Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Malay Kumar Adak | -- | 2509 | 2023-08-18 09:12:47 | | | |
| 2 | Lindsay Dong | Meta information modification | 2509 | 2023-08-21 04:48:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Adak, M.K.; Das, A.; Kundu, A.; Chatterjee, M.; Hasanuzzaman, M. Anoxia Tolerance in Rice Seeds under Submergence. Encyclopedia. Available online: https://encyclopedia.pub/entry/48210 (accessed on 07 February 2026).
Adak MK, Das A, Kundu A, Chatterjee M, Hasanuzzaman M. Anoxia Tolerance in Rice Seeds under Submergence. Encyclopedia. Available at: https://encyclopedia.pub/entry/48210. Accessed February 07, 2026.
Adak, Malay Kumar, Abir Das, Ankita Kundu, Mitali Chatterjee, Mirza Hasanuzzaman. "Anoxia Tolerance in Rice Seeds under Submergence" Encyclopedia, https://encyclopedia.pub/entry/48210 (accessed February 07, 2026).
Adak, M.K., Das, A., Kundu, A., Chatterjee, M., & Hasanuzzaman, M. (2023, August 18). Anoxia Tolerance in Rice Seeds under Submergence. In Encyclopedia. https://encyclopedia.pub/entry/48210
Adak, Malay Kumar, et al. "Anoxia Tolerance in Rice Seeds under Submergence." Encyclopedia. Web. 18 August, 2023.
Copy Citation
Submergence in rice fields creating inundation stress and realizing anoxia or hypoxia is a problem in agriculture. Seeds under this oxygen deficit are faced with fermentative respiration, where the end product would be poisoning the tissue viability. This is more aggravated in direct seeded rice cultivation with the accumulation of lactate as a poison.
tolerance
genetic variabilities
rice
1. Introduction
Rice, a semi-aquatic plant, requires a significant amount of water and tissue hydration throughout its developmental stages. The germination of rice seeds requires more soil moisture than other crops, up to the level of full submergence. Seeds of rice require hypoxia or anoxia under inundation conditions for germination [1]. The germination of rice seeds under hypoxic conditions induces one type of carbohydrate metabolism for starch and mobilization via enzymatic induction. The ability of rice seeds to tolerate hypoxia or anoxia also offers direct seeding in agronomic practices [2]. Direct seeding is more economic than seedling transplantation, where the availability of complete submergence is frequent. Herein, the chances of weed intolerance from floods are highly possible and obstructed by infestation from pests [3]. No matter the species, rice seeds are sensitive to submergence for germination and few cultivars have a better germination rate or seedling tolerance to hypoxia. Tolerant rice seeds exhibit better coleoptile elongation, the induction of carbohydrates, fermentative pathways, and reduced oxidative damages indicating stress tolerance [4].
2. Physiological Relevance of Seeds’ Response to Anoxia
The germination of seeds, establishment of seedlings, and development into plants are subjected to flooding stress. Therefore, seed development under anoxic conditions would be sensitive to anoxia or hypoxia [5]. When flooding occurs during seed germination (under complete submergence), a distinct shift into anaerobic metabolism is observed. Rice plants that are semi-aquatic in nature can experience withstanding anaerobic respiration mostly for maintaining respiration, meaning seed tissues are viable under the water. Coleoptiles of sprouting seeds are exposed to anoxia, and total carbohydrate metabolism is shifted toward anaerobic respiration, mostly via alcoholic fermentation. A number of genes like alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC) are the most important in inducing fermentation metabolism [6]. Even rice seeds, which are dormant in nature, also remain non-viable under water. The dormancy of rice seeds and their further progress under water are regulated by an endogenous ratio of abscisic acid (ABA) to gibberellic acid (GA) [7]. The major signaling gene(s) for ABA metabolism in rice show their expression with varied modalities through the anaerobic phases. The genes that are predominantly involved include OsABI5, OsDSG1, OsNCED3, OsVP1, OsPDS, and OsZDS for ABA metabolism [8]. These are actual key genes governing seed dormancy and germination, and are mostly required for the signaling and metabolism of ABA [9].
3. Hormonal Interplay between Seed Dormancy and Germination in Rice under Water
It is evident that seed dormancy and germination play a key role in plant initial survival against submergence. Growth regulators like ABA and GA and their ratio compete with each other, favoring either dormancy or germination depending on the physiological needs [10]. ABA suppresses the physiologically assisted genes for germination whereas GAs can erase the effects. Embryo activity at the cellular level overcoming mechanical distances via tests is important. All of the activity of developing embryos is regulated by GA-assisted genes. The latter, with their increasing rates of transcription and translation, may support ABA-suppressed activities of quiescence strategies [11]. In seeds, a precise concentration of GA is maintained by a balance between biosynthesis and its turnover via oxidative degradation. Regulation for de novo biosynthesis is also important in the context of GA activity on seedling development. This becomes more complicated given that under water a significant level of reactive oxygen species (ROS) and Ca2+-dependent signaling are prerequisites. This may integrate downstream pathways for anoxia tolerance and the germination of seeds [12]. This is explained more by the fact that ROS can upregulate a number of ABA-metabolizing gene (particularly ABAI3 and AP2) transcription factors (TFs). Auxins (Au) are also involved to allow the binding of a few TFs, and thereby they induce downstream genes related to ABA metabolism. Au, like GA involvement, in this aspect may concern other TFs like AP2 and DELLA and other proteins. These often cause GA oxidation pathways involving genes like GA20ox, GA3ox, and PIL5 TF [13]. After the ripening of embryos in seeds, a number of genes are involved, like RGA, RGL2, and GAI, from different insensitive mutants to GA. For the up- or downregulation of ABA-inducing genes, growth substances are involved including jasmonic acid (JA), brassinosteroid (BR), ethylene (ET), salicylic acid (SA), etc. [14]. ABA, through its auxin inductive cascade, can induce a number of genes via some auxin response factors (ARF10/16). These factors are involved to allow the binding of other TFs. Likewise, ABA can induce MYB96 factor, which otherwise induces TF ABI4. The latter is responsible for the suppression of CYP707A1/2 factor activity, which is likely to bind the α-amylase promoter and promotes seed germination [15]. ABA can induce other TFs (ABI5, BIN2, PKS5, etc.) which also inhibit seed germination. All of these are related to the precise concentration of ROS, which collectively are key balancing factors for gene regulation. ABA, under anaerobic conditions, is more active with regard to its regulation of catabolism as well. On the seeds and vegetative parts of plants, a number of ABA receptor components are grouped and these regulate protein phosphatase 2C (PP2C) when ABA is present. PP2C is inactive under aerobic conditions but is activated with sucrose non-fermenting 1 (SNF1)-related kinase 2. The latter allows for specific response element binding TFs [16]. Increased dormancy is also related to ABA, where a particular factor, DELAY OF GERMINATION 1 (DOG1), becomes the master regulator of the primary dormancy activator. This is in turn regulated by a specific PP2C, activated by ABA [17]. DOG1 modifies ABA signaling where PP2C acts on seeds, particularly regarding those depending on ABA hypersensitive germination. Even after grain development, OsDOG1-like gene expression is also involved for immature seed development. This is co-dominantly expressed with an over expression of OsNCED2 and OsABA8’OH3. Seed germination under water is independent of primary dormancy, where susceptible varieties can germinate but fail through downstream development. Not only embryos under submergence stress but also heat-stressed embryos are dependent on DNA methylation. The DNA methylation of ABA-catabolizing genes and the α-amylase promoter are also subjected to anaerobic stress [18]. Another two genes, ABA deficient 4 (ABA4) and Neoxanthin deficient 1 (NXD1), are required for ABA biosynthesis and its regulation [19]. Therefore, more studies on ABA-GA cross talk in the germination phenomenon with molecular insights into submergence or anoxic germination are required (Figure 1).
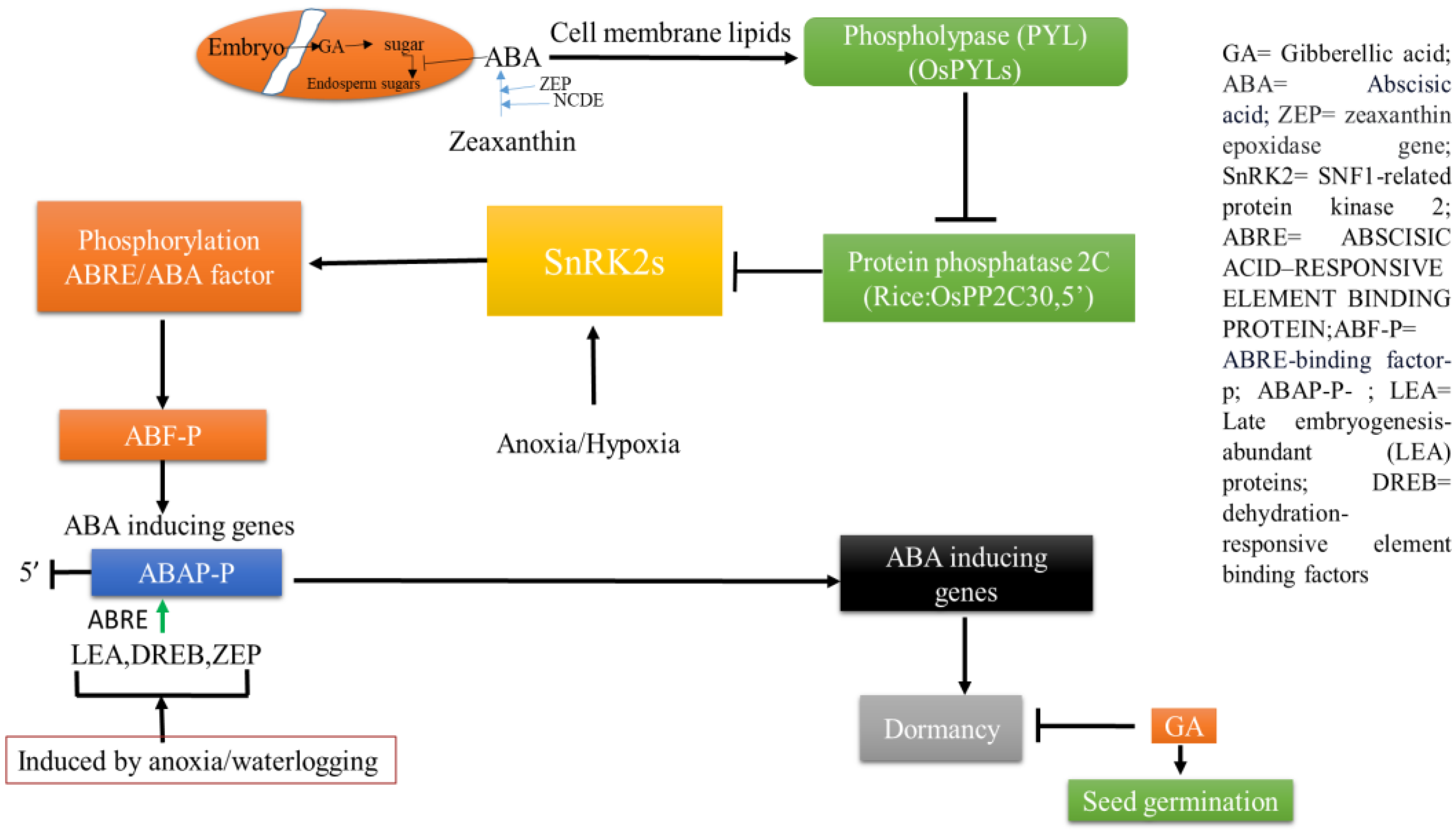
Figure 1. Hormonal interplay between seed dormancy and germination under inundation stress.
4. Development of Rice Seedlings Facing Anoxic Conditions
Soil flooding may be the most vulnerable abiotic constraint in rice cultivation after seed germination. Seedling growth becomes important with the duration and depth of water stagnation. Seeds of taller plants almost fail to germinate under anaerobic conditions whereas rice germinates in oxygen-depleted soil under partial or full submergence [20]. Rice seeds starchy in grain material could tolerate anaerobiosis, where fermentative catabolism is a prime event. Thereby, seeds can maintain a high rate of carbohydrate turnover under oxygen deficiency. A decline in oxygen partial pressure causes negative effects in other cereals like oat and barley, where root emergence is normal [21]. In contrast, even though root growth is suppressed, shoot growth increases where there is oxygen demand under submergence in the case of rice. Even depleted oxygen can increase the final length of coleoptiles that exceeds that of aerobically grown seeds. Under this condition, coleoptiles may be sustained, but the root and primarily the leaves do not grow successfully.
5. Fermentative Mechanism: Pathways for Anaerobic Seeds’ Germination in Rice
Under limited oxygen concentration, mitochondrial respiration is inversely proportional to glycosylate metabolism, which produces NADH. Following entry into mitochondria, NADH undergoes re-oxidation at the start of fermentative pathways. The lactate and toxic substrate ethanol produced with a minimum gain of ATP is a bottleneck for germination under water [22]. Lactate, a substrate of lactate dehydrogenase (LDH), is typically toxic to the aleurone membrane in inducing amylase activities by GA, whereas PCD produces ethanol along with the activity of ADH. Seeds may be tolerant to anoxia by reverting the central glycolytic pathways. Ethanol may either be oxidized to acetyl dehydrogenase or diffused out of the seed coat, accumulating within spaces of grains and glumes [23]. This also induces dormancy in terms of ecological consideration for submergence tolerance. The minimum amount of ATP (2 moles) over the normal tricarboxylic acid cycle (32 moles) is a limitation for anaerobic respiration [24]. Therefore, a specific set of protein expression and their corresponding regulation are selection criteria for the better germination of seeds in the anaerobic mode. Distinct proteins like sucrose-phosphate synthase, PDC, and ADH are the most important in accessing breeding programs where submergence induces anoxia [25]. Carbohydrate metabolism in rice seeds under prolonged anoxic conditions creates the most effective screening index. There are specific modalities of the regulation of genes on the anaerobic response elements upstream of the promoters, characterizing the coding of anaerobic proteins [26]. These, in turn, become the factors to control the paths of fermentation for specific genes. From the sequence alignment, cis-elements also bear the homology for anoxia-inducible genes in plants, similar to bacteria. Rice plants tolerant to anoxia can accommodate ATPs; however, for a long time, this has occurred via fermentative catabolism, as long as the hypoxia is maintained without affecting the embryo tissues.
6. Molecular Regulation of Major Glycolytic Flux: Amylase Activity with GA Induction
Major glycolytic flux is based on the availability of soluble sugars, which determines the rate of germination and seed growth in rice under submergence. The regulation of α-amylase expression by GA is a major control for soluble sugars as a chief fuel for glycolysis [27]. Hypoxia-induced coleoptile growth is thus matched with GA biosynthesis, catabolism, and the regulation of amylase activity [28]. The expression of the specific α amylase gene is also under activation when embryos deplete sugars from endosperm. This was well established from the expression of α amylase in rice embryos, as derived from suspension cells in aleurone, which becomes a major source of α amylases. Under two sugar-depleted culture sets of genes, α-Amy3 and α-Amy8 [29] are major amylases. This is responsible for starch breakdown below the threshold level for sugar content, which supports the germination of embryos. The sugar repression of AM3 and AM8 is based on the regulation of the transcription rate, as well as the stability of transcripts. Typical TA box (5′-TATCCA-3′) is the main sugar response element in rice embryos [30].
7. Constraints and Remediation of Pre-Harvest Sprouting
Under different environmental stresses, the production of cereal seeds is physiologically affected by grain germination on plants, called pre-harvest sprouting. It results in a significant loss of yield, reduced grain quality, and other physiological traits in a wide coverage of cereals including maize, barley, wheat, rye, oats, jowar, etc. [31]. Pre-harvest sprouting is favored with suitable temperature and moisture for seed germination. This is also associated with the energy exhausted by grains and the breakdown of high-energy-producing starch and lipids. The energy released by metabolites may allow shoots to expand under an environmental condition that supports sprouting [32]. Moreover, genetic and environmental factors independently or by interactive means may influence pre-harvest sprouting. Specific varieties have been investigated for sprouting resistance that minimize the loss of yield and grain quality. A selection procedure for the characteristics that aid sprouting resistance from a rice data base has already been identified.
8. Metabolomic Approaches for Hypoxia Tolerance in Rice
In the recent past, a detailed comparative multi-omics analysis suggested the involvement of different pathways to tolerate hypoxia for embryos and coleoptiles. Rice seeds, with their in-built tolerance to grow under oxygen deficiency, lead to a conversion to pyruvate following alcohol. From metabolomic studies, a complete set of enzymes for starch mobilization under hypoxic stress remains active [33]. Agronomically, this tolerance demonstrates rice as being a crop for direct seedlings in cultivation. The cell-wall-specific high expression of wall-modifying enzymes like trans-glycosylase as well as proteins for coleoptile growth are important [34]. The turnover of glycolytic pathways induces a higher survival of hypersensitive rice seeds to hypoxia than other intolerant ones.
9. Regulation of Transcripts in Seed Germination under Submergence
As already reported, rice seeds favor some sort of anoxia which is moderated by the adoption of a quiescence strategy. A number of bio-metabolites and hormonal influences are pre-requisites for quiescence strategy under the regulation of some genes from the whole transcriptome [35]. These genes include the enzyme system for the fermentative mechanism, where key enzymes for glycolysis are pyruvate decarboxylase (PDC), alcohol dehydrogenase (ADH), etc. [36]. At the metabolome level, alcoholic fermentation can provide adequate energy for seed germination, coleoptile growth, and ATP synthesis to facilitate the quiescence mechanism. In alcoholic fermentation, pyruvate is catalyzed into acetaldehyde by PDC. This is followed by ADH and other constitutive genes responsible for alcoholic fermentation [37]. ADH becomes a key gene for submergence tolerance, which is regulated in a feedback manner with slender rice 1 (SLR1) and slender rice like 1 (SLRL1). However, for quiescence strategies, ADH is inhibited or less active for the adh1 mutant associated with a decrease in the NADP+/NADPH ratio [38]. In this mutant, coleoptile growth and its elongation are hindered in the molecular regulation of anaerobic genes.
10. Biochemical Implications of Anoxic Seed Germination
Rice seeds, due to their anaerobic germination, show two distinct modalities for biotechnology implications, particularly in anaerobic conditions. Primarily, they have minimum or basal oxygen requirements for seed germination for dormancy-related pathways. Secondarily, the use of specific devices to maintain minimum oxygen tension and its diffusion for developing embryos is important [39]. A number of chemical elicitors to influence the metabolic expression from developing embryos have been well addressed. Seed-coat-residing phenolics and their oxidation into other residues are related with the removal of dormancy under anoxic conditions. For aerobic rice, where oxygen concentration is not a constraint, seed coat phenolic residues are also affected. In some cases, established physiological and biochemical pathways are clear, but the signaling mechanism for the soil moisture deficit, ROS, and growth regulators is not explained. Therefore, the biotechnological implication of anaerobic seed germination requires a consolidated background with plant physiology, biochemistry, ecology, and cellular and molecular biology of seeds in the plasticity of environmental constraints.
11. Conclusions
Rapid seed germination and coleoptile elongation are primary traits of anoxia and hypoxia tolerance. Seeds can overcome anerobic stress when oxidative phosphorylation can provide adequate ATPs. These are required for energy conservation when seeds are to sustain their viability by arresting its growth. This is physiologically manifested into quiescence strategies, where growth is regulated under submergence. Sugar and amino acid metabolism were features of hypoxia-tolerant cultivars that support tissue development in coleoptiles. Multi-omics research suggests that genes functioning in a differential manner for potential transcript(s) may provide tolerance. These genes are genetic resources for the mobilization of nutrients under oxygen deficits in seeds. From existing land races of rice tolerant to submergence, significant functional gene(s) are recovered using the reference genome of FR13A, etc. Therefore, hypoxic germination shares the same background of gene pools concerning oxidative redox, and these must be employed in breeding programs for direct seeding in rice.
References
- Wiraguna, E. Adaptation of legume seeds to waterlogging at germination. Crops 2022, 2, 111–119.
- Zhang, G.; Liu, Y.; Gui, R.; Wang, Z.; Li, Z.; Han, Y.; Guo, X.; Sun, J. Comparative multi-omics analysis of hypoxic germination tolerance in weedy rice embryos and coleoptiles. Genomics 2021, 113, 3337–3348.
- Damalas, C.A. Understanding benefits and risks of pesticide use. Sci. Res. Essays 2009, 4, 945–949.
- Das, A.; Uchimiya, H. Oxygen stress and adaptation of a semi-aquatic plant: Rice (Oryza sativa). J. Plant Res. 2002, 115, 315–320.
- Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 11522.
- Ventura, I.; Brunello, L.; Iacopino, S.; Valeri, M.C.; Novi, G.; Dornbusch, T.; Perata, P.; Loreti, E. Arabidopsis phenotyping reveals the importance of alcohol dehydrogenase and pyruvate decarboxylase for aerobic plant growth. Sci. Rep. 2020, 10, 16669.
- Chen, Y.; Xiang, Z.; Liu, M.; Wang, S.; Zhang, L.; Cai, D.; Huang, Y.; Mao, D.; Fu, J.; Chen, L. ABA biosynthesis gene OsNCED3 contributes to preharvest sprouting resistance and grain development in rice. Plant Cell Environ. 2022, 46, 1384–1401.
- Sohn, S.I.; Pandian, S.; Kumar, T.S.; Zoclanclounon, Y.A.B.; Muthuramalingam, P.; Shilpha, J.; Satish, L.; Ramesh, M. Seed dormancy and pre-harvest sprouting in rice—An updated overview. Int. J. Mol. Sci. 2021, 22, 11804.
- Millar, A.A.; Jacobsen, J.V.; Ross, J.J.; Helliwell, C.A.; Poole, A.T.; Scofield, G.; Reid, J.B.; Gubler, F. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8′-hydroxylase. Plant J. 2006, 45, 942–954.
- Sun, J.; Zhang, G.; Cui, Z.; Kong, X.; Yu, X.; Gui, R.; Han, Y.; Li, Z.; Lang, H.; Hua, Y.; et al. Regain flood adaptation in rice through a 14-3-3 protein OsGF14h. Nat. Commun. 2022, 13, 5664.
- Srivastava, A.K.; Suresh Kumar, J.; Suprasanna, P. Seed ‘primeomics’: Plants memorize their germination under stress. Biol. Rev. 2021, 96, 1723–1743.
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415.
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849.
- Panigrahy, M.; Singh, A.; Das, S.; Panigrahi, K.C. Co-action of ABA, brassinosteriod hormone pathways and differential regulation of different transcript isoforms during cold-and-dark induced senescence in Arabidopsis. J. Plant Biochem. Biotechnol. 2022, 31, 489–510.
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.P.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366.
- Halford, N.G.; Hey, S.J. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem. J. 2009, 419, 247–259.
- Graeber, K.; Linkies, A.; Müller, K.; Wunchova, A.; Rott, A.; Leubner-Metzger, G. Cross-species approaches to seed dormancy and germination: Conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol. Biol. 2010, 73, 67–87.
- Roychoudhury, A.; Paul, S.; Basu, S. Cross-talk between abscisic acid-dependent and abscisic-independent pathways during abiotic stress. Plant Cell Rep. 2013, 32, 985–1006.
- Neuman, H.; Galpaz, N.; Cunningham, F.X., Jr.; Zamir, D.; Hirschberg, J. The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. Plant J. 2014, 78, 80–93.
- Miro, B.; Ismail, A.M. Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Front. Plant Sci. 2013, 4, 269.
- Mejía, S.M.V.; de Francisco, A.; Bohrer, B. A comprehensive review on cereal β-glucan: Extraction, characterization, causes of degradation, and food application. Crit. Rev. Food Sci. Nutr. 2020, 60, 3693–3704.
- Yankov, D. Fermentative lactic acid production from lignocellulosic feedstocks: From source to purified product. Front. Chem. 2022, 10, 823005.
- Laville, J.; Blumer, C.; Von Schroetter, C.; Gaia, V.; Défago, G.; Keel, C.; Haas, D. Characterization of the hcnABC gene cluster encoding hydrogen cyanide synthase and anaerobic regulation by ANR in the strictly aerobic biocontrol agent Pseudomonas fluorescens CHA0. J. Bacteriol. 1998, 180, 3187–3196.
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378.
- Panda, D.; Barik, J.; Behera, P.K. Improving Submergence Tolerance in Rice: Recent Progress and Future Perspectives. In Response of Field Crops to Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2022; pp. 111–122.
- Gibbs, J.; Morrell, S.; Valdez, A.; Setter, T.L.; Greenway, H. Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. J. Exp. Bot. 2000, 51, 785–796.
- Gómez-Álvarez, E.M.; Pucciariello, C. Cereal germination under low oxygen: Molecular processes. Plants 2022, 11, 460.
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The rice alpha-amylase, conserved regulator of seed maturation and germination. Int. J. Mol. Sci. 2019, 20, 450.
- Joo, J.; Lee, Y.H.; Song, S.I. Overexpression of the rice basic leucine zipper TF OsbZIP12 confers drought tolerance to rice and makes seedlings hypersensitive to ABA. Plant Biotech. Rep. 2014, 8, 431–441.
- Gubler, F.; Kalla, R.; Roberts, J.K.; Jacobsen, J.V. Gibberellin-regulated expression of a myb gene in barley aleurone cells: Evidence for Myb transactivation of a high-pI alpha-amylase gene promoter. Plant Cell 1995, 7, 1879–1891.
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24.
- Hegde, P.S.; White, I.R.; Debouck, C. Interplay of transcriptomics and proteomics. Curr. Opin. Biotech. 2003, 14, 647–651.
- Barnes, W.J.; Anderson, C.T. Release, recycle, rebuild: Cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol. Plant 2018, 11, 31–46.
- Figueroa, C.M.; Lunn, J.E. A tale of two sugars: Trehalose 6-phosphate and sucrose. Plant Physiol. 2016, 172, 7–27.
- Zhang, P.; Lyu, D.; Jia, L.; He, J.; Qin, S. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom. 2017, 18, 649.
- Shiao, T.L.; Ellis, M.H.; Dolferus, R.; Dennis, E.S.; Doran, P.M. Overexpression of alcohol dehydrogenase or pyruvate decarboxylase improves growth of hairy roots at reduced oxygen concentrations. Biotech. Bioeng. 2022, 77, 455–461.
- Marisco, G.; Saito, S.T.; Ganda, I.S.; Brendel, M.; Pungartnik, C. Low ergosterol content in yeast adh1 mutant enhances chitin maldistribution and sensitivity to paraquat-induced oxidative stress. Yeast 2011, 28, 363–373.
- Sahi, C.; Singh, A.; Kumar, K.; Blumwald, E.; Grover, A. Salt stress response in rice: Genetics, molecular biology, and comparative genomics. Funct. Integr. Genom. 2006, 6, 263–284.
- Jurdak, R.; Launay-Avon, A.; Paysant-Le Roux, C.; Bailly, C. Retrograde signalling from the mitochondria to the nucleus translates the positive effect of ethylene on dormancy breaking of Arabidopsis thaliana seeds. New Phytol. 2021, 229, 2192–2205.
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
593
Revisions:
2 times
(View History)
Update Date:
21 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




