease has been explored to assess plaque burden, morphology, and vulnerability, aiding in risk stratification and treatment decision-making.
2.1. Fundamental Concepts and Methodological Approaches in IVUS
IVUS utilizes miniature high-frequency transducers (20–45 MHz) placed within an angiographic catheter to obtain real-time, high-resolution images of vascular structures perpendicular to the long axis during pull-back. By analyzing the strength and characteristics of the echoes, IVUS provides valuable information regarding plaque composition and allows for the determination of signs of plaque instability [9].
Virtual Histology Intravascular Ultrasound (VH-IVUS)
Although grayscale IVUS can differentiate calcified from non-calcified plaques, it cannot accurately determine non-calcified plaque tissue composition due to post-processing limitations. VH-IVUS utilizes sophisticated radiofrequency analysis of echo signals to generate multiple spectral parameters, which are then converted into color histograms for the analysis of different plaque components [10]. By employing these techniques, VH-IVUS can classify plaques into four phenotypes: fibrous plaque, fibrolipid plaque, necrotic core, and dense calcium, providing a morphological evaluation of the plaque’s evolution [11]. The accuracy of VH-IVUS was validated against histology and has shown a sensitivity, specificity, and predictive accuracy for detecting a necrotic core of 60.1%, 93.0%, and 88.9%, respectively [12].
2.2. Features of a Vulnerable Carotid Plaque: Insights from IVUS Imaging
IVUS provides valuable information for the quantitative assessment of plaque thickness, cross-sectional area, plaque burden, and the remodeling index. It also allows for qualitative assessment, including the identification of the thin fibrous cap and the analysis of plaque composition. This analysis helps differentiate plaque components and assess plaque instability [9][12] (Figure 1).
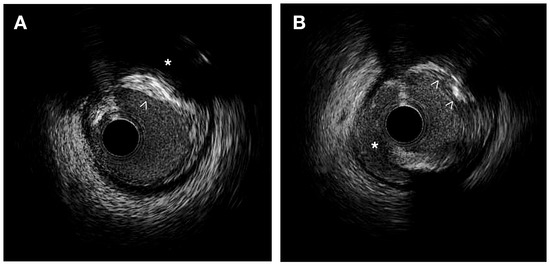
Figure 1. IVUS images. (A) Heterogeneous plaque with high echodensity areas (white arrowhead) and back shadowing (*) indicating calcification, as well as lower echodensity zones corresponding to a fibrous plaque. (B) Intimal disruption is associated with a dissection (*) and an irregular calcified plaque (white arrowheads).
Plaque Composition
Thin cap fibroatheroma (TCFA) is a type of plaque characterized by a plaque burden exceeding 40% and a large necrotic-rich core (>10%), without apparent fibrotic tissue observed in consecutive frames using VH-IVUS [13][14]. In the PROSPECT trial, the presence of a thin fibrous cap evaluated by VH-IVUS demonstrated a significant correlation with the subsequent risk of major adverse cardiac events in patients with acute coronary syndrome [14]. IVUS allows for the identification of lipid-rich plaques, which typically exhibit low echogenicity and appear as a hypoechoic or “dark” region within the vessel wall. In symptomatic patients, IVUS studies using integrated backscatter (IB) analysis have revealed higher percentages of lipid and smaller percentages of fibrous volumes, along with a greater plaque eccentricity, plaque burden, and remodeling index than in asymptomatic patients [15]. High-definition IVUS can identify IPH as an echolucent area with well-defined borders, typically appearing as a crescent-shaped region within the plaque [16]. Using grayscale IVUS, calcium appears as bright, dense, and acoustic shadowing regions and calcified nodules appear as distinct calcifications with an irregular, protruding, and convex luminal surface [17].
Plaque Morphology
IVUS allows the quantitative analysis of plaque thickness by means of the measurement of the distance between the luminal surface and the leading edge of the plaque. Comparing cross-sectional areas at different locations within the vessel helps to identify focal stenosis or diffuse disease. It also calculates the percentage of the vessel cross-sectional area occupied by the plaque corresponding to plaque burden. IVUS has confirmed that plaque erosion is characterized by an eccentric plaque with a thick fibrous cap while plaque rupture is observed when a hypoechoic cavity within the plaque is connected to the lumen [18]. Ruptured plaques typically exhibit eccentricity, reduced calcification, increased plaque burden, and positive remodeling, and are often associated with large thrombi [19].
Plaque Activity
IVUS-based assessment of inflammatory activity relies on the detection of features such as neovascularization and macrophage infiltration. IVUS can identify neovascularization as microvessels originating from the adventitial side and penetrating into the plaque. It also can calculate the remodeling index by dividing the external elastic membrane area at the site of maximal plaque burden by the average external elastic membrane area in reference segments [20]. The remodeling index reflects the adaptive response of the vessel wall to plaque formation. Numerous clinical studies have demonstrated that pre-interventional positive remodeling, as assessed by IVUS, predicts unfavorable short-term and long-term outcomes following percutaneous coronary intervention [21].
2.3. Clinical Practice Perspectives and Emerging Trends in IVUS
Diagnostic and Therapeutic Implications
IVUS plays a crucial role in guiding interventional procedures, providing real-time visualization of the vessel and the stented segment, aiding in optimal stent sizing, placement, expansion, and apposition [22]. IVUS enables the longitudinal assessment of plaque progression and regression over time and is relevant in evaluating the effectiveness of therapeutic interventions, such as lipid-lowering therapies or lifestyle modifications. Multiple studies using serial IVUS imaging have demonstrated that statin therapy can slow plaque progression and promote plaque regression in a dose-dependent manner [23][24].
In the prospective multicenter VICTORY registry study, IVUS and IVUS-VH examinations performed during carotid artery interventional therapy were deemed feasible and safe, offering valuable insights into the qualitative and quantitative composition of carotid plaques [25]. Another study conducted by Diethrich et al., entitled The Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL), demonstrated a strong correlation between VH-IVUS plaque characterization and the histological examination of plaques following endarterectomy, particularly for unstable plaque types. They found that the diagnostic accuracy varied with the composition of the plaque (from 99% in TCFA to 72% for calcified atheroma) [26].
Widespread adoption of IVUS to identify risk factors in asymptomatic patients in standard clinical practice is currently facing challenges due to its invasiveness and high cost. Although IVUS demonstrates high sensitivity and specificity in detecting large dense calcified plaques or spot calcifications [27], one of the limitations of IVUS is the limited axial resolution, ranging from 100 to 200 μm. This limitation hinders the identification of thin-fibrous-cap thickness, plaque disruption, macrophage infiltration, and thrombosis within plaques [27]. Combining multiple imaging modalities may help overcome these inherent limitations.
Advances in IVUS Technology
Traditional IVUS imaging provides two-dimensional cross-sectional images, limiting the assessment of plaque characteristics in the longitudinal plane. Three-dimensional (3D) IVUS allows for the reconstruction of volumetric images of the vessel and plaque, providing a more comprehensive assessment of plaque burden and morphology. Hybrid imaging systems that combine IVUS with NIRS or OCT catheters allow the simultaneous analysis of both vessel structure and plaque composition and have the potential to overcome the limitations of each technique, thus improving the accuracy of plaque characterization.
The integration of machine learning with artificial intelligence (AI) could be valuable in combining intravascular imaging findings with biomarkers to identify factors associated with plaque instability and progression [28].
3. Carotid Optical Coherence Tomography (OCT)
Traditionally, OCT has served as a non-invasive imaging diagnostic method that provides valuable insights into the functional blood vessels within the eye and allows for the study of various retinal conditions, such as macular telangiectasia, impaired perfusion, microaneurysms, capillary remodeling, certain types of intraretinal fluid, and neovascularization [29]. In 1991, Huang et al. introduced OCT as an intravascular imaging technique to overcome the limitations of angiography in visualizing the coronary artery [30]. Since 2010, the use of OCT has significantly expanded, particularly in the field of interventional cardiology, and by extension, in carotid artery disease.
3.1. Fundamental Concepts and Methodological Approaches in OCT
OCT is an invasive microscopic imaging technology that utilizes reflected near-infrared light and interferometry to generate high-resolution images of the tissue microstructure of the carotid artery with exceptional clarity [31][32].
The OCT system employs rapid scanning of the catheter to acquire multiple cross-sectional images, known as B-scans, along the length of the artery. The catheter is slowly withdrawn, and the scanning process is repeated at different pullback speeds to capture a three-dimensional representation of the structure of the vessel wall and assess plaque morphology, composition, and vulnerability [33] as well as stent positioning in carotid arteries [34].
3.2. Features of Vulnerable Carotid Plaque: Insights from OCT Imaging
In terms of vulnerable plaques, OCT provides visualization and assessment of various characteristics such as cap thickness (with thin caps defined as those ≤65 μm), lipid core detection, calcification, cholesterol crystallization, IPH, plaque erosion, plaque rupture or thrombi, and neovascularization. Moreover, OCT allows for the observation and quantification of inflammation within unstable plaques by measuring macrophage infiltration in the fibrous cap and subintimal lipid accumulation [35]. Previous studies have validated the use of OCT for assessing various characteristics of atherosclerotic plaque using histologic controls. [31][35][36] (Figure 2).
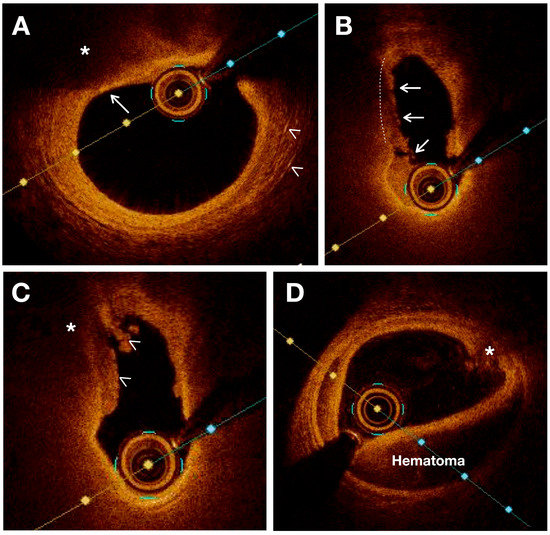
Figure 2. OCT images showing various characteristics of vulnerable plaques. (A) Thin fibrous plaque (white arrow) overlying a large lipid pool or necrotic core (*). White arrowheads indicate the presence of cholesterol crystals. (B) Plaque rupture (white lines) is sealed with thrombus (white arrows). (C) Intraluminal thrombus (white arrowheads) covers a disrupted plaque with necrotic core (*) (D) Intramural hematoma and intimal disruption (*) are observed.
Plaque Composition
Lipid cores appear as low-intensity regions with distinct borders on OCT images. Necrotic cores appear as a low-signal area in OCT imaging with an indistinct border, an absence of backscattering signal, and a rapid signal drop-off. OCT also demonstrates high sensitivity and specificity in detecting lipid-rich plaques, as verified by autopsy specimens (90–94% and 90–92%, respectively) [37]. OCT can also visualize fresh and organized intraplaque hemorrhage as high-intensity regions within the plaque due to the presence of red blood cells. Unlike IVUS, OCT can penetrate plaque calcification and provide detailed information regarding its thickness, area, and volume [38]. As a result, the calcified nodules are distinctly delineated from other components of the plaque with a very high sensitivity (96%) and specificity (97%) [36][39].
Plaque Morphology
OCT provides a highly detailed visualization of intimal thickening, where the intima layer exhibits a strong backscattering signal at its internal boundary, gradually decreasing in intensity towards the outer layers [40]. Measurements of cap thickness by OCT have been associated with the prevalence of plaque rupture [22]. The hallmark of plaque erosion is represented by a thrombus covering a non-disrupted fibrous cap. On the other hand, plaque ulceration is observed in OCT imaging as an intra-plaque cavity while plaque erosion can occur without involvement of the lesion’s lipid core [39].
Plaque Activity
In OCT imaging, inflammation is characterized by a highly intense speckle signal observed in regions adjacent to the fibroatheroma, varying in size. It is crucial to differentiate these areas from cholesterol crystals, elastic lamina, or calcium deposits [41]. It should be noted that OCT indications of inflammation can only be interpreted when a fibrous plaque is present, as there is currently no confirmed information regarding the significance of images suggesting macrophage accumulation in other regions of the vascular wall [41].
3.3. Clinical Practice Perspectives and Emerging Trends in OCT
Diagnostic and Therapeutic Implications
The clinical applications of OCT in carotid stenosis encompass both diagnostic and therapeutic purposes. The use of OCT in characterizing vulnerable carotid plaques has significant clinical implications for risk stratification and patient management. By providing detailed information on plaque morphology, fibrous cap thickness, lipid-rich regions, and surface features, OCT can help to identify high-risk plaques that are prone to rupture and subsequent ischemic events. This information can assist in determining the optimal treatment strategy for individual patients and the need for invasive interventions.
OCT plays a crucial role in guiding therapeutic interventions for carotid artery disease. It provides real-time feedback during interventions, revealing features that were not visualized using other imaging modalities like CT and MR angiography, such as free intraluminal thrombus, dissection, TCFA with an underlying ulcerative plaque and filling defects inside or adjacent to stents, or an undersized stent [42]. In view of this, several studies have described the use of OCT imaging to guide appropriate endovascular therapy, allowing for accurate stent placement and the detection of plaque prolapse and stent strut malposition during CAS [42][43][44][45][46].
However, the prognostic value of plaque morphology and composition in terms of stroke risk has not been established in large prospective studies. Therefore, it remains challenging to set up OCT in routine clinical practice and determine its impact on patient outcomes.
Advances in OCT Technology
Improvements in imaging devices, including a higher resolution and faster acquisition rates, are expanding the capabilities of OCT in visualizing microstructural details. Additionally, the development of advanced image analysis algorithms and machine learning techniques has enabled automated identification and quantification of plaque features, reducing the subjectivity and time required for interpretation. Regardless, there is a need to develop a validated algorithm for plaque characterization that can help to facilitate the standardization of OCT image detection of plaque instability. This goal has been achieved by He et al., who designed a machine learning algorithm for the characterization of atherosclerotic plaque components by intravascular OCT using ex vivo carotid plaque tissue samples [47]. A total of 31 patients underwent carotid endarterectomy and the ex vivo carotid plaques were imaged with OCT. The algorithm was validated against histology slices, and it was capable of characterizing the fibrous, calcified, and lipid tissue of the carotid plaque with an excellent accuracy using the combined feature set [47].
4. Near-Infrared Spectroscopy (NIRS)
Near-infrared spectroscopy (NIRS) is a novel imaging technique that utilizes near-infrared light to analyze the absorption pattern of cholesterol molecules within the vessel wall, enabling the detection of lipid-rich plaques with high accuracy [48]. NIRS also provides valuable information about cerebral hemodynamic conditions and has the potential to serve as a brain monitor in various clinical scenarios, especially during carotid endarterectomy [49]. While commonly used as a non-invasive technique, a catheter-based NIRS proved to be accurate in detecting high lipid core plaque in atherosclerotic plaques. In 2002, Moreno et al. reported the successful application of NIRS in detecting lipid-rich necrotic cores (LRNC) in human aortic specimens. Histological analysis demonstrated NIRS’s high sensitivity and specificity, with values of 90% and 93% for identifying lipid pools, and 77% and 93% for identifying thin caps, respectively [48].
However, there are limitations that have hindered its independent use in clinical settings. Firstly, NIRS only provides information regarding the lipid composition of plaques and does not offer a comprehensive morphological assessment. Secondly, it cannot visualize or evaluate the size of the lumen, external vessel wall, or plaque burden. Lastly, NIRS lacks the depth resolution required to precisely locate the necrotic core within the plaque and differentiate TCFA from thick-cap fibroatheromas.
It is worth noting that NIRS provides limited anatomical information and is commonly used in conjunction with intravascular ultrasound (IVUS) to generate a “chemogram” or probability map. The chemogram represents the pullback position in millimeters on the X-axis and the circumferential position in degrees on the Y-axis, resembling the longitudinal splitting of the coronary vessel. In studies comparing NIRS to IVUS alone, NIRS has demonstrated superior performance in identifying lipid core plaques [50]. Some studies have explored the combination of NIRS with OCT probes to further enhance the system’s accuracy [51]. Its integration with other imaging modalities and further advancements in technology are expected to overcome its limitations and enhance its clinical utility in the future.
5. Hybrid Intravascular Imaging Modalities
Multimodal imaging approaches, combining different imaging modalities such as IVUS, OCT, and NIRS, have emerged to overcome the limitations of individual techniques and provide a comprehensive assessment of plaque morphology and composition as well as a prediction of disease progression. Studies have shown that a hybrid approach with IVUS and NIRS imaging is particularly advantageous in identifying the distribution of lipid core plaques and exploring the relationship between vascular geometry, shear stress, and plaque composition [52]. While IVUS alone can detect fibrous atherosclerotic plaques, which may be obscured by the presence of calcification, NIRS can detect lipids even in the presence of calcifications [53]. In the PACMANAMI randomized clinical trial, the combination of IVUS and NIRS was successfully utilized to evaluate the effect of statins on plaque burden and composition [54]. The ATHEROREMO-IVUS study and other recent prospective studies have suggested that IVUS-NIRS can serve as a diagnostic tool in clinical practice for detecting unstable plaques, especially fatty plaques, and identifying patients at high risk of subsequent major adverse cardiovascular events [55].
However, IVUS-NIRS also has limitations as the low resolution of IVUS affects the evaluation of cap thickness and luminal boundary definition in the presence of thrombosis or severe intraplaque bleeding. Alternatively, it has been suggested to integrate the two approaches of IVUS and OCT in order to benefit from the deep penetration of IVUS and the high resolution of OCT [25]. The application of IVUS-OCT has demonstrated improved imaging characteristics and provided supplementary information for detecting TCFA [56]. Furthermore, OCT-NIRS catheters have been developed to acquire OCT and NIRS data in a pull-back manner, combining the advantage of NIRS in identifying lipid core components with the advantage of OCT in determining fibrous cap thickness over lipid pools [57]. Additionally, other innovative multimodal imaging techniques such as OCT–near-infrared fluorescence (NIRF), IVUS–NIRF, IVUS–intravascular photoacoustic imaging (IVPA), and IVUS–fluorescence lifetime imaging microscopy (FLIM) are currently undergoing preclinical evaluation [58][59][60][61].
6. Carotid Angiography
Carotid angiography is considered to be the gold standard in evaluating carotid artery disease. In the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and European Carotid Surgery Trial (ECST), angiography served as the reference standard for assessing luminal stenosis in carotid extracranial disease [62][63]. Based on these trials, stenosis emerged as a crucial factor in determining stroke risk.
In as early as 1978, Moore et al. noted that the presence of ulceration observed on angiography could identify patients at high risk of subsequent strokes [64]. In the initial 500 patients enrolled in NASCET, angiography was performed to detect ulceration and was subsequently compared to observations during endarterectomy. The sensitivity and specificity of angiography in detecting ulcerated plaques were 46% and 74%, respectively. The positive predictive value for identifying an ulcer was 72% [65]. A similar study design was employed in ECST, involving 1671 patients, with sensitivities and specificities for ulceration of 69% and 47%, respectively [66]. Studies that compared the radiological appearance with the histology of resected plaques showed a wide range of sensitivity and specificity, indicating substantial variability in the results [67][68][69]. Therefore, due to this variability, angiography provides little information regarding the actual risk of plaque instability.
References
- Naylor, R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice-Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81.
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177.
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111.
- Kamtchum-Tatuene, J.; Wilman, A.; Saqqur, M.; Shuaib, A.; Jickling, G.C. Carotid plaque with high-risk features in embolic stroke of undetermined source: Systematic review and meta-analysis. Stroke 2020, 51, 311–314.
- Fayad, Z.A.; Fuster, V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ. Res. 2001, 89, 305–316.
- Skagen, K.; Skjelland, M.; Zamani, M.; Russell, D. Unstable carotid artery plaque: New insights and controversies in diagnostics and treatment. Croat. Med. J. 2016, 57, 311–320.
- Nighoghossian, N.; Derex, L.; Douek, P. The vulnerable carotid artery plaque: Current imaging methods and new perspectives. Stroke 2005, 36, 2764–2772.
- Yock, P.G.; Linker, D.T.; Angelsen, B.A. Two-dimensional intravascular ultrasound: Technical development and initial clinical experience. J. Am. Soc. Echocardiogr. 1989, 2, 296–304.
- Yamagishi, M.; Tereshima, M.; Awano, K.; Kijima, M.; Nakatani, S.; Daikoku, S.; Ito, K.; Yasumura, Y.; Miyatake, K. Morphology of vulnerable coronary plaque: Insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J. Am. Coll. Cardiol. 2000, 35, 106–111.
- Cismaru, G.; Serban, T.; Tirpe, A. Ultrasound methods in the evaluation of atherosclerosis: From pathophysiology to clinic. Biomedicines 2021, 9, 418.
- Shin, E.S.; Garcia-Garcia, H.M.; Serruys, P.W. A new method to measure necrotic core and calcium content in coronary plaques using intravascular ultrasound radiofrequency-based analysis. Int. J. Cardiovasc. Imaging 2010, 26, 387–396.
- Nasu, K.; Tsuchikane, E.; Katoh, O.; Vince, D.G.; Virmani, R.; Surmely, J.F.; Murata, A.; Takeda, Y.; Ito, T.; Ehara, M.; et al. Accuracy of in vivo coronary plaque morphology assessment: A validation study of in vivo virtual histology compared with in vitro histopathology. J. Am. Coll. Cardiol. 2006, 47, 2405–2412.
- Rodriguez-Granillo, G.A.; Garcia-Garcia, H.M.; Fadden, E.P.M.; Valgimigli, M.; Aoki, J.; de Feyter, P.; Serruys, P.W. In Vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J. Am. Coll. Cardiol. 2005, 46, 2038–2042.
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristae, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235.
- Sano, K.; Kawasaki, M.; Ishihara, Y.; Okubo, M.; Tsuchiya, K.; Nishigaki, K.; Zhou, X.; Minatoguchi, S.; Fujita, H.; Fujiwara, H. Assessment of vulnerable plaques causing acute coronary syndrome using integrated backscatter intravascular ultrasound. J. Am. Coll. Cardiol. 2006, 47, 734–741.
- Ohashi, H.; Ando, H.; Otsuka, F.; Takashima, H.; Amano, T. Histopathologically confirmed intraplaque haemorrhage in a patient with unstable angina. Eur. Heart J.-Cardiovasc. Imaging 2022, 23, e165.
- Lee, J.B.; Mintz, G.S.; Lisauskas, J.B.; Biro, S.G.; Pu, J.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Goldstein, J.; Stone, G.W.; et al. Histopathologic Validation of the intravascular ultrasound diagnosis of calcified coronary artery nodules. Am. J. Cardiol. 2011, 108, 1547–1551.
- Kusama, I.; Hibi, K.; Kosuge, M.; Nozawa, N.; Ozaki, H.; Yano, H.; Sumita, S.; Tsukahara, K.; Okuda, J.; Ebina, T.; et al. Impact of plaque rupture on infarct size in ST-segment elevation anterior acute myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 1230–1237.
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From Vulnerable Plaque to Vulnerable Patient A Call for New Definitions and Risk Assessment Strategies: Part I. Circulation 2003, 108, 1664–1672.
- Yonetsu, T.; Jang, I.K. Advances in intravascular imaging: New insights into the vulnerable plaque from imaging studies. Korean Circ. J. 2010, 48, 1–15.
- Okura, H.; Morino, Y.; Oshima, A.; Hayase, M.; Ward, M.R.; Popma, J.J.; Kuntz, R.E.; Bonneau, H.N.; Yock, P.G.; Fitzgerald, P.J. Preintervention arterial remodeling affects clinical outcome following stenting: An intravascular ultrasound study. J. Am. Coll. Cardiol. 2001, 37, 1031–1035.
- Tian, J.; Ren, X.; Vergallo, R.; Xing, L.; Yu, H.; Jia, H.; Soeda, T.; McNulty, I.; Hu, S.; Lee, H.; et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: A combined optical coherence tomography and intravascular ultrasound study. J. Am. Coll. Cardiol. 2014, 63, 2209–2216.
- Nicholls, S.J.; Hsu, A.; Wolski, K.; Hu, B.; Bayturan, O.; Lavoie, A.; Uno, K.; Tuzcu, E.M.; Nissen, S.E. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J. Am. Coll. Cardiol. 2010, 55, 2399–2407.
- Ahmadi, A.; Narula, J. Primary and secondary prevention, or subclinical and clinical atherosclerosis. JACC Cardiovasc. Imaging 2017, 10, 447–450.
- Sangiorgi, G.; Bedogni, F.; Sganzerla, P.; Binetti, G.; Inglese, L.; Musialek, P.; Esposito, G.; Cremonesi, A.; Biasi, G.; Jakala, J.; et al. The Virtual histology In CaroTids Observational RegistrY (VICTORY) study: A European prospective registry to assess the feasibility and safety of intravascular ultrasound and virtual histology during carotid interventions. Int. J. Cardiol. 2013, 168, 2089–2093.
- Diethrich, E.B.; Margolis, M.P.; Reid, D.B.; Burke, A.; Ramaiah, V.; Rodriguez-Lopez, J.A.; Wheatley, G.; Olsen, D.; Virmani, R. Virtual histology intravascular ultrasound assessment of carotid artery disease: The Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) Study. J. Endovasc. Ther. 2007, 14, 676–686.
- Van Veelen, A.; Van der Sangen, N.; Delewi, R.; Beijk, M.; Henriques, J.; Claessen, B. Detection of vulnerable coronary plaques using invasive and non-invasive imaging modalities. J. Clin. Med. 2022, 11, 1361.
- Roy-Cardinal, M.H.; Destrempes, F.; Soulez, G.; Cloutier, G. Assessment of carotid artery plaque components with machine learning classification using homodyned-K parametric maps and elastograms. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 493–504.
- Kashani, A.H.; Chen, C.L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100.
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.; GChang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181.
- Kume, T.; Uemura, S. Current clinical applications of coronary optical coherence tomography. Cardiovasc. Interv. Ther. 2018, 33, 1–10.
- Spacek, M.; Zemanek, D.; Hutyra, M.; Sluka, M.; Taborsky, M. Vulnerable atherosclerotic plaque—A review of current concepts and advanced imaging. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2018, 162, 10–17.
- Xu, X.; Huang, F.; Shi, X.; Liu, R.; Han, Y.; Li, M.; Wang, F.; Yang, Q.; Zhu, W.; Ye, R.; et al. Optical Coherence Tomography Evaluation of Carotid Artery Stenosis and Stenting in Patients With Previous Cervical Radiotherapy. Front. Neurosci. 2022, 16, 861511.
- Uemura, S.; Ishigami, K.; Soeda, T.; Okayama, S.; Sung, J.H.; Nakagawa, H.; Somekawa, S.; Takeda, Y.; Kawata, H.; Horii, M.; et al. Thincap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur. Heart J. 2012, 33, 78–85.
- Tearney, G.J.; Yabushita, H.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Halpern, E.F.; Bouma, B.E. Quantification of macrophages content in atherosclerotic plaques by optical coherence tomography. Circulation 2003, 107, 113–119.
- Yabushita, H.; Bouma, B.E.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Kang, D.H.; Halpern, E.F.; et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002, 106, 1640–1645.
- Takahashi, S.; Kawasaki, M.; Miyata, S.; Suzuki, K.; Yamaura, M.; Ido, T.; Aoyama, T.; Fujiwara, H.; Minatoguchi, S. Feasibility of tissue characterization of coronary plaques using 320- detector row computed tomography: Comparison with integrated backscatter intravascular ultrasound. Heart Vessels. 2016, 31, 29–37.
- Mushenkova, N.V.; Summerhill, V.I.; Zhang, D.; Romanenko, E.B.; Grechko, A.V.; Orekhov, A.N. Current advances in the diagnostic imaging of atherosclerosis: Insights into the pathophysiology of vulnerable plaque. Int. J. Mol. Sci. 2020, 21, 2992.
- Spinu, M.; Olinic, D.M.; Olinic, M.; Homorodean, C. In vivo imaging of complicated atherosclerotic plaque—Role of optical coherence tomography (OCT). Rom. J. Morphol. Embryol. 2018, 59, 469–478.
- Otsuka, F.; Joner, M.; Prati, F.; Virmani, R.; Narula, J. Clinical classification of plaque morphology in coronary disease. Nat. Rev. Cardiol. 2014, 11, 379–389.
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072.
- Pasarikovski, C.; Ramjist, J.; da Costa, L.; Black, S.; Cardinell, J.; Yang, V. Optical coherence tomography as an adjunct during carotid artery stenting for carotid atherosclerotic disease. Clin. Neuroradiol. 2020, 30, 503–509.
- Dohad, S.; Zhu, A.; Krishnan, S.; Wang, F.; Wang, S.; Cox, J.; Henry, T.D. Optical coherence tomography guided carotid artery stent procedure: Technique and potential applications. Cathet. Cardiovasc. Intervent. 2018, 91, 521–530.
- Jones, M.R.; Attizzani, G.F.; Given, C.A., 2nd; Brooks, W.H.; Costa, M.A.; Bezerra, H.G. Intravascular frequency-domain optical coherence tomography assessment of atherosclerosis and stent-vessel interactions in human carotid arteries. AJNR Am. J. Neuroradiol. 2012, 33, 1494–1501.
- Harada, K.; Kajihara, M.; Sankoda, Y.; Taniguchi, S. Efficacy of post-dilatation during carotid artery stenting for unstable plaque using closed-cell design stent evaluated by optical coherence tomography. J. Neuroradiol. 2019, 46, 384–389.
- De Donato, G.; Setacci, F.; Sirignano, P.; Galzerano, G.; Cappelli, A.; Setacci, C. Optical coherence tomography after carotid stenting: Rate of stent malapposition, plaque prolapse and fibrous cap rupture according to stent design. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 579–587.
- He, C.; Li, Z.; Wang, J.; Huang, Y.; Yin, Y.; Li, Z. Atherosclerotic Plaque Tissue Characterization-An OCT-Based Machine Learning Algorithm With ex vivo Validation. Front. Bioeng. Biotechnol. 2020, 8, 749.
- Moreno, P.R.; Lodder, R.A.; Purushothaman, K.R.; Charash, W.E.; O’Connor, W.N.; Muller, J.E. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation 2002, 105, 923–927.
- Smith, M. Shedding light on the adult brain: A review of the clinical applications of near-infrared spectroscopy. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4452–4469.
- Kang, S.J.; Mintz, G.S.; Pu, J.; Sum, S.T.; Madden, S.P.; Burke, A.P.; Xu, K.; Goldstein, J.A.; Stone, G.W.; Muller, J.E.; et al. Combined IVUS and NIRS detection of fibroateheromas: Histopatological validation in human coronary arteries. J. Am. Coll. Cardiol. Img. 2015, 8, 184–194.
- Fard, A.M.; Vacas-Jacques, P.; Hamidi, E.; Wang, H.; Carruth, R.W.; Gardecki, J.A.; Tearney, G.J. Optical coherence tomography—near infrared spectroscopy system and catheter for intravascular imaging. Opt. Express 2013, 21, 30849–30858.
- Ono, M.; Kawashima, H.; Hara, H.; Gao, C.; Wang, R.; Kogame, N.; Takahashi, K.; Chichareon, P.; Modolo, R.; Tomaniak, M.; et al. Advances in IVUS/OCT and Future Clinical Perspective of Novel Hybrid Catheter System in Coronary Imaging. Front. Cardiovasc. Med. 2020, 7, 119, Erratum in: Front. Cardiovasc. Med. 2020, 7, 594899.
- Weng, S.T.; Lai, Q.L.; Cai, M.T.; Wang, J.J.; Zhuang, L.Y.; Cheng, L.; Mo, Y.J.; Liu, L.; Zhang, Y.X.; Qiao, S. Detecting vulnerable carotid plaque and its component characteristics: Progress in related imaging techniques. Front. Neurol. 2022, 13, 982147.
- Räber, L.; Ueki, Y.; Otsuka, T.; Losdat, S.; Häner, J.D.; Lonborg, J.; Fahrni, G.; Iglesias, J.F.; van Geuns, R.J.; Ondracek, A.S.; et al. PACMAN-AMI collaborators. Effect of Alirocumab Added to High-Intensity Statin Therapy on Coronary Atherosclerosis in Patients With Acute Myocardial Infarction: The PACMAN-AMI Randomized Clinical Trial. JAMA 2022, 327, 1771–1781.
- De Boer, S.; Baran, Y.; Garcia-Garcia, H.M.; Eskin, I.; Lenzen, M.J.; Kleber, M.E.; Regar, E.; de Jaegere, P.J.; Ligthart, J.M.; van Geuns, R.J.; et al. The European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis—Intravascular Ultrasound (ATHEROREMO-IVUS) study. EuroIntervention 2018, 14, 194–203.
- Lv, R.; Maehara, A.; Matsumura, M.; Wang, L.; Zhang, C.; Huang, M.; Guo, X.; Samady, H.; Giddens, D.P.; Zheng, J.; et al. Using optical coherence tomography and intravascular ultrasound imaging to quantify coronary plaque cap stress/strain and progression: A follow-up study using 3D thin-layer models. Front. Bioeng. Biotechnol. 2021, 9, 713525.
- Muller, J.; Madder, R. OCT-NIRS Imaging for Detection of Coronary Plaque Structure and Vulnerability. Front. Cardiovasc. Med. 2020, 7, 90.
- Yoo, H.; Kim, J.W.; Shishkov, M.; Namati, E.; Morse, T.; Shubochkin, R.; McCarthy, J.R.; Ntziachristos, V.; Bouma, B.E.; Jaffer, F.A.; et al. Intraarterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat. Med. 2011, 17, 1680–1684.
- Peng, C.; Wu, H.; Kim, S.; Dai, X.; Jiang, X. Recent advances in transducers for intravascular ultrasound (IVUS) imaging. Sensors 2021, 21, 3540.
- Sowers, T.; VanderLaan, D.; Karpiouk, A.; Onohara, D.; Schmarkey, S.; Rousselle, S.; Padala, M.; Emelianov, S. In vivo safety study using radiation at wavelengths and dosages relevant to intravascular imaging. J. Biomed. Opt. 2022, 27, 016003.
- Alfonso-Garcia, A.; Bec, J.; Weyers, B.; Marsden, M.; Zhou, X.; Li, C.; Marcu, L. Mesoscopic fluorescence lifetime imaging: Fundamental principles, clinical applications and future directions. J. Biophoton. 2021, 14, e202000472.
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial: Surgical results in 1415 patients. Stroke 1999, 30, 1751–1758.
- Warlow, C.P. Symptomatic patients: The European Carotid Surgery Trial (ECST). J. Mal. Vasc. 1993, 18, 198–201.
- Moore, W.S.; Boren, C.; Malone, J.M.; Roon, A.J.; Eisenberg, R.; Goldstone, J.; Mani, R. Natural history of nonstenotic, asymptomatic ulcerative lesions of the carotid artery. Arch. Surg. 1978, 113, 1352–1359.
- Streifler, J.Y.; Eliasziw, M.; Fox, A.J.; Benavente, O.R.; Hachinski, V.C.; Ferguson, G.G.; Barnett, H.J. Angiographic detection of carotid plaque ulceration. Comparison with surgical observations in a multicenter study. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994, 25, 1130–1132.
- Rothwell, P.M.; Gibson, R.; Warlow, C.P. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 2000, 31, 615–621.
- Lovett, J.K.; Gallagher, P.J.; Hands, L.J.; Walton, J.; Rothwell, P.M. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation 2004, 110, 2190–2197.
- O’Donnell, T.F., Jr.; Erdoes, L.; Mackey, W.C.; McCullough, J.; Shepard, A.; Heggerick, P.; Isner, J.; Callow, A.D. Correlation of B-mode ultrasound scan imaging and arteriography with pathologic findings at carotid endarterectomy. Arch. Surg. 1985, 120, 443–449.
- Kim, D.I.; Lee, S.J.; Lee, B.B.; Kim, Y.I.; Chung, C.S.; Seo, D.W.; Lee, K.H.; Ko, Y.H.; Kim, D.K.; Do, Y.S.; et al. The relationship between the angiographic findings and the clinical features of carotid artery plaque. Surg. Today 2000, 30, 37–42.
More
Information
Subjects:
Cardiac & Cardiovascular Systems; Neuroimaging
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
547
Revisions:
2 times
(View History)
Update Date:
21 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




