Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Benjamin Tawiah | -- | 4908 | 2023-08-15 13:11:49 | | | |
| 2 | Sirius Huang | Meta information modification | 4908 | 2023-08-16 03:10:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tawiah, B.; Ofori, E.A.; Bin, F. Sustainable Fire-Resistant Polysaccharide-Based Composite Aerogels. Encyclopedia. Available online: https://encyclopedia.pub/entry/48086 (accessed on 07 February 2026).
Tawiah B, Ofori EA, Bin F. Sustainable Fire-Resistant Polysaccharide-Based Composite Aerogels. Encyclopedia. Available at: https://encyclopedia.pub/entry/48086. Accessed February 07, 2026.
Tawiah, Benjamin, Emmanuel A. Ofori, Fei Bin. "Sustainable Fire-Resistant Polysaccharide-Based Composite Aerogels" Encyclopedia, https://encyclopedia.pub/entry/48086 (accessed February 07, 2026).
Tawiah, B., Ofori, E.A., & Bin, F. (2023, August 15). Sustainable Fire-Resistant Polysaccharide-Based Composite Aerogels. In Encyclopedia. https://encyclopedia.pub/entry/48086
Tawiah, Benjamin, et al. "Sustainable Fire-Resistant Polysaccharide-Based Composite Aerogels." Encyclopedia. Web. 15 August, 2023.
Copy Citation
Fire safety is a critical concern in various industries necessitating the development of sustainable and effective fire-resistant materials. Sustainable fire-resistant polysaccharide-based composite aerogels are regarded as an innovative solution in fire safety applications.
polysaccharides
flame retardants
sustainability
aerogels
fire-resistant composites
1. Introduction
Fire safety is a top priority in a variety of sectors and applications, from building construction to transportation and aircraft [1][2]. Traditional fire-resistants (mostly halogen-based such as tris(2-chloroethyl) phosphate, tris(1,3-dichloroisopropyl) phosphate, pentabromobenzyl acrylate, and tris(1-chloro-2-propyl) phosphate) frequently rely on non-renewable resources and can have negative environmental consequences. More especially, fire retardant materials derived from petrochemical sources raise significant concerns related to environmental sustainability, health, and long-term impacts [3][4]. The challenges associated with the use of these traditional flame retardants have spurred the demand for sustainable, and eco-friendly alternatives [5][6], and this has driven significant advancement in fire safety technology. One such breakthrough is the development of sustainable fire-resistant polysaccharide-based composite aerogels. These composite aerogels derived from renewable sources such as cellulose, chitosan, starch, or other polysaccharides, offer a unique combination of fire resistance, sustainability, versatility, and mechanical strength suitable for various applications. Polysaccharide-based aerogels may not naturally possess high levels of fire resistance, however, this characteristic can be enhanced because of their adaptability to changes in the aerogel preparation process. Therefore, numerous strategies have been used in the preparation of fire-resistant aerogels, including the use of additives, coatings, insertion of nanoparticles, metal and silicate oxides. Polysaccharide-based composite aerogels offer an effective and versatile solution for improving fire safety in various applications, such as building construction, transportation, and electronics [7]. Their ability to withstand high temperatures, slow down the spread of fire, and reduce smoke production makes them an essential component of modern fire safety strategies [8].
The utilization of polysaccharides in the production of fire-resistant composite aerogels addresses two critical issues of polymers; environmental sustainability and fire safety [9]. Polysaccharides are abundant in nature and derived from sources such as plants, fungi, and crustacean shells. By utilizing these naturally occurring materials, the need for non-renewable resources is reduced, thereby mitigating the environmental impact associated with conventional fire-resistant materials [10][11]. The potential of polysaccharide-based aerogels to replace conventional petrochemical-based fire retardants is mainly due to their environmentally friendly nature and the fact that these polysaccharides are renewable compared to petrochemical-based flame retardants. More so, the use of such renewable sources aligns with the global shift towards sustainable practices and the pursuit of a greener future. The fire-resistant properties of these composite aerogels are exceptional and surpass most of the conventional alternatives [11][12][13][14]. Through careful material design and composition, these aerogels can exhibit outstanding flame retardancy [15][16][17], effectively preventing the spread of fire and reducing the risk of combustion. Most of the composite aerogels have gone through rigorous testing and evaluation and have demonstrated their ability to withstand high temperatures and resist ignition, making them ideal for enhancing fire safety in a wide range of applications [18][19].
Another key advantage of sustainable fire-resistant polysaccharide-based composite aerogels lies in their lightweight and versatile nature [14]. The unique porous structure of aerogels, combined with their low density (approximately 0.001 to 0.5 g per cubic centimeter (g/cm3) depending on the material), makes them highly efficient thermal insulators [19]. The inimitable nanoporous structure of aerogels, composed of interconnected particles, creates a sponge-like material with a vast surface area and a high percentage of air-filled voids. These air-filled pores contribute to the low density, exceptional thermal insulation properties, and other unique characteristics of aerogels These aerogels can effectively reduce heat transfer and provide excellent insulation, resulting in energy conservation and improved overall energy efficiency [20][21]. Their lightweight nature also contributes to their ease of use, enabling seamless integration into various products, systems, and structures without compromising performance or adding excessive weight [22][23]. The fabrication and customization of these composite aerogels are relatively straightforward. They can be manufactured in different shapes and sizes tailored to specific application requirements. This adaptability allows for their seamless integration into existing materials or systems, as well as their incorporation into new construction projects or retrofitting applications. The versatility of these aerogels extends their potential use across diverse industries, ranging from building construction to automotive, aerospace, and beyond [24][25][26].
Furthermore, the improved thermal conductivity (range of 0.015 to 0.035 watts per meter kelvin (W/m·K) compared to the thermal conductivity of air is around 0.025 W/m·K at room temperature), the compressive mechanical strength (approximately 10 to 30 MPa depending on the composite design and reinforcement material) of sustainable fire-resistant polysaccharide-based composite aerogels has improved over the years and that has helped extend their durability in some typical applications [27]. They can withstand demanding environments, maintaining their fire-resistant properties over extended periods. This reliability and robustness make them suitable for critical applications where safety and long-term performance are paramount [28][29][30]. Sustainable fire-resistant polysaccharide-based composite aerogels represent a significant advancement in fire safety technology.
2. Technological Advancements in Sustainable Fire-Resistant Polysaccharide-Based Aerogels
The frequent occurrence of fire outbreaks in structures, industries, and other sectors has sped up the development of creative and strategic management techniques for slowing or reducing burn rates while maintaining sustainability over the course of their lifecycles. It is imperative to state that efforts aimed at improving fire resistance will remain palpably ineffective unless preferences, costs, and environmental impacts are taken into account [31]. The manufacturing of sustainable fire-resistant polysaccharide-based composite aerogels made from renewable sources, such as cellulose, chitosan, starch, or other polysaccharides, represent a breakthrough since it combines fire resistance with sustainability, adaptability, and mechanical strength in a novel way [32][33]. Therefore, the creation of renewable raw materials for housing and construction is essential for the accomplishment of the twelfth Sustainable Development Goal (“Ensure sustainable consumption and production patterns”) agreed to by more than 150 UN member states [33]. Aerogel is a unique class of solid that is synthesized using the sol-gel technique with branching, and porous nanostructures. Aerogel exhibits little shrinking because the liquid–vapor phase is absent and has an intact structure since there is no surface tension on the gel. The sol-gel process remains a highly versatile and effective method for synthesizing functional aerogels [34]. This technique enables the production of aerogels with tailored properties, making them suitable for a wide range of applications. Unlike conventional gels, where the liquid is evaporated to produce a solid material, aerogels are dried using a unique process called supercritical drying or freeze-drying. In supercritical drying, the liquid solvent is removed under carefully controlled conditions, transforming the gel into an aerogel without causing significant shrinkage or structural collapse [35]. This process preserves the highly porous structure of the aerogel, ensuring its exceptional properties, such as low density and high surface area.
Thanks to Steven Kistler’s ingenuity in 1932, composite aerogel was first produced using the supercritical drying method [36]. These composite materials have outstanding fire resistance qualities that compare with the traditional flame retardants due to meticulous material design and composition, which successfully stops the propagation of fire and lowers the risk of combustion [37]. As observed from the keyword thematic analysis, “aerogel, thermal insulation, and thermal conductivity” are the basic themes with high centrality and low density—which are considered important and not yet fully developed for the research field. Polysaccharide-based aerogel composites under this theme include chitin (CT)/chitosan (CS), hyaluronic acid (HA), chondroitin sulfate (CRS), cellulose (Cel), starch (St), pectin (Pec), pullulan (Pul), dextran (Dex), salecan (SL), and xanthan gum (XG) [38]. It is important to state that aerogels have not been developed based on all these varieties of polysaccharides, hence, a brief qualitative study of available polysaccharide-based aerogels has been reviewed.
Cellulose-based aerogels have been the front-runners in replacing the conventional petro-polymers in most applications due to their high porosity, wide specific surface area, low density, high insulation, low thermal conductivity, sustainability, biocompatibility, biodegradability, and low price [36][39]. As a result, cellulose at the nanoscale level, is a great option for replacing synthetic materials such as steel, Kevlar, poly (vinyl alcohol) (PVA), and polyurethane (PU) [39]. These nano cellulose-based aerogel composites are used in a variety of industries, including aerospace, lightweight construction, automotive interiors, packaging, insulation, flexible devices, effluent treatment, etc. [32]. The polysaccharide portion of -D-glucopyranose units, which is connected by -1,4-linkages, constitutes cellulose’s makeup as shown in Figure 1a. Sugar molecules that are both reducing and non-reducing stabilize the end terminal of cellulose polymer chains. The cellulose’s C-2, C-3, and C-6 positions have the -OH active side group, which is responsible for the chemical alteration and cellulose characteristics with each unit having three hydroxyl groups [39]. However, cellulose also has a limiting oxygen index (LOI) of 18%, making it a combustible substance [39]. Because of this, efforts to improve the flame retardancy of cellulose aerogels while preserving their distinctive characteristics have increased over the years.
Mostly, the use of materials such as clay, organic/inorganic materials [32], MOFs, and nanofiller [39] composites have become the surest way to improve their flame resistance due to their smoke suppression, sustainability, as well as their structure and chemical composition. In this regard, the cellulose-aerogel composites with a sandwich-like structure were fabricated by in situ polymerization of aniline and PDMS/CNT after freeze-drying. The fire retardancy of the composite aerogel was subjected to an open flame-retardant test and the results showed outstanding synergistic effects between the PDMS/CNT/PANI cellulose aerogel with the other flame-retardant properties improving by ca 84% [40]. Similarly, a cellulose-based MgAl-layered double hydroxide (MgAl-LDH) aerogel was fabricated and freeze-dried as shown in Figure 1b. The flame-retardant properties of the cellulose-based MgAl-LDH aerogel improved significantly with a reduction in the peak heat release rate (PHRR) by 50% and total smoke produced by 75% [32]. The composite aerogel could not be ignited easily, and the burning could not be sustained once it is moved away from the flame. The excellent flame retardancy of the composite was attributed principally to the physical barrier and carbonization impact of LDH, or dual gas-phase and condensed phase flame retardant processes.
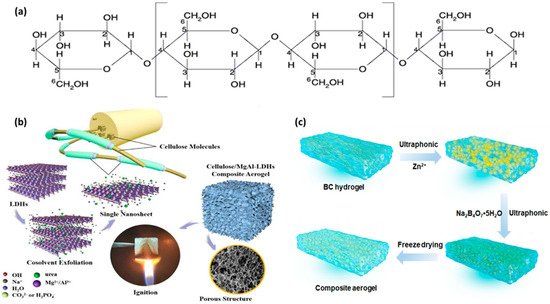
Additionally, bacterial cellulose (BC) aerogel flame retardants have become a focus of research in the search for sustainable flame-retardant options because BC is a type of maintainable material that combines the exceptional properties of extremely porous aerogels, such as being ultralight, having a highly specific surface area, and insulation, low density, and thermal conductivity, as well as advantages in biodegradability and biocompatibility. In this regard, Wang and co-workers [41] fabricated a sustainable bacterial cellulose-based retardant composite aerogel (Figure 1c) possessing heat-insulating properties by introducing zinc borate (ZB) particles into BC via an ultrasound-assistant deposition process and achieved a significant reduction in the heat release capacity (only 8 J·g−1 k=1), exhibiting excellent flame retardancy. The plausible mechanism was the dehydration of ZB particles lowering the surface temperature by releasing the bound water and simultaneously producing metallic oxides (ZnO and B2O3) for retarding the spread of heat and isolating the flammable fibrils within the combustion area. A list of other cellulose-based flame-retardant composite aerogels, their fabrication method, and flame-retardant evaluation results are presented in Table 1.
Table 1. Summarized cellulose-based composite flame-retardant aerogels.
| Type of Cellulose-Based Aerogel |
Flame Retardant Additive |
Fabrication Method | Drying Method | FR Mechanism | PHRR Reduction (%) |
LOI (%) | VBT/HBT Rating |
Ref. |
|---|---|---|---|---|---|---|---|---|
| PolyMXene (PCM) | Phosphorus and Mxene | Ice-induced assembly and in situ mineralization | Freeze-drying | Gas phase flame inhibition and intumescence effect | - | 45.3 | N/A | [42] |
| Cellulose nanofibers/Sepiolite clay | MTMS (Methyltrimethoxysilane), Sepiolite nanoclay |
Chemo-mechanical means | Freeze-drying | Barrier effect | N/A | N/A | VBT = V-0 HBT = HB |
[36] |
| Cellulose nanofibers | Phosphorus-containing flame-retardant modifier agent (DOPO-IA) |
Simple esterification | Freeze-drying | Gas phase inhibition, intumescence effect | 67.8 | 27 | VBT = V-2 | [12] |
| ZIF-8@cellulose composite aerogels |
2-methylimidazole, ZIF-8 | In situ polymerization | Freeze-drying | Barrier effect by | 49.36 | 49.36 | VBT = V-0 | [43] |
| Cellulose nanofiber (CNF)/boron phosphate (BP) hybrid aerogels |
Boron phosphorus | Cation-induced gelation | Freeze-drying | Gas phase flame inhibition and intumescent effect | 73 | 23.9 | N/A | [44] |
| Cellulose nanofibrils (CNF) |
Melamine Formaldehyde (MF) and Methyltrimethoxysilane (MTMS) | Simple crosslinking reaction | Freeze-drying | Gas phase flame inhibition and barrier effect | 50.6 | 37.1 | VBT = V-0 | [45] |
| Cellulose nanofibrils (CNFs) | Sodium alginate, boric acid | Divalent cation crosslinking | Freeze-drying | Gas phase flame inhibition and barrier effect | 44.6 | 39.5 | VBT = V-1 | [46] |
| Polyvinyl alcohol/cellulose nanofibers hybrid aerogel | Microencapsulated ammonium polyphosphate (MCAPP) | Crosslinking reaction | Freeze-drying | Intumescent effect | 48.48 | 37.5 | VBT = V-0 | [47] |
| Alginate-based | Magnesium hydroxide | Post-crosslinking method | Simple freeze-drying | Barrier effect | 28.91 | 60 | VBT = V-0 | [48] |
| Tubular cellulose aerogels (kapok) |
(NH4)2HPO4 and MMT |
Hydrogen bonding and crosslinking | Oven drying | Gas phase flame inhibition and intumescent effect | 26.64 | 43 | N/A | [49] |
| Cellulose-silica | 1-allyl-methylimidazole chloride (AmimCl), silica | In situ formation | Supercritical CO2 drying | Barrier effect and gas phase inhibition | 15.51 | 34 | N/A | [13] |
LOI = Limiting Oxygen Index, VBT = Vertical Burning Test, HBT = Horizontal Burning Test.
Starch is also an interesting material for preparing aerogels because it is biobased and biodegradable [50][51]. Additionally, it is widely used owing to its excellent mechanical properties, low density, environmental friendliness, biodegradability, and is abundantly available from bioresources [39]. Starch is the main storage carbohydrate in higher plants. Starch contains two D-glucan biopolymers, i.e., amylose, a relatively linear 1,4-α-D-glucan with a small number of long branches; and amylopectin, mainly a 1,4-α-D-glucan containing high-density branches (ca. 5% of glycosidic bonds are α-1,6) [52][53] as shown in Figure 2a.
The performance of starch differs greatly depending on the composition of amylose and amylopectin. Because the heterogeneous structure of pure amylose cannot withstand the pressure during the freezing process, it cannot form aerogels. However, the inclusion of amylose can enhance the specific surface area of the aerogel while decreasing its density. Furthermore, the kind of starch and amylose/amylopectin ratio is critical for the microstructure of aerogels, resulting in varied characteristics. The amylose content of starch, for example, might influence the mechanical characteristics of aerogels [54]. Starch, on the other hand, is typically regarded as a naturally flame-retardant substance due to its good char-forming characteristics, which emits CO2, CO, and creates a carbon layer upon combustion, hence limiting the spread of heat and oxygen [55]. Despite the outstanding advantages of starch aerogel, there remain some drawbacks, such as low hardness. However, the mechanical property can be improved through start materials modified in the preparation process of aerogels [55][56]. Hence, different modification techniques have been used to alter starch-based products to be good as flame retardant materials. Therefore, Glenn and Irving [50] reported the preparation of unmodified wheat starch (B28% amylose and B72% amylopectin), corn starch (B28% amylose and B72% amylopectin), and high-amylose corn starch (B70% amylose and B30% amylopectin) based aerogels. These aerogels were conditioned at 50% relative humidity for at least 48 h before analysis. Among these starch-based aerogels, the corn starch with a high-amylose content aerogel had a nanostructured morphology, whereas corn starch and wheat starch-based aerogels showed 2D sheet-like morphology with macropores and flame-retardant properties and thermal insulation properties. In general, starch-based aerogel can be fabricated in two steps, starch-based hydrogel formation and drying [57]. The supercritical CO2 drying-based technique is commonly used for production [54] similar to the freeze-drying technique. However, the information regarding the production, processing, properties, and uses of starch-based aerogels is rather scattered.
In a recent study, the use of starch as an intumescent flame-retardant synergist for replacing petroleum-based char-forming agents through modifications with expandable graphite (EG) and H3PO4 for the preparation of aerogel by hot vacuum drying has been reported [58]. A portentous decline in peak heat release rate (PHRR) (33.5%) was achieved—attributable to the conjunct physicochemical action of EG and H3PO4 as shown in the quality of the charring layer in Figure 2b.
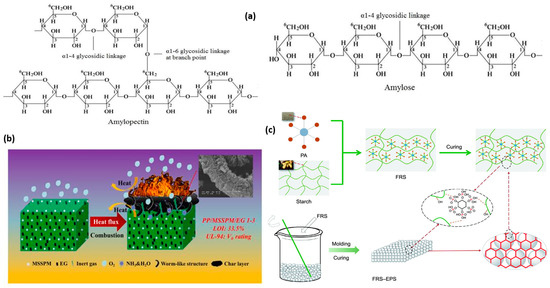
Similarly, a porous bio-based flame-retardant coating via an esterification interaction designed from starch modified with phytic acid (PA) (see Figure 2c) for reducing the flammability of expanded polystyrene aerogel that acts as both a flame retardant and an adhesive was reported [59]. The resultant FR coated aerogel showed an 83.3% reduction in peak heat release and smoke production, indicating its excellent fire retardancy. It also exhibited excellent self-extinguishing behavior in the vertical burning tests with an LOI of 35.5%. Equally, eco-friendly aerogels with high mechanics and fire resistance from naturally occurring pea starch were used to fabricate composite aerogels via a freeze–drying method resulting in the formation of a multi-crosslinked hybrid network and the application of borax and polyvinyl alcohol (PVA) as an additive in enhancing its performance [55]. The peak heat release rate decreased by 74.5%. Not only did the experiment result in the formation of borate ester bonds acting as covalent linkages, but also, the multi-crosslinked hybrid network structure resulted in high mechanics and good thermal insulation. It is important to indicate that research on starch-based materials for the fabrication of sustainable fire-resistant aerogel is still in the infant stages and more research will be needed in this direction to realize the full potential of this important polysaccharide-based resource.
Chitosan-based flame-retardant composite aerogels have emerged as a promising and innovative fire safety solution at the molecular scale [60]. Chitosan, a biopolymer derived from chitin found in the exoskeletons of crustaceans and insects, possesses inherent flame-retardant properties [61]. When combined with other additives and engineered into an aerogel form, chitosan-based composites exhibit enhanced flame retardancy and mechanical strength, making them suitable for diverse applications, including thermal insulation, protective coatings, and fire-resistant barriers [62][63]. This aspect provides a snippet of the properties, production methods, and applications of chitosan as a great promise in mitigating fire hazards and advancing the realm of flame-retardant technology. Chitin/chitosan is a family of linear polysaccharides made up of different proportions of N-acetyl-2 amino-2-deoxy-D-glucose (glucosamine, GlcN) and 2-amino-2-deoxy-D-glucose (N-acetyl-glucosamine, GlcNAc) residues known as chitin and its deacetylated derivative, chitosan [64][65][66] (See Figure 3a). Chitosan is soluble in aqueous acidic conditions by primary amine protonation as well as in several crustacean derivatives.
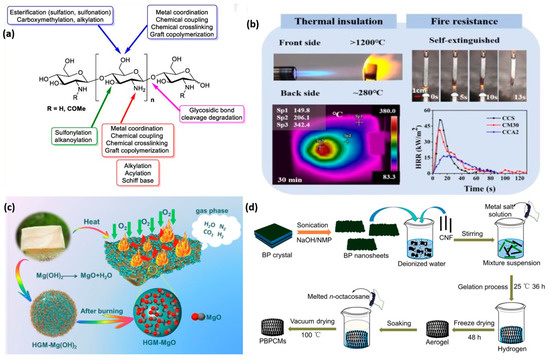
Because of the presence of abundant hydroxyl and amino groups, carboxymethyl chitosan (CCS) is a promising alternative to conventional flame retardants. This gives CCS aerogel superior charring and thermal insulation properties. Additionally, when exposed to a flame, CCS can more securely connect with different 2D nanomaterials to create compact char layers, which will shield the aerogel from burning easily [68]. Biopolymer aerogels, such as CCS aerogel, are typically more plastically bendable and less brittle than inorganic aerogels. To achieve this objective, an ultrasensitive fire-warning and high fire-resistance chitosan/montmorillonite/carbon was fabricated via a freeze-drying method at a temperature of 45 °C [67]. The PHRR was reduced by ca 73% and a V = 0 rating with the composite aerogel serving as a heat sink as shown in Figure 3b.
Similarly, Zhu et al. [61] prepared a sustainable novel porous, yet mechanically tough, vertical directional channel composite aerogel by incorporating Mg (OH)2 coated hollow glass microspheres (HGM) into a chitosan (CSA) matrix and crosslinking it with glutaraldehyde as shown in Figure 3c. The composite aerogel obtained a V =0 rating with a substantial reduction in PHRR with the potential for scaling up for large-scale production. To expand the application possibilities of these sustainable aerogels, Du et al. [44] developed flame-retardant and form-stable phase change composites based on black phosphorus nanosheets/cellulose nanofiber aerogels (See Figure 3d) with extremely high energy storage density and superior solar-thermal conversion efficiency. The form-stable phase change composites aerogel had extremely high n-alkane loading capacity and thermal storage density of ca 247.0–251.6 J g−1. The thermal conductivity and the solar-thermal conversion and storage efficiency of the composite aerogel increased by 89.0% and 87.6%, respectively, whereas the PHRR decreased considerably with a marginal improvement in the LOI value. Further, Yang and co-workers [69] designed and synthesized intrinsic flame-retardant and thermal-conductive vanillin-based epoxy/graphene aerogel (GA) composites with excellent flexural strength and modulus in addition to excellent flame retardancy. Table 2 provides summarized works by other scholars on chitosan/chitin-based flame-retardant composite aerogels.
Table 2. Summarized works by other scholars on chitosan/chitin-based flame-retardant composite aerogels.
| Chitosan/Chitin-Based Aerogel | Flame Retardant Additives | Fabrication Method | Drying Method | FR Mechanism | PHRR Reduction (%) |
LOI (%) | UL-94 Rating (VBT/HBT) |
Ref. |
|---|---|---|---|---|---|---|---|---|
| Phosphorylatedchitosan (PCS) | H3PO4, Chitosan | Freeze thawing | Freeze-drying and Freeze thawing | Gas phase flame inhibition and barrier effect | 91 | 80 | VBT = V-0 | [70] |
| Sodium alginate chitosan, sodium carboxymethylcellulose | Sodium hypophosphiteAlginate, chitosan | Post-crosslinking | Freeze-drying | Gas phase flame inhibition and barrier effect | 19.94 | 37.7 | VBT = V-0 | [28] |
| Carboxymethylchitosan (CCS) | Montmorillonite (MMT), carbon nanotubes | Ionic interaction | Freeze-drying | Barrier effect | - | 85 | VBT = V-0 | [71] |
| Chitosan nanofiller (CNF) | Montmorillonite (MMT) powder | Chemicalcrosslinking via Schiff base reaction | Directional freezing, and Freeze-drying | Barrier effect, slightly gas phase phenomenon | - | 43 | N/A | [63] |
| Chitosan-aluminum/PVA | Aluminum, chitosan | Crosslinking and chemical vapor deposition | Freeze-drying | Gas phase dilution and barrier effect | 77.68 | 41 | VBT = V-0 | [72] |
| Chitosan (CS) hydrogels | MXene, CS | Facile water evaporation-induced self-assembly | Freeze-drying | Barrier effect | 25.6 | N/A | N/A | [73] |
| Biopolymer chitosan (CS) and ammonium polyphosphate (APP) | Boric acid, APP, CS | Crosslinking | Freeze-drying | Gas phase inhibition, barrier effect | 23.0 | N/A | VBT = V-0 | [74] |
| Chitosan (CS)/APP | APP, CS | Layer-by-Layer (LbL) assembly | Vacuum oven drying | Gas phase, barrier effect | 27.6 | 23.8 | N/A | [75] |
| Hydroxyapatite (HAP) and chitosan (CS) | HAP | Chemical crosslinking | UnidirectionalFreeze-drying methods | Barrier effect | - | - | - | [76] |
| Chitosan (CS) | APP and polyethyleneimine (BPEI) | Strong hydrogenbonding | Simple drying | Gas phase flame inhibition, barrier effect | 28.87 | 32.7 | N/A | [77] |
LOI = Limiting Oxygen Index, VBT = Vertical Burning Test, HBT = Horizontal Burning Test.
3. Processing Conditions for Drying of Polysaccharide-Based Composite Aerogels and Structural Properties
In the fabrication of polysaccharide-based composite aerogels, material drying plays a crucial role in the achievement of structurally tough and functional composite aerogel. The wet gel’s solvent is eliminated during the drying phase leaving a highly porous solid structure with a low-density aerogel behind [78][79]. For the aerogel to stay intact and retain its desirable qualities, the drying process must be controlled properly [78][80]. Gel formation is usually obtained through a simple process, but the solvent exchange and drying processes are more challenging, time-consuming, and can cause structural damage. Typically, the supercritical CO2 drying process requires careful alteration of experimental process to ensure the aerogels’ rheological behavior can be managed such that the dried aerogel will be structurally intact [81]. The drying conditions for polysaccharide-based aerogels must be carefully controlled to minimize structural collapse, cracking, or shrinking due to sensitivity [82]. Typically, polar solvents with high surface tension are employed to dissolve polymers; and as a result, direct drying of these solvents results in significant shrinkage due to capillary pressure during the drying process [83]. The most effective and widely adopted method for solving this problem is supercritical CO2 drying (SCD) [84][85][86] where the solvent within the gel is converted into a supercritical fluid by adjusting temperature and pressure conditions above its critical point. The drawbacks of this process include expensive prices and potential safety hazards, as well as the need for extremely harsh drying conditions. The freeze-drying approach is another regularly used method that involves freezing a wet gel to create a solid ice matrix and then sublimating that ice directly into vapor under reduced pressure, by skipping the liquid phase [87][88]. Lyophilization and pre-freezing are two categories of freeze-drying. The liquid in the wet gel is either swiftly frozen using liquid at 196 °C for 10 s or slowly frozen over the course of 24 h using a freezer at 18 °C in the pre-freezing process. The next process is lyophilization, which involves desorption and reduction in moisture content from 7% to between 0.5 and 2.0%. The generated aerogels tend to be fragile due to the rearrangement of water molecules during freeze-drying, and the drying period is typically very long even though the technique preserves the porous structure of the gel and produces a lightweight aerogel with minimum shrinkage [87][88]. Vacuum drying (VD) and ambient pressure drying (APD) are the most practical and economical choices [89][90]. These techniques are difficult to apply because wet gel pores typically cannot withstand the strong capillary forces created when solvents are evaporated inside of them. This causes significant shrinkage and the loss of the porous structure. To lessen the effects of the solid–solvent interaction and meniscus force of deformation during drying, APD, and VD procedures often rely on surface modification and/or solvent exchange by a liquid with low surface tension.
Structurally, most aerogels contain 99% air, making them one of the lightest substance known to man [82][91]. The density of aerogel can be as low as 0.004–0.5 g/cm3 with a high porosity of up to 99.8% and a large specific surface area of 100–1600 m2/g [78]. To view the network structure and pore morphologies in the aerogel, SEM is mostly utilized. The drying process and the aerogel component concentrations are two examples of variables that affect the properties of pore size and distribution. The crucial network structure that connects the components and creates the porous structure is critically examined [92]. By observing the evolving morphology across several works, it is possible to determine the effectiveness of drying during aerogel creation. The morphological structure of most aerogels can be damaged during the drying–adsorption cycle, particularly when the free-drying techniques are employed, because of the quick transition from liquid to crystal form. Network structural changes may also result from the addition of additives to the aerogel structure. Lately, a variety of crosslinking chemicals, including aldehydes, divinylsulfone, cellulose nanofibrils, borax, CaCl2, and bio-based gelatin, are used to improve the mechanical properties of polymer aerogels [93]. For instance, Jaafar et al. found that the viscosity of the XG/CNC colloidal dispersion increased when xyloglucan (XG) was added to a cellulose nanocrystal (CNC) aerogel, causing the morphology to change from a lamellar to an alveolar form [94].
Common characteristics of flame-retardant polysaccharide composite aerogels are their enhanced porosity, which is closely related to their morphologies and mechanical properties, thermal stability, flame retardant performance, dimensional stability, and moisture resistance rate [95]. Hence, the mechanical properties of aerogels are improved using additives. The compressive stress of ZIF-8@cellulose composite aerogel, showed significant improvement due to the dense structure of ZIF-8 with NC serving as cellulose skeleton. The ZIF-8 reinforcement protects the porous structures from collapsing, thereby improving the mechanical properties [43]. Additionally, the addition of inorganic clay fillers as additives is claimed to improve aerogel’s compressive modulus and also improves the FR performance aerogels [49]. A tubular composite aerogel morphology with enhanced compressive strength, high density, thermal-insulating and flame-retardant properties has been reported by the incorporation of NC and nanoparticles [50]. Similarly, silica is reported to significantly improve the compressive strength of aerogels when combined with NC and other flame-retardant materials [13]. Typically, composite aerogels with high silica content have outstanding toughness because they can be crushed without rupturing more than 50%. Generally, aerogels with honeycomb-like structure enhance low-frequency sound absorption capabilities with an anisotropic structure that supports the mechanical, thermal, and heat-resistant properties of aerogels.
4. Challenges and Opportunities in Sustainable Fire-Resistant Polysaccharide-Based Aerogels
Polysaccharide aerogels made of cellulose, chitosan, and starch have a lot of potential as environmentally friendly substitutes for fire-resistant materials. These materials provide several benefits, including their accessibility, biodegradability, and inherent non-toxicity [96]. However, some obstacles reduce their full potential from being realized as fire-resistant aerogels for many applications. The poor mechanical strength of polysaccharide aerogels has been identified as one of the main development concerns. These aerogels frequently show brittleness and fragility when compared to conventional insulation materials [96][97]. Crosslinking and composite creation are two strengthening techniques that are being investigated and used more frequently to improve materials’ mechanical strength to make them suitable for real-world applications. Additionally, the extended thermal stability of polysaccharide aerogels presents a substantial additional obstacle. As a result, these materials are less useful in fire-resistant applications at extremely high temperatures due to their susceptibility to thermal degradation. For this reason, material additives found extensive application in composite aerogel matrixes to help them tolerate extended heat (temperature) exposure.
Moreover, many polysaccharide aerogels are hygroscopic, making them capable of absorbing and holding onto moisture from their surroundings, as shown in numerous research articles [98][99]. Their moisture sensitivity adversely affects their fire-resistance qualities, causing them to lower their ignition point and hasten to burn. Research must thus overcome this obstacle by creating moisture-resistant coatings or adding hydrophobic chemicals to the aerogel matrices. The most often employed compounds for increasing the hydrophobicity of hydrophilic surfaces are silanes [100].
Large-scale manufacturing of polysaccharide aerogels has proven challenging, despite the encouraging results of laboratory-scale production. For these materials to be used commercially, the scalability of manufacturing techniques and the accompanying cost-effectiveness must be addressed. To make polysaccharide aerogels commercially feasible, efforts should be directed at streamlining the manufacturing process, lowering the cost of raw materials, and improving production techniques.
With regards to the opportunities, most polysaccharides are readily accessible renewable resources that can be used to meet the demands of sustainability and the public’s urge to practice climate justice. The abundance of these raw minerals offers a great chance to lessen reliance on fossil fuels and help create a more sustainable future. By exploiting leftover biomass or agricultural byproducts for fire-resistant aerogels, one can contribute to the development of a circular economy. Compared to conventional insulation materials, polysaccharide aerogels have significant environmental benefits. They are produced using renewable resources, biodegrade, and do not produce or burn toxic volatile organic compounds (VOCs). Utilizing polysaccharide aerogels can help foster a greener, more sustainable construction sector by reducing the environmental effect of insulation materials [101]. Although polysaccharide aerogels may not naturally have a high level of fire resistance, this property can be improved due to their versatility of modification. Their fire-resistant performance can be improved chemically through surface functionalization or nanoparticle doping. The use of flame-retardant chemicals can also improve the materials’ fire resistance. Beyond insulation and fire resistance, polysaccharide aerogels offer the potential for multifunctionality that can be of use in a variety of industrial applications with the proper adjustments. Their adaptability creates a variety of opportunities for many businesses and enables the creation of effective and sustainable solutions in fire science in numerous fields.
References
- Shafique, M.; Luo, X. Nanotechnology in transportation vehicles: An overview of its applications, environmental, health and safety concerns. Materials 2019, 12, 2493.
- Mouritz, A.; Gibson, A. Fire safety regulations. In Fire Properties of Polymer Composite Materials; Springer: Dordrecht, The Netherlands, 2006; pp. 313–323.
- North, E.J.; Halden, R.U. Plastics and environmental health: The road ahead. Rev. Environ. Health 2013, 28, 1–8.
- Proshad, R.; Kormoker, T.; Islam, M.S.; Haque, M.A.; Rahman, M.M.; Mithu, M.M.R. Toxic effects of plastic on human health and environment: A consequences of health risk assessment in Bangladesh. Int. J. Health 2018, 6, 1–5.
- Horrocks, A.R. The potential for bio-sustainable organobromine-containing flame retardant formulations for textile applications—A review. Polymers 2020, 12, 2160.
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R. K Sustainable flame-retardant polyurethanes using renewable resources. Ind. Crops Prod. 2018, 123, 480–488.
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydr. Polym. 2019, 226, 115242.
- Biswal, T.; Sahoo, P.K. Fire Retardancy of Polysaccharide-Based Polyurethane Foams. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 2: Green Flame Retardants; ACS Publications: Washington, DC, USA, 2021; pp. 13–29.
- Wang, Y.T.; Zhao, H.B.; Guo, M.L.; Degracia, K.; Sun, H.; Sun, M.Z.; Zhao, Z.Y.; Schiraldi, D.A.; Wang, Y.Z. Rigid and fire-resistant all-biomass aerogels. ACS Sustain. Chem. Eng. 2022, 10, 12117–12126.
- Liu, C.; Ye, S.; Feng, J. The Preparation of Compressible and Fire-Resistant Sponge-Supported Reduced Graphene Oxide Aerogel for Electromagnetic Interference Shielding. Chem.–Asian J. 2016, 11, 2586–2593.
- Madyaratri, E.W.; Ridho, M.R.; Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Nawawi, D.S.; Antov, P.; Kristak, L.; Majlingová, A.; Fatriasari, W. Recent advances in the development of fire-resistant biocomposites—A review. Polymers 2022, 14, 362.
- Yu, Z.L.; Ma, Z.Y.; Yao, H.X.; Qin, B.; Gao, Y.C.; Xia, Z.J.; Huang, Z.H.; Yin, Y.C.; Tu, H.; Ye, H.; et al. Economical Architected Foamy Aerogel Coating for Energy Conservation and Flame Resistance. ACS Mater. Lett. 2022, 4, 1453–1461.
- Yuan, B.; Zhang, J.; Mi, Q.; Yu, J.; Song, R.; Zhang, J. Transparent cellulose–silica composite aerogels with excellent flame retardancy via an in situ sol-gel process. ACS Sustain. Chem. Eng. 2017, 5, 11117–11123.
- Huang, J.; Wang, X.; Guo, W.; Niu, H.; Song, L.; Hu, Y. Eco-friendly thermally insulating cellulose aerogels with exceptional flame retardancy, mechanical property, and thermal stability. J. Taiwan Inst. Chem. Eng. 2022, 131, 104159.
- Wang, D.; Peng, H.; Yu, B.; Zhou, K.; Pan, H.; Zhang, L.; Li, M.; Liu, M.; Tian, A.; Fu, S. Biomimetic structural cellulose nanofiber aerogels with exceptional mechanical, flame-retardant and thermal insulating properties. Chem. Eng. J. 2020, 389, 124449.
- Cao, M.; Liu, B.W.; Zhang, L.; Peng, Z.C.; Zhang, Y.Y.; Wang, H.; Zhao, H.B.; Wang, Y.Z. Fully biomass-based aerogels with ultrahigh mechanical modulus, enhanced flame retardancy, and great thermal insulation applications. Compos. Part B Eng. 2021, 225, 109309.
- Tian, J.; Yang, Y.; Xue, T.; Chao, G.; Fan, W.; Liu, T. Highly flexible and compressible polyimide/silica aerogels with integrated double network for thermal insulation and fire-retardancy. J. Mater. Sci. Technol. 2022, 105, 194–202.
- Xue, T.; Fan, W.; Zhang, X.; Zhao, X.; Yang, F.; Liu, T. Layered double hydroxide/graphene oxide synergistically enhanced polyimide aerogels for thermal insulation and fire-retardancy. Compos. Part B Eng. 2021, 219, 108963.
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631.
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. Aerogel based thermal insulating cementitious composites: A review. Energy Build. 2021, 245, 111058.
- Thapliyal, P.C.; Singh, K. Aerogels as promising thermal insulating materials: An overview. J. Mater. 2014, 2014, 127049.
- Guo, J.; Fu, S.; Deng, Y.; Xu, X.; Laima, S.; Liu, D.; Zhang, P.; Zhou, J.; Zhao, H.; Yu, H.; et al. Hypocrystalline ceramic aerogels for thermal insulation at extreme conditions. Nature 2022, 606, 909–916.
- Ramesh, M.; Rajeshkumar, L.; Balaji, D. Aerogels for insulation applications. Mater. Res. Found. 2021, 98, 57–76.
- Geng, H. A facile approach to light weight, high porosity cellulose aerogels. Int. J. Biol. Macromol. 2018, 118, 921–931.
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342.
- Maleki, H.; Durães, L.; García-González, C.A. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27.
- Akimov, Y.K. Fields of application of aerogels. Instrum. Exp. Tech. 2003, 46, 287–299.
- He, H.; Wang, Y.; Yu, Z.; Liu, J.; Zhao, Y.; Ke, Y. Ecofriendly flame-retardant composite aerogel derived from polysaccharide: Preparation, flammability, thermal kinetics, and mechanism. Carbohydr. Polym. 2021, 269, 118291.
- Cruz, L.G.D.L.; Schiraldi, D.A. Thermal, electrical, insulation and fire resistance properties of polysaccharide and protein-based aerogels. Biobased Aerogels Polysacch. Protein-Based Mater. 2018, 58, 158.
- Wei, P.; Cai, J.; Zhang, L. High-strength and tough crystalline polysaccharide-based materials. Chin. J. Chem. 2020, 38, 761–771.
- Storesund, K.; Amon, F.; Steen-Hansen, A.; Haghighatpanah, S.; Larsson, I. Fire safe, sustainable loose furnishing. Fire Mater. 2021, 45, 181–190.
- Luo, X.; Shen, J.; Ma, Y.; Liu, L.; Meng, R.; Yao, J. Robust, sustainable cellulose composite aerogels with outstanding flame retardancy and thermal insulation. Carbohydr. Polym. 2019, 230, 115623.
- Moreno, P.; Villamizar, N.; Perez, J.; Bayona, A.; Roman, J.; Moreno, N.; Cardozo, N.S.M. Fire-Resistant Cellulose Boards from Waste Newspaper, Boric Acid Salts and Protein Binders. Clean Technol. Environ. Policy 2021, 23, 1537–1546.
- Frenzer, G.; Maier, W.F. Amorphous porous mixed oxides: Sol-gel ways to a highly versatile class of materials and catalysts. Annu. Rev. Mater. Res. 2006, 36, 281–331.
- Yang, H.; Zhu, M.; Li, Y. Sol-gel research in China: A brief history and recent research trends in synthesis of sol-gel derived materials and their applications. J. Sol-Gel Sci. Technol. 2023, 106, 406–421.
- Gupta, P.; Verma, C.; Maji, P.K. Flame retardant and thermally insulating clay based aerogel facilitated by cellulose nanofibers. J. Supercrit. Fluids 2019, 152, 104537.
- Farooq, M.; Sipponen, M.H.; Seppälä, A.; Österberg, M. Eco-friendly Flame-Retardant Cellulose Nanofibril Aerogels by Incorporating Sodium Bicarbonate. ACS Appl. Mater. Interfaces 2018, 10, 27407–27415.
- Ghiorghita, C.A.; Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Polysaccharide-Based Composite Hydrogels as Sustainable Materials for Removal of Pollutants from Wastewater. Molecules 2022, 27, 8574.
- Taib, M.N.A.M.; Hamidon, T.S.; Garba, Z.N.; Trache, D.; Uyama, H.; Hussin, M.H. Recent progress in cellulose-based composites towards flame retardancy applications. Polymer 2022, 244, 124677.
- Chen, J.; Zhu, Z.; Zhang, H.; Fu, S. Sustainable cellulose-based multifunctional material for electromagnetic shielding, flame retardancy and antibacterial. Int. J. Biol. Macromol. 2023, 230, 123295.
- Wang, Z.; E, Y.Y.; Li, J.; Du, T.; Wang, K.; Yao, X.; Jiang, J.; Wang, M. Sustainable Bacterial Cellulose-Based Composite Aerogels with Excellent Flame Retardant and Heat Insulation; Preprint (Version 1); Research Square: Ibaraki, Tsukuba, 2022.
- Zhao, Y.; Zeng, Q.; Lai, X.; Li, H.; Zhao, Y.; Li, K.; Jiang, C.; Zeng, X. Multifunctional cellulose-based aerogel for intelligent fire fighting. Carbohydr. Polym. 2023, 316, 121060.
- Nabipour, H.; Nie, S.; Wang, X.; Song, L.; Hu, Y. Highly flame retardant zeolitic imidazole framework-8@cellulose composite aerogels as absorption materials for organic pollutants. Cellulose 2020, 27, 2237–2251.
- Du, X.; Qiu, J.; Deng, S.; Du, Z.; Cheng, X.; Wang, H. Flame-retardant and form-stable phase change composites based on black phosphorus nanosheets/cellulose nanofiber aerogels with extremely high energy storage density and superior solar-thermal conversion efficiency. J. Mater. Chem. A 2020, 8, 14126–14134.
- Yue, X.; Zhang, S.; He, J.; Wang, Z. Fabrication of Flame Retarded Cellulose Aerogel with Hydrophobicity via MF/MTMS Double Cross-Linking. J. Nat. Fibers 2023, 20, 2133053.
- Jiang, S.; Zhang, M.; Li, M.; Zhu, J.; Ge, A.; Liu, L.; Yu, J. Cellulose-based composite thermal-insulating foams toward eco-friendly, flexible and flame-retardant. Carbohydr. Polym. 2021, 273, 118544.
- Huang, Y.; Zhou, T.; He, S.; Xiao, H.; Dai, H.; Yuan, B.; Yang, X. Flame-retardant polyvinyl alcohol/cellulose nanofibers hybrid carbon aerogel by freeze drying with ultra-low phosphorus. Appl. Surf. Sci. 2019, 497, 143775.
- Shang, K.; Liao, W.; Wang, J.; Wang, Y.T.; Wang, Y.Z.; Schiraldi, D.A. Nonflammable Alginate Nanocomposite Aerogels Prepared by a Simple Freeze-Drying and Post-Cross-Linking Method. ACS Appl. Mater. Interfaces 2016, 8, 643–650.
- Sun, J.; Wu, Z.; An, B.; Ma, C.; Xu, L.; Zhang, Z.; Luo, S.; Li, W.; Liu, S. Thermal-insulating, flame-retardant and mechanically resistant aerogel based on bio-inspired tubular cellulose. Compos. Part B Eng. 2021, 220, 108997.
- Muthuraj, R.; Jimenez-Saelices, C.; Grohens, Y.; Seantier, B. Applications of Polysaccharide and Protein Based Aerogels in Thermal Insulation; Royal Society of Chemistry: London, UK, 2018; pp. 261–294.
- Zheng, Z.; Liao, C.; Xia, Y.; Liu, Y.; Dai, B.; Li, A. Co-microencapsulation of biomass-based char source and melamine polyphosphate and investigation for their synergistic action in flame-retarding polypropylene. Polym. Test. 2020, 90, 106741.
- Maningat, C.C.; Seib, P.A.; Bassi, S.D.; Woo, K.S.; Lasater, G.D. Chapter 10—Wheat Starch: Production, Properties, Modification and Uses, in Starch, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 441–510.
- Villa Zabala, C.C. An Overview on Starch Structure and Chemical Nature. In Starch-Based Nanomaterials; Zabala, C.C.V., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–9.
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10.
- Shi, S.; Jiang, Y.; Ji, Q.; Xing, Y.; Ma, X.; Xia, Y. Multi-crosslinked, ecofriendly flame-retardant starch-based composite aerogels with high compression-resistance. Polym. Eng. Sci. 2022, 63, 154–166.
- Abhari, N.; Madadlou, A.; Dini, A. Structure of starch aerogel as affected by crosslinking and feasibility assessment of the aerogel for an anti-fungal volatile release. Food Chem. 2017, 221, 147–152.
- Zheng, Q.; Tian, Y.; Ye, F.; Zhou, Y.; Zhao, G. Fabrication and application of starch-based aerogel: Technical strategies. Trends Food Sci. Technol. 2020, 99, 608–620.
- Xia, Y.; Chai, W.; Liu, Y.; Su, X.; Liao, C.; Gao, M.; Li, Y.; Zheng, Z. Facile fabrication of starch-based, synergistic intumescent and halogen-free flame retardant strategy with expandable graphite in enhancing the fire safety of polypropylene. Ind. Crops Prod. 2022, 184, 115002.
- Li, M.E.; Zhao, H.B.; Cheng, J.B.; Wang, T.; Fu, T.; Zhang, A.N.; Wang, Y.Z. An Effective Green Porous Structural Adhesive for Thermal Insulating, Flame-Retardant, and Smoke-Suppressant Expandable Polystyrene Foam. Engineering 2022, 17, 151–160.
- Chang, X.; Chen, D.; Jiao, X. Chitosan-based aerogels with high adsorption performance. J. Phys. Chem. B 2008, 112, 7721–7725.
- Zhu, Z.; Niu, Y.; Wang, S.; Su, M.; Long, Y.; Sun, H.; Liang, W.; Li, A. Magnesium hydroxide coated hollow glass microspheres/chitosan composite aerogels with excellent thermal insulation and flame retardancy. J. Colloid Interface Sci. 2022, 612, 35–42.
- Tang, W.; Zhang, A.; Cheng, Y.; Dessie, W.; Liao, Y.; Chen, H.; Qin, Z.; Wang, X.; Jin, X. Fabrication and application of chitosan-based biomass composites with fire safety, water treatment and antibacterial properties. Int. J. Biol. Macromol. 2023, 225, 266–276.
- Sun, Y.; Chu, Y.; Deng, C.; Xiao, H.; Wu, W. High-strength and superamphiphobic chitosan-based aerogels for thermal insulation and flame retardant applications. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129663.
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174.
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of chitosan aerogels: Three-dimensional pore control for tailored applications. Angew. Chem. Int. Ed. 2021, 60, 9828–9851.
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256.
- Chen, J.; Xie, H.; Lai, X.; Li, H.; Gao, J.; Zeng, X. An ultrasensitive fire-warning chitosan/montmorillonite/carbon nanotube composite aerogel with high fire-resistance. Chem. Eng. J. 2020, 399, 125729.
- Xiao, Y.; Zheng, Y.; Wang, X.; Chen, Z.; Xu, Z. Preparation of a chitosan-based flame-retardant synergist and its application in flame-retardant polypropylene. J. Appl. Polym. Sci. 2014, 131.
- Yang, W.; Ding, H.; Liu, T.; Ou, R.; Lin, J.; Puglia, D.; Xu, P.; Wang, Q.; Dong, W.; Du, M.; et al. Design of intrinsically flame-retardant vanillin-based epoxy resin for thermal-conductive epoxy/graphene aerogel composites. ACS Appl. Mater. Interfaces 2021, 13, 59341–59351.
- Cui, H.; Wu, N.; Ma, X.; Niu, F. Superior intrinsic flame-retardant phosphorylated chitosan aerogel as fully sustainable thermal insulation bio-based material. Polym. Degrad. Stab. 2023, 207, 110213.
- Jiang, X.; Zhang, J.; You, F.; Yao, C.; Yang, H.; Chen, R.; Yu, P. Chitosan/clay aerogel: Microstructural evolution, flame resistance and sound absorption. Appl. Clay Sci. 2022, 228, 106624.
- Yang, Z.; Li, H.; Niu, G.; Wang, J.; Zhu, D. Poly(vinylalcohol)/chitosan-based high-strength, fire-retardant and smoke-suppressant composite aerogels incorporating aluminum species via freeze drying. Compos. Part B Eng. 2021, 219, 108919.
- Gong, Y.L.; Jiang, B. Mechanically Flexible and Flame-Retardant Cellulose Nanofibril-Based Films Integrated with MXene and Chitosan. Soft Sci. 2022.
- Luo, M.; Xu, J.; Lv, S.; Yuan, X.; Liang, X. Enhanced Thermal Insulation and Flame-Retardant Properties of Polyvinyl Alcohol-Based Aerogels Composited with Ammonium Polyphosphate and Chitosan. Int. J. Polym. Sci. 2021, 2021, 5555916.
- Deng, S.B.; Liao, W.; Yang, J.C.; Cao, Z.J.; Wang, Y.Z. Flame-Retardant and Smoke-Suppressed Silicone Foams with Chitosan-Based Nanocoatings. Ind. Eng. Chem. Res. 2016, 55, 7239–7248.
- Zhu, J.; Xiong, R.; Zhao, F.; Peng, T.; Hu, J.; Xie, L.; Xie, H.; Wang, K.; Jiang, C. Lightweight, High-Strength, and Anisotropic Structure Composite Aerogel Based on Hydroxyapatite Nanocrystal and Chitosan with Thermal Insulation and Flame Retardant Properties. ACS Sustain. Chem. Eng. 2020, 8, 71–83.
- Chen, J.; Huang, W.; Chen, Y.; Zhou, Z.; Liu, H.; Zhang, W.; Huang, J. Facile Preparation of Chitosan-Based Composite Film with Good Mechanical Strength and Flame Retardancy. Polymers 2022, 14, 1337.
- Muhammad, A.; Lee, D.; Shin, Y.; Park, J. Recent Progress in Polysaccharide Aerogels: Their Synthesis, Application, and Future Outlook. Polymers 2021, 13, 1347.
- Lee, D.H.; Jo, M.J.; Han, S.W.; Yu, S.; Park, H. Polyimide aerogel with controlled porosity: Solvent-induced synergistic pore development during solvent exchange process. Polymer 2020, 205, 122879.
- Zou, F.; Budtova, T. Polysaccharide-based aerogels for thermal insulation and superinsulation: An overview. Carbohydr. Polym. 2021, 266, 118130.
- Tabernero, A.; Baldino, L.; Misol, A.; Cardea, S.; del Valle, E.M.M. Role of rheological properties on physical chitosan aerogels obtained by supercritical drying. Carbohydr. Polym. 2020, 233, 115850.
- El-Naggar, M. Synthesis, Drying Process and Medical Application of Polysaccharide-Based Aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128.
- Leventis, N.; Palczer, A.; McCorkle, L.; Zhang, G.; Sotiriou-Leventis, C. Nanoengineered Silica-Polymer Composite Aerogels with No Need for Supercritical Fluid Drying. J. Sol-Gel Sci. Technol. 2005, 35, 99–105.
- Maleki, H.; Durães, L.; Portugal, A. Synthesis of lightweight polymer-reinforced silica aerogels with improved mechanical and thermal insulation properties for space applications. Microporous Mesoporous Mater. 2014, 197, 116–129.
- Guo, H.; Dewey, O.S.; McCorkle, L.S.; Meador, M.A.B.; Pasquali, M. Polyimide Aerogels as Lightweight Dielectric Insulators for Carbon Nanotube Cables. ACS Appl. Polym. Mater. 2019, 1, 1680–1688.
- Mosanenzadeh, S.G.; Saadatnia, Z.; Shi, F.; Park, C.B.; Naguib, H.E. Structure to properties relations of BPDA and PMDA backbone hybrid diamine polyimide aerogels. Polymer 2019, 176, 213–226.
- Zhang, X.; Li, W.; Song, P.; You, B.; Sun, G. Double-cross-linking strategy for preparing flexible, robust, and multifunctional polyimide aerogel. Chem. Eng. J. 2020, 381, 122784.
- Simón-Herrero, C.; Chen, X.-Y.; Ortiz, M.L.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Linear and crosslinked polyimide aerogels: Synthesis and characterization. J. Mater. Res. Technol. 2019, 8, 2638–2648.
- Chen, D.; Dong, K.; Gao, H.; Zhuang, T.; Huang, X.; Wang, G. Vacuum-dried flexible hydrophobic aerogels using bridged methylsiloxane as reinforcement: Performance regulation with alkylorthosilicate or alkyltrimethoxysilane co-precursors. New J. Chem. 2019, 43, 2204–2212.
- Hasegawa, G.; Shimizu, T.; Kanamori, K.; Maeno, A.; Kaji, H.; Nakanishi, K. Highly Flexible Hybrid Polymer Aerogels and Xerogels Based on Resorcinol-Formaldehyde with Enhanced Elastic Stiffness and Recoverability: Insights into the Origin of Their Mechanical Properties. Chem. Mater. 2017, 29, 2122–2134.
- Zu, G.; Kanamori, K.; Maeno, A.; Kaji, H.; Nakanishi, K.; Shen, J. Ambient-dried highly flexible copolymer aerogels and their nanocomposites with polypyrrole for thermal insulation, separation, and pressure sensing. Polym. Chem. 2019, 10, 4980–4990.
- Zhang, H.; Wang, J.; Xu, G.; Xu, Y.; Wang, F.; Shen, H. Ultralight, hydrophobic, sustainable, cost-effective and floating kapok/microfibrillated cellulose aerogels as speedy and recyclable oil superabsorbents. J. Hazard. Mater. 2021, 406, 124758.
- Batista, M.P.; Gonçalves, V.S.S.; Gaspar, F.B.; Nogueira, I.D.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782.
- Jaafar, Z.; Quelennec, B.; Moreau, C.; Lourdin, D.; Maigret, J.E.; Pontoire, B.; D’orlando, A.; Coradin, T.; Duchemin, B.; Fernandes, F.M.; et al. Plant cell wall inspired xyloglucan/cellulose nanocrystals aerogels produced by freeze-casting. Carbohydr. Polym. 2020, 247, 116642.
- Wu, N.; Deng, S.; Wang, F.; Wang, M.; Xia, M.; Cui, H.; Jia, H. Highly Efficient Flame-Retardant and Enhanced PVA-Based Composite Aerogels through Interpenetrating Cross-Linking Networks. Polymers 2023, 15, 657.
- Fu, Y.; Guo, Z. Natural polysaccharide-based aerogels and their applications in oil-water separations: A review. J. Mater. Chem. A 2022, 10, 8129–8158.
- Illera, D.; Mesa, J.; Gomez, H.; Maury, H. Cellulose aerogels for thermal insulation in buildings: Trends and challenges. Coatings 2018, 8, 345.
- Chang, K.J.; Wang, Y.Z.; Peng, K.C.; Tsai, H.S.; Chen, J.R.; Huang, C.T.; Ho, K.S.; Lien, W.F. Preparation of silica aerogel/polyurethane composites for the application of thermal insulation. J. Polym. Res. 2014, 21, 338.
- Wu, K.; Wu, H.; Wang, R.; Yan, X.; Sun, W.; Liu, Y.; Kuang, Y.; Jiang, F.; Chen, S. The use of cellulose fiber from office waste paper to improve the thermal insulation-related property of konjac glucomannan/starch aerogel. Ind. Crops Prod. 2022, 177, 114424.
- Sanz-Moral, L.M.; Rueda, M.; Nieto, A.; Novak, Z.; Knez, Ž.; Martín, Á. Gradual hydrophobic surface functionalization of dry silica aerogels by reaction with silane precursors dissolved in supercritical carbon dioxide. J. Supercrit. Fluids 2013, 84, 74–79.
- Lizundia, E.; Luzi, F.; Puglia, D. Organic waste valorisation towards circular and sustainable biocomposites. Green Chem. 2022, 24, 5429–5459.
More
Information
Subjects:
Materials Science, Composites
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
16 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




