
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jevan Cevik | -- | 2085 | 2023-08-15 12:58:04 | | | |
| 2 | Lindsay Dong | Meta information modification | 2085 | 2023-08-17 02:49:42 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2085 | 2023-08-17 02:50:18 | | |
Video Upload Options
Breast reconstruction is an essential component in the multidisciplinary management of breast cancer patients. Over the years, preoperative planning has played a pivotal role in assisting surgeons in planning operative decisions prior to the day of surgery. The evolution of preoperative planning can be traced back to the introduction of modalities such as ultrasound and colour duplex ultrasonography, enabling surgeons to evaluate the donor site’s vasculature and thereby plan operations more accurately. However, the limitations of these techniques paved the way for the implementation of modern three-dimensional imaging technologies. With the advancements in 3D imaging, including computed tomography and magnetic resonance imaging, surgeons gained the ability to obtain detailed anatomical information. Moreover, numerous adjuncts have been developed to aid in the planning process. The integration of 3D-printing technologies has made significant contributions, enabling surgeons to create complex haptic models of the underlying anatomy. Direct infrared thermography provides a non-invasive, visual assessment of abdominal wall vascular physiology. Additionally, augmented reality technologies are poised to reshape surgical planning by providing an immersive and interactive environment for surgeons to visualize and manipulate 3D reconstructions. Still, the future of preoperative planning in breast reconstruction holds immense promise.
1. Introduction
2. Early Techniques
3. Emergence of Advanced Imaging Modalities
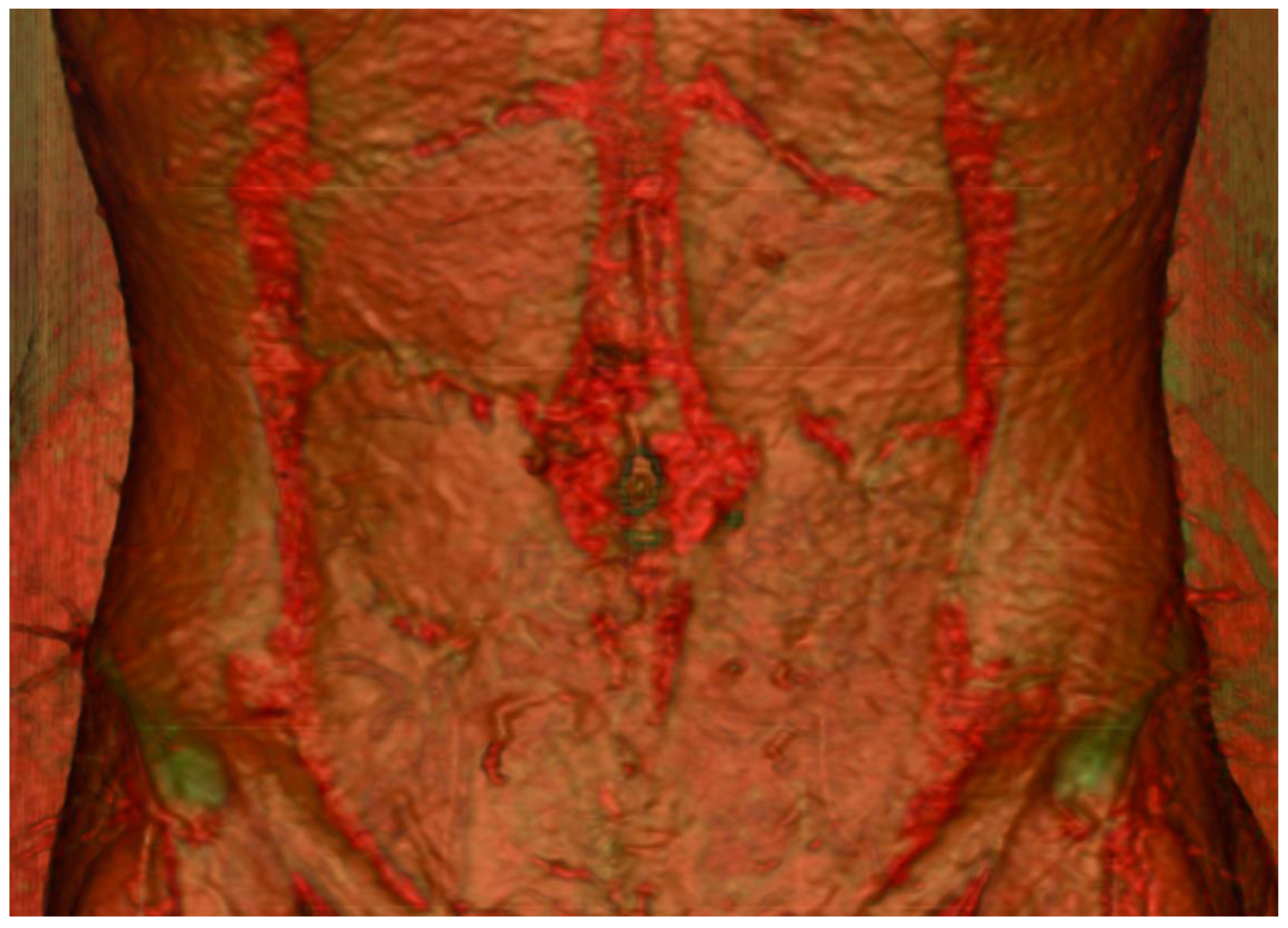
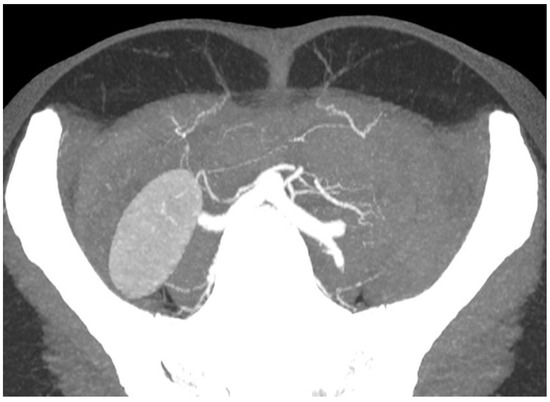
4. Development of Imaging Adjuncts
4.1. Direct Infrared Thermography (DIRT)
4.2. Three-Dimensional Printing
4.3. Augmented Reality
4.4. Contrast-Enhanced Ultrasound
5. Artificial Intelligence in Pre-Operative Planning
References
- Cevik, J.; Hunter-Smith, D.J.; Rozen, W.M. Current Advances in Breast Reconstruction. J. Clin. Med. 2022, 11, 3328.
- Seth, I.; Seth, N.; Bulloch, G.; Rozen, W.M.; Hunter-Smith, D.J. Systematic Review of Breast-Q: A Tool to Evaluate Post-Mastectomy Breast Reconstruction. Breast Cancer Targets Ther. 2021, 13, 711–724.
- Edwards, B.F. Teflon-silicone breast implants. Plast. Reconstr. Surg. 1963, 32, 519–526.
- Gersuny, R. The classic reprint. Concerning a subcutaneous prosthesis: Robert Gersuny. (Uber eine subcutane Prothese. Zeitschrift f. Heilkunde Wien u Leipzig 21:199, 1900). Translated from the German by Miss Rita Euerle. Plast. Reconstr. Surg. 1980, 65, 525–527.
- Cronin, T.D.; Gerow, F.J. Augmentation mammaplasty: A new. “natural feel” prostheses. Excerpta Medica Int. Congr. Ser. 1963, 66, 41–46.
- Daniel, R.K.; Taylor, G.I. Distant transfer of an island flap by microvascular anastomoses. A clinical technique. Plast. Reconstr. Surg. 1973, 52, 111–117.
- Taylor, G.I.; Daniel, R.K. The free flap: Composite tissue transfer by vascular anastomosis. Aust. N. Z. J. Surg. 1973, 43, 1–3.
- Fujino, T.; Harasina, T.; Aoyagi, F. Reconstruction for aplasia of the breast and pectoral region by microvascular transfer of a free flap from the buttock. Plast. Reconstr. Surg. 1975, 56, 178–181.
- Allen, R.J.; Treece, P. Deep inferior epigastric perforator flap for breast reconstruction. Ann. Plast. Surg. 1994, 32, 32–38.
- Rozen, W.M.; Bhullar, H.K.; Hunter-Smith, D. How to assess a CTA of the abdomen to plan an autologous breast reconstruction. Gland Surg. 2019, 8, S291–S296.
- Boyd, J.B.; Taylor, G.I.; Corlett, R. The vascular territories of the superior epigastric and the deep inferior epigastric systems. Plast. Reconstr. Surg. 1984, 73, 1–16.
- Tansatit, T.; Chokrungvaranont, P.; Sanguansit, P.; Wanidchaphloi, S. Neurovascular anatomy of the deep inferior epigastric perforator flap for breast reconstruction. J. Med. Assoc. Thai. 2006, 89, 1630–1640.
- Rozen, W.M.; Grinsell, D.; Koshima, I.; Ashton, M.W. Dominance between angiosome and perforator territories: A new anatomical model for the design of perforator flaps. J. Reconstr. Microsurg. 2010, 26, 539–545.
- Rozen, W.M.; Ashton, M.W.; Pan, W.R.; Taylor, G.I. Raising perforator flaps for breast reconstruction: The intramuscular anatomy of the deep inferior epigastric artery. Plast. Reconstr. Surg. 2007, 120, 1443–1449.
- Satomura, S.; Matsubara, S.; Yoshioka, M. A new method of mechanical vibration measurement and its application. Mem. Inst. Sci. Ind. Res. Osaka Univ. 1956, 13, 125–133.
- Satomura, S. Ultrasonic Doppler method for the inspection of cardiac function. J. Acoust. Soc. Am. 1957, 29, 1181–1185.
- Yoshida, T.; Mori, M.; Nimura, Y.; Okimura, M.; Hikita, G. Studies on the examination of the heart with the ultrasonic Doppler method III— Variety Doppler signals IV—Clinical application. Jpn. Circ. J. 1956, 20, 227.
- Aoyagi, F.; Fujino, T.; Ohshiro, T. Detection of small vessels for microsurgery by a Doppler flowmeter. Plast. Reconstr. Surg. 1975, 55, 372–373.
- Blondeel, P.N.; Beyens, G.; Verhaeghe, R.; Van Landuyt, K.; Tonnard, P.; Monstrey, S.J.; Matton, G. Doppler flowmetry in the planning of perforator flaps. Br. J. Plast. Surg. 1998, 51, 202–209.
- Giunta, R.E.; Geisweid, A.; Feller, A.M. The value of preoperative Doppler sonography for planning free perforator flaps. Plast. Reconstr. Surg. 2000, 105, 2381–2386.
- Taylor, G.I.; Doyle, M.; McCarten, G. The Doppler probe for planning flaps: Anatomical study and clinical applications. Br. J. Plast. Surg. 1990, 43, 1–16.
- Cheng, M.H.; Chen, H.C.; Santamaria, E.; Chen, H.S.; Kuo, Y.R.; Coessens, B.; Wei, F.C. Preoperative ultrasound Doppler study and clinical correlation of free posterior interosseous flap. Changgeng Yi Xue Za Zhi 1997, 20, 258–264.
- Yu, P.; Youssef, A. Efficacy of the handheld Doppler in preoperative identification of the cutaneous perforators in the anterolateral thigh flap. Plast. Reconstr. Surg. 2006, 118, 928–933.
- Stekelenburg, C.M.; Sonneveld, P.M.; Bouman, M.B.; van der Wal, M.B.; Knol, D.L.; de Vet, H.C.; van Zuijlen, P.P. The hand held Doppler device for the detection of perforators in reconstructive surgery: What you hear is not always what you get. Burns 2014, 40, 1702–1706.
- Khan, U.D.; Miller, J.G. Reliability of handheld Doppler in planning local perforator-based flaps for extremities. Aesthet. Plast. Surg. 2007, 31, 521–525.
- Shaw, R.J.; Batstone, M.D.; Blackburn, T.K.; Brown, J.S. Preoperative Doppler assessment of perforator anatomy in the anterolateral thigh flap. Br. J. Oral Maxillofac. Surg. 2010, 48, 419–422.
- Rozen, W.M.; Phillips, T.J.; Ashton, M.W.; Stella, D.L.; Gibson, R.N.; Taylor, G.I. Preoperative imaging for DIEA perforator flaps: A comparative study of computed tomographic angiography and Doppler ultrasound. Plast. Reconstr. Surg. 2008, 121, 9–16.
- Wade, R.G.; Watford, J.; Wormald, J.C.R.; Bramhall, R.J.; Figus, A. Perforator mapping reduces the operative time of DIEP flap breast reconstruction: A systematic review and meta-analysis of preoperative ultrasound, computed tomography and magnetic resonance angiography. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 468–477.
- Salgarello, M.; Visconti, G. Designing Lateral Thoracic Wall Perforator Flaps for Breast Reconstruction Using Ultrasound. J. Reconstr. Microsurg. 2022, 38, 228–232.
- Perez-Iglesias, C.T.; Laikhter, E.; Kang, C.O.; Nassar, A.H.; Maselli, A.M.; Cauley, R.; Lee, B.T. Current Applications of Ultrasound Imaging in the Preoperative Planning of DIEP Flaps. J. Reconstr. Microsurg. 2022, 38, 221–227.
- Curry, G.R.; White, D.N. Color coded ultrasonic differential velocity arterial scanner (Echoflow). Ultrasound Med. Biol. 1978, 4, 27–35.
- Eyer, M.K.; Brandestini, M.A.; Phillips, D.J.; Baker, D.W. Color digital echo/Doppler image presentation. Ultrasound Med. Biol. 1981, 7, 21–31.
- Brandestini, M. Topoflow—A digital full range Doppler velocity meter. IEEE Trans. Sonics Ultrasonics 1978, 25, 287–292.
- Hallock, G.G. Evaluation of fasciocutaneous perforators using color duplex imaging. Plast. Reconstr. Surg. 1994, 94, 644–651.
- Hallock, G.G. Doppler sonography and color duplex imaging for planning a perforator flap. Clin. Plast. Surg. 2003, 30, 347–357.
- Chang, B.W.; Luethke, R.; Berg, W.A.; Hamper, U.M.; Manson, P.N. Two-dimensional color Doppler imaging for precision preoperative mapping and size determination of TRAM flap perforators. Plast. Reconstr. Surg. 1994, 93, 197–200.
- Lethaus, B.; Loberg, C.; Kloss-Brandstätter, A.; Bartella, A.K.; Steiner, T.; Modabber, A.; Hölzle, F.; Teichmann, J. Color duplex ultrasonography versus handheld Doppler to plan anterior lateral thigh flaps. Microsurgery 2017, 37, 388–393.
- Ensat, F.; Babl, M.; Conz, C.; Rueth, M.J.; Greindl, M.; Fichtl, B.; Herzog, G.; Ussmueller, J.; Spies, M. The efficacy of color duplex sonography in preoperative assessment of anterolateral thigh flap. Microsurgery 2012, 32, 605–610.
- Debelmas, A.; Camuzard, O.; Aguilar, P.; Qassemyar, Q. Reliability of Color Doppler Ultrasound Imaging for the Assessment of Anterolateral Thigh Flap Perforators: A Prospective Study of 30 Perforators. Plast. Reconstr. Surg. 2018, 141, 762–766.
- Ogawa, R.; Hyakusoku, H.; Murakami, M. Color Doppler ultrasonography in the planning of microvascular augmented “super-thin” flaps. Plast. Reconstr. Surg. 2003, 112, 822–828.
- Berg, W.A.; Chang, B.W.; DeJong, M.R.; Hamper, U.M. Color Doppler flow mapping of abdominal wall perforating arteries for transverse rectus abdominis myocutaneous flap in breast reconstruction: Method and preliminary results. Radiology 1994, 192, 447–450.
- Hallock, G.G. Acoustic Doppler sonography, color duplex ultrasound, and laser Doppler flowmetry as tools for successful autologous breast reconstruction. Clin. Plast. Surg. 2011, 38, 203–211.
- Napel, S.; Marks, M.P.; Rubin, G.D.; Dake, M.D.; McDonnell, C.H.; Song, S.M.; Enzmann, D.R.; Jeffrey, R.B., Jr. CT angiography with spiral CT and maximum intensity projection. Radiology 1992, 185, 607–610.
- Schwartz, R.B.; Jones, K.M.; Chernoff, D.M.; Mukherji, S.K.; Khorasani, R.; Tice, H.M.; Kikinis, R.; Hooton, S.M.; Stieg, P.E.; Polak, J.F. Common carotid artery bifurcation: Evaluation with spiral CT. Work in progress. Radiology 1992, 185, 513–519.
- Masia, J.; Clavero, J.A.; Larrañaga, J.R.; Alomar, X.; Pons, G.; Serret, P. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 594–599.
- Rozen, W.M.; Phillips, T.J.; Ashton, M.W.; Stella, D.L.; Taylor, G.I. A new preoperative imaging modality for free flaps in breast reconstruction: Computed tomographic angiography. Plast. Reconstr. Surg. 2008, 122, 38e–40e.
- Mihara, M.; Nakanishi, M.; Nakashima, M.; Narushima, M.; Koshima, I. Utility and anatomical examination of the DIEP flap’s three-dimensional image with multidetector computed tomography. Plast. Reconstr. Surg. 2008, 122, 40e–41e.
- Alonso-Burgos, A.; García-Tutor, E.; Bastarrika, G.; Cano, D.; Martínez-Cuesta, A.; Pina, L.J. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: Imaging findings and initial experience. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 585–593.
- Cevik, J.; Rozen, W. A Novel optimization technique of Computed Tomography Angiographic 3D-reconstructions for pre-operative planning of DIEP flaps. JPRAS Open 2023, 35, 38–41.
- Fitzgerald O’Connor, E.; Rozen, W.M.; Chowdhry, M.; Band, B.; Ramakrishnan, V.V.; Griffiths, M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surg. 2016, 5, 93–98.
- Rozen, W.M.; Ashton, M.W.; Grinsell, D.; Stella, D.L.; Phillips, T.J.; Taylor, G.I. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery 2008, 28, 306–313.
- Clavero, J.A.; Masia, J.; Larrañaga, J.; Monill, J.M.; Pons, G.; Siurana, S.; Alomar, X. MDCT in the preoperative planning of abdominal perforator surgery for postmastectomy breast reconstruction. AJR Am. J. Roentgenol. 2008, 191, 670–676.
- Scott, J.R.; Liu, D.; Said, H.; Neligan, P.C.; Mathes, D.W. Computed tomographic angiography in planning abdomen-based microsurgical breast reconstruction: A comparison with color duplex ultrasound. Plast. Reconstr. Surg. 2010, 125, 446–453.
- Teunis, T.; Heerma van Voss, M.R.; Kon, M.; van Maurik, J.F. CT-angiography prior to DIEP flap breast reconstruction: A systematic review and meta-analysis. Microsurgery 2013, 33, 496–502.
- Minqiang, X.; Lanhua, M.; Jie, L.; Dali, M.; Jinguo, L. The value of multidetector-row CT angiography for pre-operative planning of breast reconstruction with deep inferior epigastric arterial perforator flaps. Br. J. Radiol. 2010, 83, 40–43.
- Smit, J.M.; Dimopoulou, A.; Liss, A.G.; Zeebregts, C.J.; Kildal, M.; Whitaker, I.S.; Magnusson, A.; Acosta, R. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1112–1117.
- Fansa, H.; Schirmer, S.; Frerichs, O.; Gehl, H.B. . Handchir. Mikrochir. Plast. Chir. 2011, 43, 81–87.
- Casey, W.J., 3rd; Chew, R.T.; Rebecca, A.M.; Smith, A.A.; Collins, J.M.; Pockaj, B.A. Advantages of preoperative computed tomography in deep inferior epigastric artery perforator flap breast reconstruction. Plast. Reconstr. Surg. 2009, 123, 1148–1155.
- Gacto-Sánchez, P.; Sicilia-Castro, D.; Gómez-Cía, T.; Lagares, A.; Collell, T.; Suárez, C.; Parra, C.; Leal, S.; Infante-Cossío, P.; De La Higuera, J.M. Computed tomographic angiography with VirSSPA three-dimensional software for perforator navigation improves perioperative outcomes in DIEP flap breast reconstruction. Plast. Reconstr. Surg. 2010, 125, 24–31.
- Ghattaura, A.; Henton, J.; Jallali, N.; Rajapakse, Y.; Savidge, C.; Allen, S.; Searle, A.E.; Harris, P.A.; James, S.E. One hundred cases of abdominal-based free flaps in breast reconstruction. The impact of preoperative computed tomographic angiography. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1597–1601.
- Rozen, W.M.; Anavekar, N.S.; Ashton, M.W.; Stella, D.L.; Grinsell, D.; Bloom, R.J.; Taylor, G.I. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction? Microsurgery 2008, 28, 516–523.
- Lauterbur, P.C. Image Formation by Induced Local Interactions: Examples Employing Nuclear Magnetic Resonance. Nature 1973, 242, 190–191.
- Ahn, C.Y.; Narayanan, K.; Shaw, W.W. In vivo anatomic study of cutaneous perforators in free flaps using magnetic resonance imaging. J. Reconstr. Microsurg. 1994, 10, 157–163.
- Rozen, W.M.; Stella, D.L.; Bowden, J.; Taylor, G.I.; Ashton, M.W. Advances in the pre-operative planning of deep inferior epigastric artery perforator flaps: Magnetic resonance angiography. Microsurgery 2009, 29, 119–123.
- Chernyak, V.; Rozenblit, A.M.; Greenspun, D.T.; Levine, J.L.; Milikow, D.L.; Chia, F.A.; Erhard, H.A. Breast reconstruction with deep inferior epigastric artery perforator flap: 3.0-T gadolinium-enhanced MR imaging for preoperative localization of abdominal wall perforators. Radiology 2009, 250, 417–424.
- Haider, C.R.; Glockner, J.F.; Stanson, A.W.; Riederer, S.J. Peripheral vasculature: High-temporal- and high-spatial-resolution three-dimensional contrast-enhanced MR angiography. Radiology 2009, 253, 831–843.
- Vasile, J.V.; Levine, J.L. Magnetic resonance angiography in perforator flap breast reconstruction. Gland Surg. 2016, 5, 197–211.
- Greenspun, D.; Vasile, J.; Levine, J.L.; Erhard, H.; Studinger, R.; Chernyak, V.; Newman, T.; Prince, M.; Allen, R.J. Anatomic imaging of abdominal perforator flaps without ionizing radiation: Seeing is believing with magnetic resonance imaging angiography. J. Reconstr. Microsurg. 2010, 26, 37–44.
- Newman, T.M.; Vasile, J.; Levine, J.L.; Greenspun, D.T.; Allen, R.J.; Chao, M.T.; Winchester, P.A.; Prince, M.R. Perforator flap magnetic resonance angiography for reconstructive breast surgery: A review of 25 deep inferior epigastric and gluteal perforator artery flap patients. J. Magn. Reson. Imaging 2010, 31, 1176–1184.
- Thimmappa, N.D.; Vasile, J.V.; Ahn, C.Y.; Levine, J.L.; Prince, M.R. MRA of the skin: Mapping for advanced breast reconstructive surgery. Clin. Radiol. 2019, 74, 13–28.
- Schaverien, M.V.; Ludman, C.N.; Neil-Dwyer, J.; McCulley, S.J. Contrast-enhanced magnetic resonance angiography for preoperative imaging of deep inferior epigastric artery perforator flaps: Advantages and disadvantages compared with computed tomography angiography: A United Kingdom perspective. Ann. Plast. Surg. 2011, 67, 671–674.
- Pauchot, J.; Aubry, S.; Kastler, A.; Laurent, O.; Kastler, B.; Tropet, Y. Preoperative imaging for deep inferior epigastric perforator flaps: A comparative study of computed tomographic angiography and magnetic resonance angiography. Eur. J. Plast. Surg. 2012, 35, 795–801.
- Cina, A.; Barone-Adesi, L.; Rinaldi, P.; Cipriani, A.; Salgarello, M.; Masetti, R.; Bonomo, L. Planning deep inferior epigastric perforator flaps for breast reconstruction: A comparison between multidetector computed tomography and magnetic resonance angiography. Eur. Radiol. 2013, 23, 2333–2343.
- Ring, E.F. The historical development of thermal imaging in medicine. Rheumatology 2004, 43, 800–802.
- Theuvenet, W.J.; Koeyers, G.F.; Borghouts, M.H. Thermographic assessment of perforating arteries. A preoperative screening method for fasciocutaneous and musculocutaneous flaps. Scand. J. Plast. Reconstr. Surg. 1986, 20, 25–29.
- Salmi, A.M.; Tukiainen, E.; Asko-Seljavaara, S. Thermographic mapping of perforators and skin blood flow in the free transverse rectus abdominis musculocutaneous flap. Ann. Plast. Surg. 1995, 35, 159–164.
- de Weerd, L.; Weum, S.; Mercer, J.B. The value of dynamic infrared thermography (DIRT) in perforatorselection and planning of free DIEP flaps. Ann. Plast. Surg. 2009, 63, 274–279.
- Whitaker, I.S.; Lie, K.H.; Rozen, W.M.; Chubb, D.; Ashton, M.W. Dynamic infrared thermography for the preoperative planning of microsurgical breast reconstruction: A comparison with CTA. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 130–132.
- Weum, S.; Mercer, J.B.; de Weerd, L. Evaluation of dynamic infrared thermography as an alternative to CT angiography for perforator mapping in breast reconstruction: A clinical study. BMC Med. Imaging 2016, 16, 43.
- Yamamoto, T.; Todokoro, T.; Koshima, I. Handheld thermography for flap monitoring. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1747–1748.
- de Weerd, L.; Miland, A.O.; Mercer, J.B. Perfusion dynamics of free DIEP and SIEA flaps during the first postoperative week monitored with dynamic infrared thermography. Ann. Plast. Surg. 2009, 62, 42–47.
- Smit, J.M.; Negenborn, V.L.; Jansen, S.M.; Jaspers, M.E.H.; de Vries, R.; Heymans, M.W.; Winters, H.A.H.; van Leeuwen, T.G.; Mullender, M.G.; Krekel, N.M.A. Intraoperative evaluation of perfusion in free flap surgery: A systematic review and meta-analysis. Microsurgery 2018, 38, 804–818.
- de Weerd, L.; Mercer, J.B.; Setså, L.B. Intraoperative dynamic infrared thermography and free-flap surgery. Ann. Plast. Surg. 2006, 57, 279–284.
- Hennessy, O.; Potter, S.M. Use of infrared thermography for the assessment of free flap perforators in autologous breast reconstruction: A systematic review. JPRAS Open 2020, 23, 60–70.
- Hardwicke, J.T.; Osmani, O.; Skillman, J.M. Detection of Perforators Using Smartphone Thermal Imaging. Plast. Reconstr. Surg. 2016, 137, 39–41.
- Mu, X.; Zhang, J.; Jiang, Y. 3D Printing in Breast Reconstruction: From Bench to Bed. Front. Surg. 2021, 8, 641370.
- Hummelink, S.; Verhulst, A.C.; Maal, T.J.J.; Hoogeveen, Y.L.; Schultze Kool, L.J.; Ulrich, D.J.O. An innovative method of planning and displaying flap volume in DIEP flap breast reconstructions. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 871–875.
- Chae, M.P.; Ganhewa, D.; Hunter-Smith, D.J.; Rozen, W.M. Direct augmented reality computed tomographic angiography technique (ARC): An innovation in preoperative imaging. Eur. J. Plast. Surg. 2018, 41, 415–420.
- Rahman, O.F.; Nahabedian, M.Y.; Sinkin, J.C. Augmented Reality and Wearable Technology in Image-guided Navigation and Preoperative Planning. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1057.
- Wesselius, T.S.; Meulstee, J.W.; Luijten, G.; Xi, T.; Maal, T.J.J.; Ulrich, D.J.O. Holographic Augmented Reality for DIEP Flap Harvest. Plast. Reconstr. Surg. 2021, 147, 25e–29e.
- Pratt, P.; Ives, M.; Lawton, G.; Simmons, J.; Radev, N.; Spyropoulou, L.; Amiras, D. Through the HoloLens™ looking glass: Augmented reality for extremity reconstruction surgery using 3D vascular models with perforating vessels. Eur. Radiol. Exp. 2018, 2, 2.
- Su, W.; Lu, L.; Lazzeri, D.; Zhang, Y.X.; Wang, D.; Innocenti, M.; Qian, Y.; Agostini, T.; Levin, L.S.; Messmer, C. Contrast-enhanced ultrasound combined with three-dimensional reconstruction in preoperative perforator flap planning. Plast. Reconstr. Surg. 2013, 131, 80–93.
- Varoquaux, G.; Cheplygina, V. Machine learning for medical imaging: Methodological failures and recommendations for the future. npj Digit. Med. 2022, 5, 48.
- Aggarwal, R.; Sounderajah, V.; Martin, G.; Ting, D.S.W.; Karthikesalingam, A.; King, D.; Ashrafian, H.; Darzi, A. Diagnostic accuracy of deep learning in medical imaging: A systematic review and meta-analysis. npj Digit. Med. 2021, 4, 65.
- Kim, M.; Yun, J.; Cho, Y.; Shin, K.; Jang, R.; Bae, H.J.; Kim, N. Deep Learning in Medical Imaging. Neurospine 2019, 16, 657–668.
- O’Neill, A.C.; Yang, D.; Roy, M.; Sebastiampillai, S.; Hofer, S.O.P.; Xu, W. Development and Evaluation of a Machine Learning Prediction Model for Flap Failure in Microvascular Breast Reconstruction. Ann. Surg. Oncol. 2020, 27, 3466–3475.




