Early techniques focused primarily on the use of prosthetic implants for aesthetic reconstruction, yet, following significant breakthroughs in our understanding of vascular anatomy and microsurgical tissue transfer, a resurgence of autologous breast reconstruction took place
[3][4][5][6][7][8][3,4,5,6,7,8]. Numerous donor sites have been reported, yet, of the options available, the abdominal wall became one of the more popular sites for autologous tissue given the frequent presence of excess soft tissue and its ability to closely resemble native breast tissue
[9][10][9,10]. In 1994, Allen and Treece introduced the deep inferior epigastric artery perforator (DIEP) flap, revolutionising the field of breast reconstruction
[9]. Unlike traditional muscle-based myocutaneous flaps, the DIEP flap spares the rectus abdominis muscle by utilising only the skin and fat tissue, resulting in reduced donor site morbidity and improved abdominal wall function. However, perforator-based flaps of the abdominal wall, such as the DIEP flap, exhibit significant variability in their vascular supply, confronting surgeons with a new challenge and necessitating precise preoperative planning to ensure the viability and success of the reconstructive procedure
[11][12][13][14][11,12,13,14].
2. Early Techniques
With the advent of perforator-based flaps, the visualisation and identification of perforators was initially performed using Doppler ultrasound. First introduced to the medical field in the 1950s, Doppler ultrasound was primarily used for cardiovascular applications, exploring the function of the heart and flow of blood in the human body
[15][16][17][15,16,17]. By the 1970s, the medical field began recognising its potential applications in other areas, including reconstructive microsurgery
[18]. As surgeons realised the importance of understanding the vascular architecture for successful breast reconstruction, Doppler ultrasound emerged as a non-invasive, real-time imaging technique with which to identify perforators and map vascular anatomy, enabling more accurate surgical planning
[19][20][19,20].
As the only available option for surgeons early on, Doppler ultrasound showed promise as a fast, readily available, and simple method of perforator identification. Early research suggested that Doppler ultrasound had encouraging accuracy in detecting perforators preoperatively
[19][20][21][22][19,20,21,22]. However, while it offers some insight into the vascular network, it does not provide a comprehensive three-dimensional map, differs in results depending on the user, and can occasionally miss smaller vessels or misinterpret the flow of superficial main vessels as perforators
[23][24][25][23,24,25]. This is highlighted in studies that reveal the Doppler probe’s occasional inaccuracies when juxtaposed with surgical observations or more modern imaging techniques, particularly with regard to smaller, deeply located vessels
[26][27][28][26,27,28]. Nevertheless, ultrasound remains a popular and widely utilised preoperative imaging modality for breast reconstruction planning
[29][30][29,30].
Building upon Doppler ultrasound, colour duplex ultrasonography evolved to offer even further-improved visualisation. Introduced in the late 1970s, this technology combines the principles of Doppler ultrasound with conventional imaging, providing a two-dimensional anatomical representation of structures while simultaneously mapping the flow of blood
[31][32][33][31,32,33]. The colour-coding adds an additional layer of information, distinguishing between vessels with blood flowing towards and away from the probe, and providing an immediate, visual understanding of the vascular territory
[34][35][36][34,35,36]. Colour duplex ultrasonography improved the precision and accuracy of preoperative planning, proving accurate in identifying perforating vessels and in-fact exhibiting greater precision when compared with the standard Doppler ultrasound
[37][38][39][40][41][42][37,38,39,40,41,42].
3. Emergence of Advanced Imaging Modalities
One of the largest and most influential advances in the preoperative planning of breast reconstruction was the introduction of computed tomography angiography (CTA) to this field. CTA, first introduced in the early 1990s, utilizes ionising radiation in combination with intravenously administered contrast media to obtain high-resolution images of the body’s vascular system
[43][44][44,45]. As CTA became more refined over the decades, its utilisation in preoperative imaging for breast reconstruction became more evident
[45][46][47][46,47,48]. In particular, it has proven indispensable for autologous breast reconstructions using perforator flaps such as the DIEP flap, where it aids in visualising and mapping the intricate network of perforator vessels
[10][48][49][50][51][52][10,49,50,51,52,53]. In the current day, CTA has become the gold-standard option for preoperative planning prior to surgery.
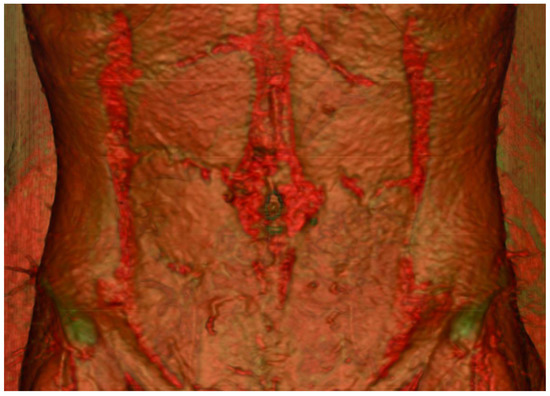
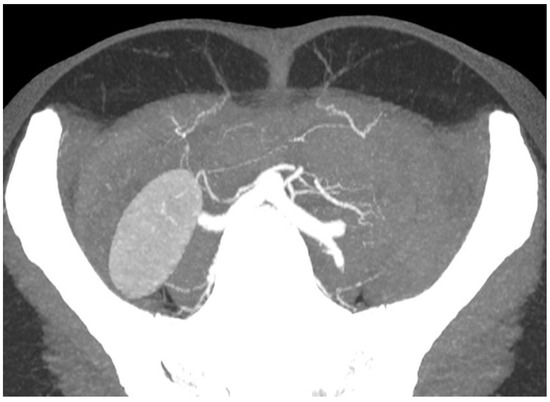
CTA’s utility lies in its ability to provide a detailed three-dimensional visualisation of the vasculature. It has become readily available in most areas, remains relatively cheap and generates consistent images regardless of the observer. Via specialised scanning protocols and advanced rendering software, accurate post-processing reconstructions of real anatomical structures can be performed (
Figure 1). Furthermore, the use of maximum-intensity projection techniques can help delineate the accurate intramuscular course of perforators to guide intra-operative dissection (
Figure 2).
Figure 1. Three-dimensional reconstruction of the abdominal wall displaying the perforating vessels originating from the deep inferior epigastric artery.
Figure 2. Axial CTA slice of the abdomen with maximum-intensity projection applied displaying the vasculature of the anterior abdominal wall and the intramuscular course of the medial row perforators.
CTA has several benefits that differentiate it from its predecessors of Doppler ultrasound or colour duplex ultrasonography. In comparison to these former imaging modalities, it has been shown to be more accurate, have a shorter scanning time, display smaller-diameter vessels with greater detail and provide information of the entire vascular tree, including the intramuscular and subcutaneous course of perforating vessels
[27][53][54][27,54,55]. However, CTA has additional limitations not present in earlier imaging techniques, particularly the use of ionising radiation which can have a cumulative effect across multiple CT scans significantly increasing the risk of cancer and other diseases. Furthermore, the reliance on iodinated contrast is problematic, given that it is contraindicated in patients with renal impairment. Despite these limitations, numerous studies have displayed the benefits of utilising CTA preoperatively on several operative and postoperative factors including shorter operative times and a reduced length of hospital admission
[50][55][56][57][51,56,57,58]. While some studies suggest a positive impact on postoperative complications such as flap failure, fat necrosis or donor site morbidity, this remains debated
[58][59][60][61][59,60,61,62].
Advancements in preoperative imaging for breast reconstruction did not stop with CTA. Magnetic resonance imaging (MRI), developed in the 1970s, was introduced for the identification of abdominal perforators by Ahn et al. in 1994
[62][63][65,66]. Unlike CTA, MRI uses a strong magnetic field and radio waves to produce detailed images of blood vessels without the use of ionising radiation, preventing potential harm to patients. However, MRI alone produces relatively low-resolution images, yet, when supplemented with contrast material such as gadolinium, can produce detail rivalling that of CTA
[64][67]. This technique, named magnetic resonance angiography (MRA), emerged as another ground-breaking imaging technology in the late 1980s
[64][65][66][67,68,69]. Since its inception, MRA has seen increasing application in preoperative planning for breast reconstruction
[67][68][69][70][70,71,72,73]. As with CTA, MRA provides valuable information about the vascular network, allowing for the precise planning of flap-based reconstruction. Moreover, MRA’s ability to offer high-quality soft tissue contrast and its non-reliance on ionising radiation or iodinated contrast make it an attractive option in certain clinical scenarios. Studies have shown a relative equivalence between CTA and MRA in mapping the arterial perforators of the deep inferior epigastric artery (DIEA)
[71][72][73][74,75,76].
45. Development of Imaging Adjuncts
4.1. Direct Infrared Thermography (DIRT)
5.1. Direct Infrared Thermography (DIRT)
The field of preoperative planning in breast reconstruction has seen the emergence of numerous technological adjuncts that augment the process of preoperative imaging. One such technique is direct infrared thermography (DIRT).
DIRT has a history that reaches back to the 1950s
[74][82], with its main function being to measure and visualize the distribution of heat across the body’s surface, and it has found a significant role in microvascular free flap reconstruction with some of its earliest applications being described by Theuvenet et al. in 1986
[75][83]. Since then, DIRT has grown in popularity and its use in autologous breast reconstruction has increased
[76][77][78][79][84,85,86,87]. The mechanism of DIRT utilises infrared cameras to detect the infrared radiation emitted from the skin, then convert this into a colour-coded visual map, displaying temperature variations. In a preoperative setting, it allows surgeons to assess areas of ‘hot spots’ which indicate the location of underlying perforators. The application of DIRT is typically dynamic; it begins with a “cold challenge” where the skin is cooled, and then the infrared camera monitors the pattern of subsequent reheating.
The benefits of DIRT include its non-invasiveness, easy reproducibility and interpretability, and real-time dynamic feedback. It is also an imaging technique that is available throughout all stages of the surgical procedure including pre, peri and postoperatively. This allows for the additional benefit of not only helping with the planning of the flap but also as an adjunct to assess flap perfusion intraoperatively and postoperatively
[80][81][82][83][88,89,90,91]. However, its limitations are substantial too. It is sensitive to environmental conditions and the topography of the body surface, which may lead to misinterpretation
[79][84][87,92].
In the modern day, DIRT has advanced to the point where infrared cameras have been developed that can simply connect to the everyday smartphone. Hardwicke et al. in 2016 demonstrated that perforators of the abdomen and thigh can be effectively and accurately localised using one such smartphone-compatible camera—Flir One
[85][93]. This camera costs just over 200 USD and is easily portable given its small size.
4.2. Three-Dimensional Printing
5.2. Three-Dimensional Printing
Another technological adjunct in the armament of surgeons when planning breast reconstruction preoperatively is three-dimensional (3D) printing. This innovative technology has expanded rapidly since its inception in 1986 with applications across numerous medical disciplines
[86][95]. In the preoperative planning of breast reconstruction, it enables the creation of physical models from digital files, typically derived from a patient’s CT or MRI scans.
4.3. Augmented Reality
5.3. Augmented Reality
Augmented reality (AR) is a recent addition to the surgical planning field, offering a means to visualize anatomical structures superimposed on the patient’s body in real-time. It integrates computer-generated images with the surgeon’s view of the patient, making the unseen seen. Like other advances in preoperative imaging, the benefits include improved anatomical understanding, intra-operative guidance, potentially shorter surgical times and assistance with surgical education
[87][88][89][104,105,106]. In the realm of breast reconstruction, wearable augmented reality devices such as HoloLens™ (Redmond, WA, USA) have been demonstrated to offer surgeons the ability to visualise perforators and anatomy before and during an operation
[90][91][107,108].
4.4. Contrast-Enhanced Ultrasound
5.4. Contrast-Enhanced Ultrasound
The field of preoperative planning in reconstructive surgery has seen a resurgence of older methods in recent years. For instance, contrast-enhanced ultrasound, as described by Su et al. in 2013, has emerged as a novel technique, building upon the original technology
[92][111]. The addition of intravenous ultrasound contrast agents provides additional reflective material in the vasculature and thereby increases the accuracy of the imaging modality in identifying perforators
[92][111].
56. Artificial Intelligence in Pre-Operative Planning
Artificial intelligence (AI) represents the most cutting-edge technology being integrated into pre-operative planning. AI describes a system that allows computers to display human-like intelligence. Machine learning (ML), a branch of AI, has seen applications in multiple areas of medical imaging
[93][114]. These models detect certain features from images, such as textures or shapes, and apply statistical formulae to detect patterns that indicate anatomical structures, diseases, or conditions. A subset of ML, deep learning (DL), is also often applied to medical imaging. DL models, built to resemble the human structure and the function of the human brain, consist of interconnected “neurons”
[94][95][115,116].
AI has had a wide impact in the field of plastic surgery, with evidence supporting its use for a multitude of various applications. For instance, O’Neill et al. in 2020 demonstrated the use of a ML algorithm at identifying those at high risk of experiencing flap failure in their cohort
[96][117].