Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Young-Chul Lee | -- | 2239 | 2023-08-18 02:25:15 | | | |
| 2 | Jessie Wu | -9 word(s) | 2230 | 2023-08-18 03:44:08 | | | | |
| 3 | Jessie Wu | Meta information modification | 2230 | 2023-08-18 03:46:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ngoc, L.T.N.; Moon, J.; Lee, Y. Mechanisms and Classification of Bioactive Peptides. Encyclopedia. Available online: https://encyclopedia.pub/entry/48199 (accessed on 16 January 2026).
Ngoc LTN, Moon J, Lee Y. Mechanisms and Classification of Bioactive Peptides. Encyclopedia. Available at: https://encyclopedia.pub/entry/48199. Accessed January 16, 2026.
Ngoc, Le Thi Nhu, Ju-Young Moon, Young-Chul Lee. "Mechanisms and Classification of Bioactive Peptides" Encyclopedia, https://encyclopedia.pub/entry/48199 (accessed January 16, 2026).
Ngoc, L.T.N., Moon, J., & Lee, Y. (2023, August 18). Mechanisms and Classification of Bioactive Peptides. In Encyclopedia. https://encyclopedia.pub/entry/48199
Ngoc, Le Thi Nhu, et al. "Mechanisms and Classification of Bioactive Peptides." Encyclopedia. Web. 18 August, 2023.
Copy Citation
Bioactive peptides have gained significant attention in the cosmetic industry due to their potential in enhancing skin health and beauty. These small protein fragments exhibit various biological activities, such as antioxidant, anti-aging, anti-inflammatory, and antimicrobial activities, making them ideal ingredients for cosmetic formulations. These bioactive peptides are classified into four categories: signal, carrier, neurotransmitter-inhibitory, and enzyme-inhibitory peptides.
classification of peptides
mechanisms of action
natural sources
safety
carrier
1. Classification of Cosmetic Peptides
It has been demonstrated that bioactive peptides can exert their biological as well as cosmetic functions in different ways; thus, they are commonly classified into four categories according to their most outstanding features which includes signal peptides, carrier peptides, neurotransmitter-inhibitory peptides, and enzyme-inhibitory peptides (Figure 1) [1][2].
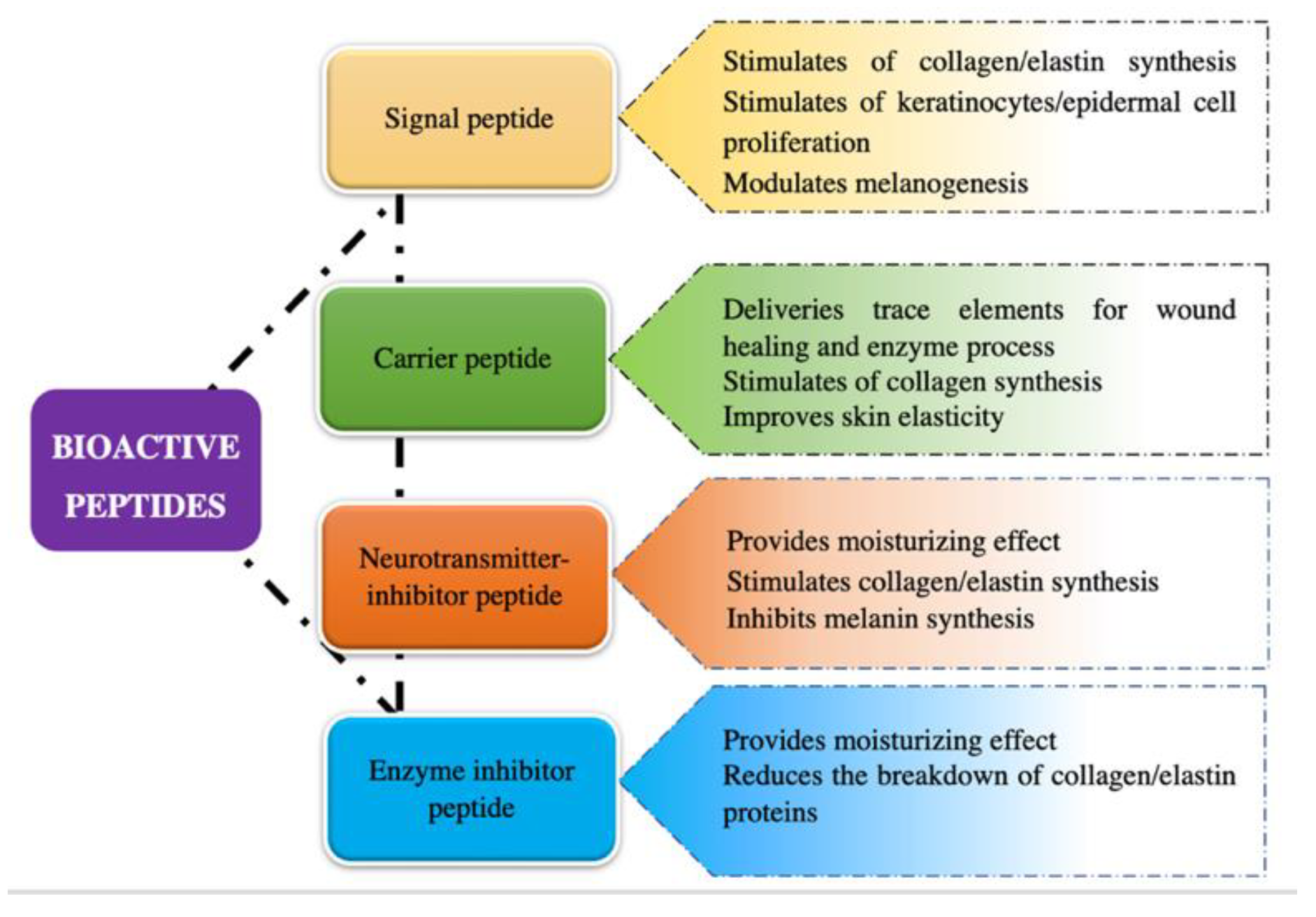
Figure 1. Classification of bioactive peptides based on cosmetic properties.
1.1. Signal Peptides
Signal peptides are active compounds that can prevent aging by stimulating skin fibroblasts, resulting in increased biological responses such as collagen, elastin, fibronectin, glycosaminoglycan, and proteoglycan production [2]. They may act as growth factors to activate protein kinase C which is mainly responsible for cell growth and migration [2].
One of the first cosmetic signal peptides is the palmitoyl peptide (Pal–Lys–Thr–Thr–Lys–Ser), which shows collagen modulating capabilities for anti-wrinkle and wound healing [1][2]. It is a sub-fragment of the carboxyl terminal pro-peptide of type I collagen that can dramatically enhance extracellular matrix production in fibroblasts [3][4], thereby effectively stimulating collagen (I, II, and III) and fibronectin production. Aruan et al. (2023) conducted a double-blind, split-face, placebo-controlled, and left-right randomized trial with 21 female subjects to assess the clinical efficacy of anti-wrinkle cream containing the peptide [4]. The result showed that in the course of a 12-week clinical trial, topical application of 3 ppm palmitoyl peptide reduced facial wrinkles/fine lines [4]. Another signal peptide that stimulates collagen synthesis is palmitoyl tripeptide-5 (palmitoyl–Lys–Val–Lys), which can mimic the effect of thrombospondin-1, a naturally occurring molecule that causes the sequence Lys–Arg–Phe–Lys to bind to the inactive transforming growth factor-β (TGF-β), consequently, promoting the release of the active form of TGF-β [5]. Thereafter, activated TGF-β causes a constant increase in the amount of type I and III collagen produced in dermal fibroblasts [5]. A number of studies have demonstrated that palmitoyl tripeptide-3/5 enhances collagen synthesis and reduces collagen breakdown by interfering with MMP-1 and MMP-3 collagen degradation, leading thereby to improvements in aging signals [2][5]. For example, a controlled trial was conducted on 60 Chinese volunteers treated with palmitoyl pentapeptide-5 (2.5%) cream compared with a placebo cream (for 84 days and applied twice daily) [5]. It was confirmed that palmitoyl tripeptide-5 significantly reduced skin roughness, exhibiting a greater anti-wrinkle efficacy than the placebo or pal-KTTKS-containing creams [5]. Other commercialized signal peptides modulating collagen synthesis are described in terms of their anti-aging properties and mechanisms.
Regarding the enhancement of elastin contents in the skin, several signal peptides (e.g., dipeptide-2/valy tryptophan, Val–Gly–Val–Ala–Pro–Gly, and palmitoyl oligopeptide) have been developed to stimulate elastin synthesis, leading to improved skin aging signals. For instance, hexapeptide Val–Gly–Val–Ala–Pro–Gly and its modified sequence palmitoyl hexapeptide-12 are highly specific to elastin molecules that stimulate collagen and elastin fibroblasts, as well as develop glycosaminoglycans and fibronectin [2]. The intracellular mechanism refers to the way in which they can reduce the production of proinflammatory mediators (e.g., IL-1, IL-6, and IL-8) and ultimately slow down the skin matrix’s degradation [5]. Another signal peptide, palmitoyl oligopeptide, which contains such an elastin fragment, has been incorporated into cosmetic products to promote the proliferation of collagen, elastin, and hyaluronic acid, a role which suggests “reconstruction of the dermis” and “chemotaxis for restructuring and repair” properties [2]. Hahn et al. (2016) produced an anti-aging facial cream containing 1% palmitoyl oligopeptide, Silybum marianum seed oil, vitamin E, and other functional ingredients to combat facial wrinkles [6]. After 4 weeks of application, the volunteers’ crow’s feet wrinkles were reduced by 14.07% compared to pre-application, and the skin elasticity was observed to have increased by 8.79%. They were able to confirm, therefore, that a blend of palmitoyl oligopeptide and other cosmetics has a beneficial effect on facial wrinkles, elasticity, and skin tone.
1.2. Carrier Peptides
Carrier peptides have been designed to deliver essential wound healing cofactors for enzymatic processing and wound repair [2][7]. The first commercialized carrier peptide was designed to deliver copper, a trace element necessary for wound healing, into the wounded tissue.
Copper is not only an essential trace element for wound healing but also a cofactor for enzymes lysyl oxidase, superoxide dismutase, and tyrosinase, which are important for collagen synthesis, superoxide dismutation (antioxidant action), and melanogenesis, respectively [2][8]. The first copper tripeptide Cu–GHK, potentially performs a role in the extracellular matrix by promoting regular collagen, elastin, glycosaminoglycan, and proteoglycan synthesis. This leads to the stimulation of cellular regulation molecules, and the regeneration and healing of skin and other tissues [8]. In particular, Siméon et al. (2000) demonstrated that Cu–GHK effectively promotes MMP-2 synthesis in skin fibroblasts, as represented by an increased expression of MMP-2 mRNA and the secretion of TIMP-1 and TIMP-2, resulting in fibroblast wound healing [8]. Pickart et al. (2015) demonstrated that Cu–GHK treatment remarkably reduced TNF-α levels induced by cytokines IL-6 and increased expression of various DNA repair genes [9]. It can be concluded that Cu–GHK contributes to a beneficial anti-aging effect through a number of mechanisms, especially by promoting regeneration, healing, and repair of damage. In fact, many studies have confirmed the clinical effects of Cu–GHK as a functional ingredient. Liu et al. (2023) tested the anti-wrinkle activity of a Cu–GHK formulation to improve skin elasticity, skin moisture, and skin-smoothing by enhancing collagen synthesis, thereby diminishing facial wrinkles and fine lines [10].
Another transition metal, manganese, is an essential nutrient involved in amino acid, cholesterol, antioxidant protection, and carbohydrate metabolism [11]. In addition, manganese-superoxide dismutase is considered to be very important in the defense against UV-induced photoaging [11][12]. It has been found that the level of manganese-superoxide dismutase is increased through the action of inflammatory mediators (e.g., IL-1 and TNF-α) during UV irradiation [12]. Therefore, manganese tripeptide-1 (Mn–GHK) was formulated to provide a similar functionality to that of Cu–GHK in the photoaging treatment. Hussain and Goldberg (2007) evaluated the effects of a Mn–GHK-containing facial serum formulation in the treatment of various signs of photodamage for 12 weeks [11]. At the end of the treatment, volunteers noted an improvement in the appearance of cutaneous photodamage signs, the photodamage ranking having changed from moderate to mild, as well as the lack of any side effects such as inflammation [11].
1.3. Neurotransmitter-Inhibitor Peptides
Some of the most common signs of aging (e.g., wrinkles and fine lines) have also been controlled through strategies of muscle contraction regulated by neurotransmitters released from neurons through the use of neurotransmitter-inhibitor peptides [5][7]. In particular, the muscle contraction process occurs when vesicles containing the neurotransmitter acetylcholine join the neuron in order to break into two separate fragments including the vesicle and acetylcholine (ACh)-a neurotransmitter of the parasympathetic nervous system [5][7]. The vesicle is captured with SNARE complexes (soluble N-ethylmaleimide-sensitive factor activating protein receptor) and then fused with the neuron membrane, while the ACh is released in neuromuscular junctions between the muscle and the nerve [13]. The released ACh binds to the acetylcholine receptors that are present on the muscle cell’s surface, leading to muscle contraction. It is reported that the entire process is regulated by SNAP-25, a receptor protein present in the neuronal membrane, which is associated with the vesicle and directly regulates binding with the SNARE complex as well as the fusion with the membrane of the vesicle [13]. According to the contraction mechanism, a number of peptides have been developed with sequences similar to SNAP-25 proteins, which can compete for the binding sites of SNARE complexes, leading to their structural instability and inhibition of the release of ACh at the neuromuscular junction, inducing muscle relaxation. These synthetic peptides (e.g., acetyl hexapeptide-3, pentapeptide-3, pentapeptide-18, and tripeptide-3) exhibit specific neuro-suppressive abilities; accordingly, they are called neurotransmitter-inhibitor peptides [13].
One of the most popular commercialized neurotransmitter-inhibitor peptides is acetyl hexapeptide-3 (Argireline®), offering advanced properties in anti-wrinkle and moisturizing effects. This peptide, which is similar to botulinum toxin, is able to mimic the N-terminal end of the SNAP-25 protein and compete for a site in the SNARE complex, resulting in destabilization of its formation as well as inhibition of ACh release and, eventually, decreased muscle contraction [7][14][15]. A clinical study by Ruiz et al. (2010) investigated the anti-wrinkle benefits of an oil in water (O/W) emulsion containing acetyl hexapeptide-3 in 20 human subjects for 30 days of topical application. It showed positive signs with a reduction in wrinkle depth and size by 59% and 41%, respectively, compared with the placebo control [16].
1.4. Enzyme-Inhibitory Peptides
Enzyme-inhibitory peptides can directly or indirectly inhibit enzymes that break down collagen and other proteins and interfere with that process. A number of enzyme-inhibitory peptides such as soy oligopeptides, rice-derived peptides and silk fibroin peptides have been used as inactive ingredients for skincare products (Table S1) [7][17].
Soybean-derived peptides, comprised of 3–6 amino acids, possess various biological activities including antioxidative, blood-lipid-lowering, and blood-pressure-lowering effects. These biological properties show an overwhelming effect in increasing levels of proapoptotic Bcl-2 protein and decreasing cyclobutene pyrimidine dimer-positive cells, apoptotic cells, expression of Bax and p53 proteins in the epidermis due to UVB irradiation [7][17]. As a result, they are frequently used as anti-aging agents, skin moisturizers, hair growth promoters, and cleaning detergents. A pseudo-randomized clinical study of ten Caucasian women, confirmed the superiority of soybean peptide (2%) emulsion in increasing the amount of extracellular matrix components such as collagen and glycosaminoglycan contents [18].
Rice-derived peptides (molecular weight < 300 Da) obtained after special processing of rice-bran protein, greatly inhibit activity of MMPs and stimulate the expression of hyaluronan synthase 2 genes in human keratinocytes cells [19]. Manosroi et al. (2012) successfully produced formulas containing niosomes encapsulated in rice-bran peptides, and demonstrated that they have ideal clinical anti-aging properties [20].
Silk fibroin peptide is obtained from the silkworm Bombyx mori. This peptide is able to inhibit inflammation, specifically by increasing the anti-inflammatory activity of tTAT-superoxide dismutase, which has been reported to effectively penetrate into skin cells and tissues and to exert anti-oxidant effects in an inflamed-mouse model [21].
2. Mechanisms of Action
Bioactive peptides, with their powerful single/multifunctional biological properties (e.g., antimicrobial, antioxidant, anti-aging, and anti-inflammatory activities) have been widely applied as functional ingredients in the dermatology and cosmetology fields. The peptides can improve skin health in a number of aspects, including extracellular matrix synthesis, innate immunity, inflammation, and pigmentation [22][23][24][25][26]. The detailed mechanisms of each cosmetic property are fully described in Table 1 and Figure 2.

Figure 2. Schematic summary of the mechanism of bioactive peptides related to their potential cosmetic application. ROS: Reactive oxygen species; MAPK: mitogen-activated protein kinases; ERK: Extracellular-signal-regulated kinases; JNK: c-jun N-terminal kinases; MMPs: matrix metalloproteinases; cAMP: cyclic adenosine monophosphate; MITF: microphthalmia-associated transcription factor; TNF-α: tumor necrosis factor-α; IL-1β: interleukin-1β; IL-6: interleukin-6 [22].
Table 1. Intracellular mechanisms of cosmetic properties.
| Cosmetic Properties | Mechanism | Effective Factors |
|---|---|---|
| Antioxidant activity | Prevents the deleterious effects of oxidative stress caused by overproduction of ROS in the skin [27] Act as antioxidants through hydrogen atom transfer, single electron transfer, and chelating transition pro-oxidant metals [27] |
Antioxidant properties depend on their structural properties: molecular weight, hydrophobicity, and amino acid sequence (Pro, His, Cys, Phe, Try, and Tyr) [24] Peptides with lower molecular weight show effective antioxidant properties [28] |
| Anti-inflammatory activity | Possesses anti-inflammatory capacity mediated by the inhibition and induction of the immune systems in cell lines [29] Downregulates pro-inflammatory mediators (e.g., TNF-α, IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-12, and IFN-γ receptors) and regulates immune system [29] |
Anti-inflammatory activity is related to their ability to bind to the lipid A moiety of lipopolysaccharides (LPS) and interference with LPS-CD14 interactions by competing with the LPS-binding peptide [30] |
| Antimicrobial activity | Provides antimicrobial activity based on membrane lytic mechanisms whereby peptides can directly affect cell membrane integrity through the formation of transmembrane channels, resulting in cytoplasm leakage and cell death [31] Involved with the inhibition of intracellular activities in nucleic acid, protein and cell-wall synthesis, protein folding, lipopolysaccharide formation, and cell-division progress [31] Induces a loss of regulated iron transport, leading to membrane permeation and DNA damage, and subsequently to bacterial destruction [22] |
Peptides with cationic charge (from +2 to +9) show a strong ability to interact with the negatively charged membranes of microorganisms [32] |
| Anti-aging properties | Collagenase inhibition Inhibits mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF- κB) signaling pathways, and histone modification [33] Suppresses the activities and expressions of MMP by elevating tissue inhibitors of matrix metalloproteinases (TIMP) levels and blocking activation of MAPK signaling pathway [34] |
Low molecular weight peptides (<1 kDa) possess higher inhibitory activity against MMP, p-JNK, p-p38, and p-ERK in MAPK signaling pathways than that of larger molecular weight peptides [35] |
| Hyaluronidase inhibition Inhibits the degradation of hyaluronic acid for protecting skin [36] |
Hyaluronidase inhibitor capacity depends on molecular weights as follows: large molecular peptides (3–10 kDa) > medium molecular peptides (1–3 kDa) > small molecular peptides (<1 kDa) [37] | |
| Tyrosinase inhibition Blocks the active site or chelates copper ions of tyrosinase to inhibit tyrosinase activity [38] Downregulates the activation of microphthalmia-associated transcription factor (MITF), an important event during melanogenesis, to suppress melanin synthesis [39] Downregulates cAMP signaling pathway as an anti-melanogenic activity to inhibit melanin synthesis [22][38][39] |
Peptides consisting of amino acids with hydroxyl groups (Ser and Thr), aliphatic amino acid residues (Val, Ala, and Leu), and hydrophobic compounds exhibit great tyrosinase inhibitory activities [40] Peptides with molecular weight < 3 kDa show higher tyrosinase activity that that of the whole collagen hydrolysate [22] |
|
| Elastase inhibition Downregulates the activation of elastase enzyme to protect mechanical properties of skin tissues that are impaired by overproduction of the enzyme elastase [41] |
--- |
References
- Lima, T.N.; Moraes, C.A.P. Bioactive peptides: Applications and relevance for cosmeceuticals. Cosmetics 2018, 5, 21.
- Pai, V.V.; Bhandari, P.; Shukla, P. Topical peptides as cosmeceuticals. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 9.
- Bégin, P.; Callum, J.; Jamula, E.; Cook, R.; Heddle, N.M.; Tinmouth, A.; Zeller, M.P.; Beaudoin-Bussières, G.; Amorim, L.; Bazin, R.; et al. Convalescent plasma for hospitalized patients with COVID-19: An open-label, randomized controlled trial. Nat. Med. 2021, 27, 2012–2024.
- Aruan, R.R.; Hutabarat, H.; Widodo, A.A.; Firdiyono, M.T.C.C.; Wirawanty, C.; Fransiska, L. Double-blind, Randomized Trial on the Effectiveness of Acetylhexapeptide-3 Cream and Palmitoyl Pentapeptide-4 Cream for Crow’s Feet. J. Clin. Aesthet. Dermatol. 2023, 16, 37.
- Zhao, X.; Zhang, X.; Liu, D. Collagen peptides and the related synthetic peptides: A review on improving skin health. J. Funct. Foods 2021, 86, 104680.
- Hahn, H.J.; Jung, H.J.; Schrammek-Drusios, M.C.; Lee, S.N.; Kim, J.; Kwon, S.B.; An, I.; An, S.; Ahn, K.J. Instrumental evaluation of anti-aging effects of cosmetic formulations containing palmitoyl peptides, Silybum marianum seed oil, vitamin E and other functional ingredients on aged human skin. Exp. Ther. Med. 2016, 12, 1171–1176.
- Schagen, S.K. Topical peptide treatments with effective anti-aging results. Cosmetics 2017, 4, 16.
- Siméon, A.; Emonard, H.; Hornebeck, W.; Maquart, F.-X. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci. 2000, 67, 2257–2265.
- Pickart, L.; Schagen, S. New data of the Cosmeceutical and tripeptide GHK. SOFW J. 2015, 9, 141.
- Liu, T.; Hu, L.; Lu, B.; Bo, Y.; Liao, Y.; Zhan, J.; Pei, Y.; Sun, H.; Wang, Z.; Guo, C. A novel delivery vehicle for copper peptides. New J. Chem. 2023, 47, 75–83.
- Hussain, M.; Goldberg, D.J. Topical manganese peptide in the treatment of photodamaged skin. J. Cosmet. Laser Ther. 2007, 9, 232–236.
- Bresciani, G.; da Cruz, I.B.M.; González-Gallego, J. Manganese superoxide dismutase and oxidative stress modulation. Adv. Clin. Chem. 2015, 68, 87–130.
- Zhang, Y.; Zhang, H.; Jiang, B.; Yan, S.; Lu, J. A promising therapeutic target for psoriasis: Neuropeptides in human skin. Int. Immunopharmacol. 2020, 87, 106755.
- Wang, Y.; Wang, M.; Xiao, X.S.; Huo, J.; Zhang, W.D. The anti-wrinkle efficacy of Argireline. J. Cosmet. Laser Ther. 2013, 15, 237–241.
- Wang, Y.; Wang, M.; Xiao, S.; Pan, P.; Li, P.; Huo, J. The Anti-Wrinkle Efficacy of Argireline, a Synthetic Hexapeptide, in Chinese Subjects. Am. J. Clin. Dermatol. 2013, 14, 147–153.
- Ruiz, M.A.; Clares, B.; Morales, M.E.; Gallardo, V. Evaluation of the anti-wrinkle efficacy of cosmetic formulations with an anti-aging peptide (Argireline®). Ars. Pharm. 2010, 50, 168–176.
- Fries, K.S.; Heldreth, B. Safety Assessment of Soy Proteins and Peptides as Used in Cosmetics. Int. J. Toxicol. 2023, 42, 102S–113S.
- Andre-Frei, V.; Perrier, E.; Augustin, C.; Damour, O.; Bordat, P.; Schumann, K.; Förster, T.H.; Waldmann-Laue, M. A comparison of biological activities of a new soya biopeptide studied in an in vitro skin equivalent model and human volunteers. Int. J. Cosmet. Sci. 1999, 21, 299–311.
- Maeda, K. Skin-moisturizing effect of collagen peptides taking orally. J. Nutr. Food Sci. 2018, 8, 2.
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, W.; Manosroi, J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm. Biol. 2012, 50, 208–224.
- Cermeno, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052.
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170.
- Kobayashi, T.; Nagao, K. “Deepening” Insight on Skin Aging and Anti-microbial Immunity. Cell Metab. 2019, 29, 515–517.
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72.
- Yu, W.; Field, C.J.; Wu, J. Purification and identification of anti-inflammatory peptides from spent hen muscle proteins hydrolysate. Food Chem. 2018, 253, 101–107.
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients 2021, 13, 203.
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820.
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337.
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531.
- Bamdad, F.; Bark, S.; Kwon, C.H.; Suh, J.-W.; Sunwoo, H. Anti-inflammatory and antioxidant properties of peptides released from β-lactoglobulin by high hydrostatic pressure-assisted enzymatic hydrolysis. Molecules 2017, 22, 949.
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16.
- Taniguchi, M.; Kameda, M.; Namae, T.; Ochiai, A.; Saitoh, E.; Tanaka, T. Identification and characterization of multifunctional cationic peptides derived from peptic hydrolysates of rice bran protein. J. Funct. Foods 2017, 34, 287–296.
- Lu, J.; Hou, H.; Fan, Y.; Yang, T.; Li, B. Identification of MMP-1 inhibitory peptides from cod skin gelatin hydrolysates and the inhibition mechanism by MAPK signaling pathway. J. Funct. Foods 2017, 33, 251–260.
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. B Biol. 2016, 165, 34–41.
- Yeo, I.; Lee, Y.-J.; Song, K.; Jin, H.-S.; Lee, J.-E.; Kim, D.; Lee, D.-W.; Kang, N.J. Low-molecular weight keratins with anti-skin aging activity produced by anaerobic digestion of poultry feathers with Fervidobacterium islandicum AW-1. J. Biotechnol. 2018, 271, 17–25.
- Han, Q.-Y.; Koyama, T.; Watabe, S.; Nagashima, Y.; Ishizaki, S. Isolation and Characterization of Collagen and Collagen Peptides with Hyaluronidase Inhibition Activity Derived from the Skin of Marlin (Istiophoridae). Molecules 2023, 28, 889.
- Nakchum, L.; Kim, S.M. Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep. Biochem. Biotechnol. 2016, 46, 123–130.
- Xue, W.; Liu, X.; Zhao, W.; Yu, Z. Identification and molecular mechanism of novel tyrosinase inhibitory peptides from collagen. J. Food Sci. 2022, 87, 2744–2756.
- Zhao, Y.; Zhang, T.; Ning, Y.; Wang, D.; Li, F.; Fan, Y.; Yao, J.; Ren, G.; Zhang, B. Identification and molecular mechanism of novel tyrosinase inhibitory peptides from the hydrolysate of ’Fengdan’ peony (Paeonia ostii) seed meal proteins: Peptidomics and in silico analysis. LWT 2023, 180, 114695.
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Mlouka, M.A.B.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Limam, F. Antioxidant, antityrosinase and antibiofilm activities of synthesized peptides derived from Vicia faba protein hydrolysate: A powerful agents in cosmetic application. Ind. Crops Prod. 2017, 109, 310–319.
- Leirós, G.J.; Kusinsky, A.G.; Balañá, M.E.; Hagelin, K. Triolein reduces MMP-1 upregulation in dermal fibroblasts generated by ROS production in UVB-irradiated keratinocytes. J. Dermatol. Sci. 2017, 85, 124–130.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.0K
Revisions:
3 times
(View History)
Update Date:
18 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




