Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeffrey Scott Cross | -- | 5698 | 2023-08-15 10:54:19 | | | |
| 2 | Jessie Wu | Meta information modification | 5698 | 2023-08-16 03:03:25 | | | | |
| 3 | Jessie Wu | Meta information modification | 5698 | 2023-08-16 03:05:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Classification of Green Solvents. Encyclopedia. Available online: https://encyclopedia.pub/entry/48075 (accessed on 11 March 2026).
Usman M, Cheng S, Boonyubol S, Cross JS. Classification of Green Solvents. Encyclopedia. Available at: https://encyclopedia.pub/entry/48075. Accessed March 11, 2026.
Usman, Muhammad, Shuo Cheng, Sasipa Boonyubol, Jeffrey S. Cross. "Classification of Green Solvents" Encyclopedia, https://encyclopedia.pub/entry/48075 (accessed March 11, 2026).
Usman, M., Cheng, S., Boonyubol, S., & Cross, J.S. (2023, August 15). Classification of Green Solvents. In Encyclopedia. https://encyclopedia.pub/entry/48075
Usman, Muhammad, et al. "Classification of Green Solvents." Encyclopedia. Web. 15 August, 2023.
Copy Citation
Green solvents, such as bio-based (derived from renewable sources), water-based (dissolved in water), supercritical fluids (above their critical point), and deep eutectic solvents (formed by mixing two or more components), offer alternatives to conventional organic solvents for bio-oil extraction. These solvents are characterized by being non-toxic, non-volatile, recyclable, and biodegradable.
green solvents
extraction
bio-based solvents
water-based solvents
supercritical fluids
deep eutectic solvents
1. Introduction
Green solvents can be classified into different categories based on their properties and origins. One common classification scheme divides green solvents into four main categories: bio-based solvents, water-based solvents, supercritical fluids, and deep eutectic solvents [1], as shown in Figure 1.
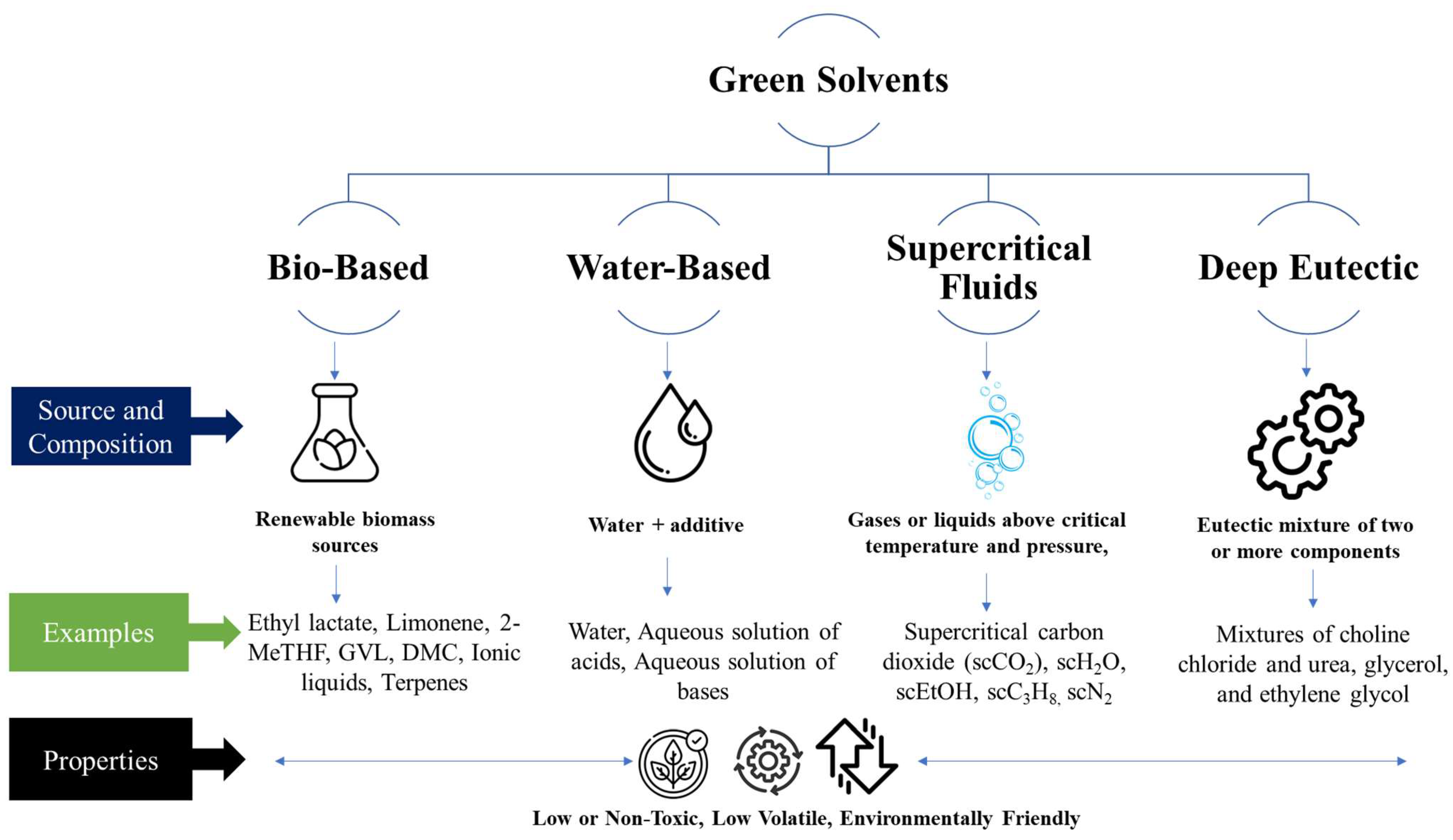
Figure 1. The scheme of green solvent classification.
Bio-based Solvents:
-
Composition: Bio-based solvents are derived from renewable biomass sources, such as plants, algae, or microorganisms.
-
Properties: Bio-based solvents are typically non-toxic and biodegradable, and have low VOC content. They offer favorable health and environmental profiles compared to petroleum-based solvents.
Water-based Solvents:
-
Composition: Water is the primary component of water-based solvents, often supplemented with small amounts of other solvents or additives.
-
Properties: Water-based solvents are non-toxic, non-flammable, and have low VOC emissions. They are readily available, inexpensive, and have high heat capacity.
Supercritical Fluids:
-
Composition: Supercritical fluids are typically gases or liquids that are above their critical temperature and pressure, resulting in a distinct supercritical state.
-
Properties: Supercritical fluids are a hybrid between a gas and a liquid. Their solvating power is adjustable, they have low viscosity, and high diffusivity. The most popular choice for supercritical fluids is supercritical carbon dioxide (CO2).
Deep Eutectic Solvents (DES):
-
Composition: DES are liquid solvents composed of a eutectic mixture of two or more components, typically a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA) [8]. These components can include both bio-based and non-bio-based compounds.
-
Properties: They often have low toxicity, low volatility, and high thermal stability. DES can be tailored to possess specific characteristics, such as tunable polarity, viscosity, and solubility, by selecting different combinations of HBD and HBA components [9].
-
Applications: DES find applications across various industries and processes. They have been used as green solvents in extraction processes for natural products, such as extraction of bioactive compounds from plant materials. DES have also shown promise in catalysis and electrochemistry, and as reaction media for organic synthesis. In addition, DES have been explored for their potential in industrial applications such as metal processing, biomass conversion, and separation processes [10].
Listed below is an in-depth description of each class of environmentally friendly solvents.
2. Bio-Based Solvents
The term “bio-based extraction solvents” refers to solvents that are produced from renewable biomass sources. These solvents are utilized in extraction processes to separate desired compounds or components from various materials. Bio-based extraction solvents offer several advantages over conventional solvents, including lower environmental impact, reduced toxicity, and potential biodegradability [3][4]. There are several bio-based extraction solvents, such as ethyl lactate [11], limonene [12], 2-methyltetrahydrofuran (2-MeTHF) [13], γ-valerolactone (GVL) [14], dimethyl carbonate (DMC) [15], Ionic liquids (ILs) [16], terpenes (e.g., pinene, terpinene, etc.) [17] which are reported in literature. The detailed explanation about these solvents is discussed below.
2.1. Ethyl Lactate
Ethyl lactate is primarily composed of two isomers: L-lactic acid and D-lactic acid, which are produced through the fermentation of renewable resources. The esterification of lactic acid with ethanol yields ethyl lactate. The schematic diagram of the ethyl lactate production is shown in Figure 2.
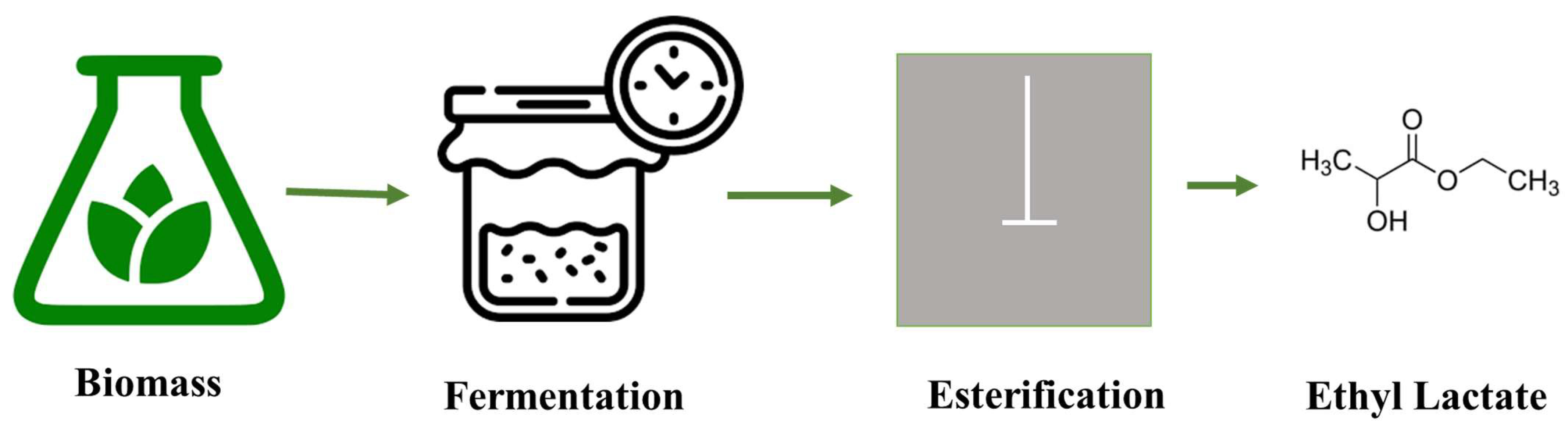
Figure 2. The production of ethyl lactate process from renewable sources.
This solvent exhibits a unique combination of desirable properties, making it suitable for various extraction processes. Ethyl lactate possesses excellent solvating power and can dissolve a wide range of organic compounds, including natural products, essential oils, and pharmaceutical compounds. It is commonly used as a solvent in the extraction of natural products, such as plant-derived bioactive compounds, due to its ability to selectively extract target compounds while leaving behind unwanted impurities. One of the advantages of ethyl lactate is its low toxicity compared to traditional solvents. It has a relatively low vapor pressure and does not contribute significantly to volatile organic compound (VOC) emissions. This makes it safer for workers and reduces potential harm to the environment. Additionally, ethyl lactate is biodegradable, further enhancing its sustainability profile [11].
2.2. Limonene
Limonene is obtained through the steam distillation or cold-press extraction of citrus peels. It is composed of two isomers: d-limonene and l-limonene. D-limonene is the more common and commercially available isomer. Due to its natural origin, limonene is considered a sustainable and renewable solvent option. Limonene exhibits excellent solvency power and can dissolve a wide range of organic compounds. It is particularly effective in extracting non-polar compounds, such as essential oils, terpenes, and hydrocarbons. This makes it a suitable solvent for various applications, including natural product extraction, flavor and fragrance production, and cleaning formulations. One of the notable advantages of limonene is its low toxicity and environmentally friendly characteristics. It has low volatility and low vapor pressure, reducing the risk of inhalation exposure. The United States Food and Drug Administration (FD) has classified limonene as a “Generally Recognized as Safe (GRAS)” substance, meaning it is safe for use in food and cosmetics. Moreover, limonene is readily biodegradable, which means it can break down naturally in the environment without causing long-term harm. This biodegradability contributes to its reduced environmental impact compared to petroleum-based solvents [12].
2.3. 2-Methyltetrahydrofuran (2-MeTHF)
2-MeTHF is typically produced through the hydrogenation of levulinic acid, as depicted in Figure 3. The production process involves several steps. First, biomass feedstock, such as lignocellulosic materials or waste streams, is subjected to pretreatment to break down the complex polymers into simpler sugars. These sugars are then fermented to produce levulinic acid through microbial or enzymatic processes. Once levulinic acid is obtained, it undergoes hydrogenation in the presence of a catalyst to convert it into 2-MeTHF.
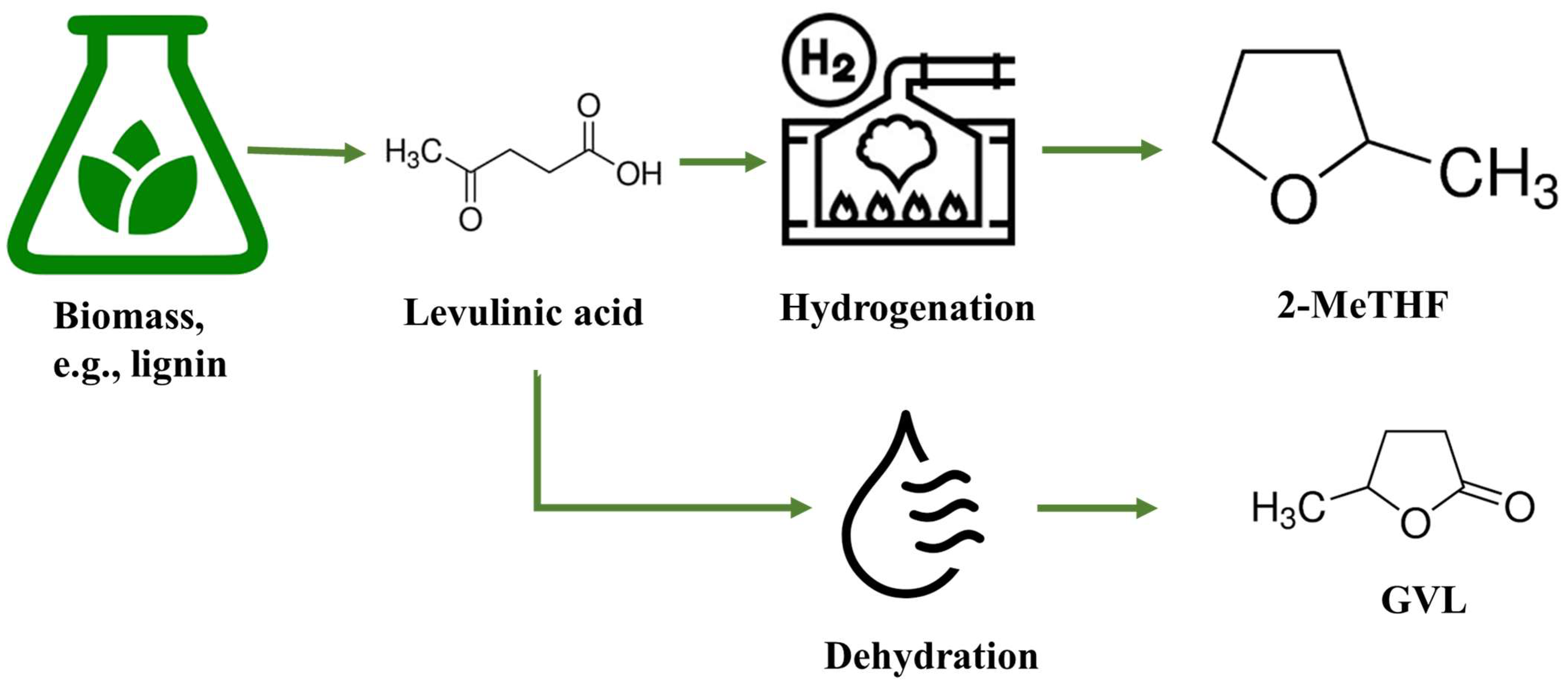
Figure 3. The schematic diagram of 2-MeTHF and GVL production from biomass.
This synthesis route allows for the utilization of renewable feedstocks, reducing reliance on fossil resources. One of the key advantages of 2-MeTHF is its excellent solvency power for a wide range of organic compounds. It is useful in many extraction processes because it dissolves both polar and non-polar substances. It has been used in the extraction of bioactive compounds, natural products, and chemicals from biomass. Compared to its predecessor, THF, 2-MeTHF offers improved stability and lower flammability, making it a safer alternative. It has a higher boiling point, wider liquid range, and is less prone to form peroxides, enhancing its handling and storage characteristics. 2-MeTHF also exhibits lower toxicity compared to other commonly used solvents. It has a lower vapor pressure, reducing the risk of inhalation exposure. Studies have shown that 2-MeTHF is less toxic to aquatic organisms and has a lower impact on the environment compared to traditional solvents [13][18].
2.4. γ-Valerolactone (GVL)
GVL can be made by dehydrating levulinic acid derived from biomass in the presence of an acid catalyst (as shown in Figure 4). It can also be obtained as a co-product of the sugar platform, where glucose is converted into levulinic acid and GVL in the presence of acid catalysts. This synthesis route allows for the utilization of renewable resources and offers a sustainable alternative to petroleum-based solvents. One of the significant advantages of GVL is its versatile solvency power. It is effective at dissolving both polar and non-polar organic compounds. This makes it suitable for various extraction processes, such as the extraction of lignin, cellulose, and other valuable compounds from biomass. GVL exhibits a high boiling point, which allows for its efficient recovery and recycling during extraction processes. It has a lower vapor pressure compared to traditional solvents, reducing the risk of inhalation exposure and improving workplace safety. Furthermore, GVL has been recognized for its low toxicity compared to other commonly used solvents. It has a lower impact on human health and the environment, making it a more sustainable option. GVL is biodegradable, which means it can naturally break down in the environment, minimizing its long-term ecological impact [14][19].
2.5. Dimethyl Carbonate (DMC)
Dimethyl carbonate (DMC) is a bio-based extraction solvent that has gained attention as a green solvent alternative to traditional petroleum-based solvents. It is a clear, colorless liquid with a pleasant odor. DMC is produced through the reaction of methanol with carbon dioxide (CO2), making it a sustainable option as it utilizes CO2 as a feedstock and reduces reliance on fossil resources. DMC offers several advantages as a green solvent. It has a low toxicity profile and is considered safe for use in various applications. It has a low vapor pressure and low volatility, reducing the risk of inhalation exposure. DMC also exhibits good solvating power for a wide range of organic compounds and can dissolve both polar and non-polar substances effectively.
One of the significant advantages of DMC is its environmentally friendly nature. It is biodegradable, meaning it can naturally break down in the environment without causing long-term harm. Additionally, DMC has a low impact on air quality and does not contribute significantly to VOCs emissions. These properties make DMC a desirable option for industries seeking to reduce their environmental footprint. DMC finds application as a solvent in various fields, including pharmaceuticals, coatings, adhesives, and cleaning products. It is also used as a reactant in the synthesis of chemicals and as a fuel additive due to its high oxygen content. Its compatibility with existing infrastructure and processes further supports its potential as a green solvent alternative [15][20][21].
2.6. Ionic Liquids (ILs)
The special properties of ionic liquids (ILs) have made them a popular choice as a green solvent for bio-based extraction processes. ILs are composed of ions, typically consisting of organic cations and inorganic or organic anions. Their name, “ionic liquids”, comes from the fact that they are liquids at or near room temperature. Advantages of ILs include high solubility for a wide variety of compounds, low volatility, and the ability to modify the IL’s properties. They have low vapor pressure, reducing the risk of inhalation exposure and making them safer to handle. Additionally, ILs have high thermal stability, allowing them to be used at elevated temperatures without significant degradation.
One of the notable advantages of ILs is their tunable nature. The combination of different cations and anions provides the ability to tailor the physical and chemical properties of ILs to suit specific applications. This includes adjusting their solvating power, polarity, viscosity, and other characteristics to meet the requirements of different extraction processes. ILs exhibit high solubility for a wide range of compounds, including polar and non-polar substances. This property makes them suitable for the extraction of various target compounds from different sources, such as natural products, pharmaceuticals, and biofuels. ILs can selectively dissolve specific compounds, allowing for efficient separation and purification. Moreover, ILs are considered environmentally friendly due to their negligible volatility and low toxicity. They have a reduced impact on air quality, as they do not readily evaporate into the atmosphere. ILs are also biodegradable, and some ILs derived from renewable sources offer a sustainable alternative to traditional solvents [16][22][23].
2.7. Terpenes
Terpenes are naturally occurring compounds found in the essential oils of plants. They are typically extracted from plant material through methods such as steam distillation or cold-press extraction. Pinene and terpinene are examples of terpenes that have been investigated for their potential as green solvents. Terpenes offer several advantages as extraction solvents. They have low toxicity profiles, making them safer to handle compared to traditional solvents. They have excellent solvating power and can dissolve many different kinds of organic compounds, both polar and non-polar. That is why they are great for obtaining the desired results when it comes to natural remedies, essential oils, and bioactive compounds.
One of the notable features of terpenes is their pleasant aroma. Their characteristic scents can be beneficial in applications where odor is a consideration, such as the formulation of fragrances, cosmetics, and personal care products. Terpenes can also contribute to the sensory experience of food and beverage products. In addition to their low toxicity and biodegradability, terpenes are derived from renewable plant sources, making them a sustainable alternative to petroleum-based solvents. The use of plant sources for their production is consistent with green chemistry and aids in the transition to less resource-intensive methods [17].
3. Water-Based Solvents
Water-based green solvents refer to solvents where water acts as the primary solvent, providing a sustainable and environmentally friendly alternative to traditional organic solvents. These solvents leverage the unique properties of water, such as its abundance, low cost, low toxicity, and high boiling point, making them suitable for various applications while minimizing the environmental impact. Water-based green solvents offer several advantages over organic solvents, including reduced flammability, reduced health and safety risks, and lower emissions of VOCs. They are widely used in green chemistry practices, as they align with the principles of sustainability, waste reduction, and hazard minimization [6][24]. There are several water-based solvents such as water [6][25], aqueous solution of acids (e.g., acetic acid, citric acid) [26][27], aqueous solution of bases (e.g., sodium hydroxide, ammonia) [28][29], aqueous alcohol solution (e.g., ethanol, methanol) [28][30][31][32] and aqueous organic solvents (e.g., water/ethanol, water/acetone) [29][33] which are reported in the literature and a brief description is given below.
3.1. Water
Water-based extraction involves utilizing water as the primary solvent for extracting target compounds from various sources, such as plants, food products, and natural materials. Water acts as a polar solvent, making it particularly effective in extracting polar compounds, including sugars, organic acids, phenolic compounds, and hydrophilic bioactive compounds. One of the advantages of water as an extraction solvent is its low toxicity. Compared to organic solvents, water is generally considered safe for human consumption and has minimal health risks. This is especially important in applications where the extracted compounds are intended for use in food, pharmaceuticals, or cosmetics. Water-based extraction methods can be performed under mild conditions, reducing the need for high temperatures or harsh chemical reagents. This not only saves energy but also helps preserve the integrity and bioactivity of the extracted compounds. Water-based extraction is often preferred for sensitive compounds that may degrade under harsher extraction conditions. Furthermore, water is a sustainable and readily available solvent. Its abundance and low cost contribute to its attractiveness as an extraction solvent, providing economic benefits and reducing reliance on non-renewable resources [6][25].
3.2. Aqueous Solution of Acids
Aqueous solutions of acids are commonly used as extraction solvents due to their ability to selectively dissolve and extract specific compounds. The acid component provides acidic conditions, which can influence the solubility and extraction efficiency of different substances. Acids such as acetic acid and citric acid are particularly popular due to their low toxicity, wide availability, and compatibility with food and pharmaceutical applications. In the case of acetic acid, its presence in water forms acetic acid–water mixtures that can enhance the solubility of polar and semi-polar compounds. Acetic acid–water mixtures can be used for the extraction of various natural products, including organic acids, polyphenols, and pigments.
Similarly, citric acid, a weak organic acid, can form aqueous solutions with desirable extraction properties. Citric acid solutions are known for their chelating abilities and pH-dependent extraction selectivity. These solutions can be used for the extraction of metal ions, alkaloids, and other compounds that exhibit pH-dependent solubility characteristics. The pH of the acid solution plays a crucial role in the extraction process, as it can influence the solubility and interaction of the target compounds. By adjusting the pH of the acid solution, specific compounds can be selectively extracted or precipitated, providing a means to isolate desired components from complex mixtures [26][27].
3.3. Aqueous Solutions of Bases
Aqueous solutions of bases are commonly used in extraction processes due to their ability to alter pH and enhance solubility of target compounds. Sodium hydroxide and ammonia are frequently utilized bases in aqueous solutions for extraction purposes. Sodium hydroxide (NaOH) is a strong base that can increase the pH of an aqueous solution, resulting in alkaline conditions. Alkaline solutions can enhance the extraction efficiency of certain compounds, particularly those that are acidic in nature. Alkaline conditions can deprotonate acidic functional groups, thereby increasing their solubility. This is especially useful for the extraction of organic acids, phenolic compounds, and other acid-sensitive substances.
Ammonia (NH3) is a weak base that can also be used in aqueous solutions for extraction purposes. Similar to sodium hydroxide, ammonia can modify pH and solubility of compounds. Ammonia-based solutions are particularly effective in the extraction of nitrogen-containing compounds, such as alkaloids and amines. The alkaline conditions provided by ammonia can facilitate the solubility and extraction of these nitrogen-rich compounds. By adjusting the concentration of the base and the pH of the solution, aqueous solutions of bases allow for selective extraction of specific compounds. The pH-dependent solubility of various substances can be exploited to selectively extract desired components from complex mixtures [28][29].
3.4. Aqueous Alcohol Solutions
Aqueous alcohol solutions combine the properties of water and alcohol, providing an effective solvent system for extraction. Ethanol and methanol are commonly used alcohols in aqueous solutions for extraction purposes. The presence of alcohol in the aqueous solution can enhance the solubility of both polar and non-polar compounds. Alcohols can disrupt intermolecular interactions, allowing for the extraction of a wider range of compounds compared to water alone. This makes aqueous alcohol solutions particularly suitable for the extraction of various natural products, including bioactive compounds, phenolic compounds, and essential oils.
Ethanol, as a polar alcohol, can effectively solubilize polar and semi-polar compounds. It is commonly used in extraction processes for its ability to extract a diverse range of compounds, including flavonoids, alkaloids, and terpenes. Ethanol also acts as a co-solvent with water, further expanding its solubilizing power. Methanol, another commonly used alcohol, is highly polar and can efficiently dissolve both polar and non-polar compounds. Methanol-based extraction solvents are often employed in the extraction of bioactive compounds, natural products, and metabolites.
The use of aqueous alcohol solutions allows for the adjustment of the polarity of the solvent system by changing the concentration of alcohol. This flexibility provides control over the extraction selectivity and efficiency, allowing for the extraction of specific compounds or classes of compounds from complex mixtures [28][30][31][32].
3.5. Aqueous Organic Solvents
Aqueous organic solvents are solvent systems that consist of a combination of water and an organic solvent, such as ethanol or acetone. These mixtures provide a versatile extraction solvent option, leveraging the solubilizing power of both water and organic solvents.
Water/ethanol mixtures are commonly used as extraction solvents due to their ability to solubilize a wide range of compounds. Ethanol, as an organic solvent, can dissolve both polar and non-polar compounds, including bioactive compounds, flavonoids, and phenolic compounds. The addition of water to ethanol improves the solubility of polar compounds, expands the range of compounds that can be extracted, and provides a more environmentally friendly solvent system.
Water/acetone mixtures are also utilized as extraction solvents in various applications. Acetone, an organic solvent, is particularly effective at dissolving non-polar compounds and lipophilic substances. The addition of water to acetone enhances its solubility for polar compounds and expands its extraction capabilities.
Aqueous organic solvent systems allow for control over the polarity and solubility characteristics of the solvent system by adjusting the ratio of water to organic solvent. This flexibility enables tailored extraction selectivity and efficiency, making these solvent systems suitable for the extraction of specific compounds or classes of compounds from complex mixtures. The use of aqueous organic solvents offers several advantages, including enhanced extraction efficiency, expanded solubility range, and reduced reliance on purely organic solvents. These solvents also align with the principles of green chemistry by reducing the use of hazardous organic solvents and promoting sustainable extraction processes [28][32][33].
4. Supercritical Fluids
The properties of both liquids and gases are present in supercritical fluids, which are substances that are kept above their critical temperature and pressure. In the context of green solvents, supercritical fluids, particularly supercritical carbon dioxide (scCO2), are considered environmentally friendly solvents for various applications. Supercritical fluids, including scCO2, offer several advantages as green solvents. They have low toxicity and are non-flammable, making them safer to handle compared to traditional organic solvents. Additionally, supercritical fluids can be easily separated from the extracted compounds, allowing for their efficient recovery and recycling.
Both polar and non-polar compounds are soluble in supercritical fluids due to their exceptional solvating properties. By adjusting the temperature and pressure, the solvating power of supercritical fluids can be fine-tuned, enabling selective extraction of target compounds while leaving impurities behind. One of the key environmental benefits of supercritical fluids is their low environmental impact. Supercritical carbon dioxide is abundant, non-toxic, and non-polluting, making it a sustainable alternative to organic solvents derived from fossil fuels. Furthermore, the use of supercritical fluids eliminates or significantly reduces the need for VOCs, leading to reduced emissions and air pollution [1][2][3][4][7][33].
4.1. Supercritical Carbon Dioxide (scCO2)
Supercritical carbon dioxide (scCO2) is carbon dioxide above its critical temperature (31.1 °C) and critical pressure (74 bar), where it possesses properties of both a gas and a liquid. These conditions enable scCO2 to function as an efficient and selective extraction solvent. One of the major advantages of scCO2 is its low critical temperature, which allows for the extraction of temperature-sensitive compounds without degradation. This makes it particularly suitable for extracting heat-sensitive bioactive compounds and natural products, such as essential oils and flavors. scCO2’s solvating power is so high that it can dissolve both polar and non-polar compounds. Extraction conditions, such as pressure, temperature, and the addition of a co-solvent, can be adjusted to maximize its solvating power. This versatility enables the selective extraction of specific compounds from complex matrices [34][35].
Another advantage of scCO2 is its environmental friendliness. Carbon dioxide is a byproduct of many manufacturing procedures, so it is abundant, safe, and nonflammable. It can replace dangerous organic solvents in the extraction process, lowering both the environmental and health risks. scCO2 also offers advantages in terms of easy solvent removal and product recovery. The extracted compounds and scCO2 can be easily separated by lowering the pressure after extraction. This facilitates downstream processing and minimizes the need for extensive post-extraction purification steps [33][34][35].
4.2. Supercritical Water (scH2O)
Supercritical water (scH2O) offers several advantages as an extraction solvent. Firstly, its high temperature and pressure (p > 22.064 MPa, T > 373.946 °C) enhance the solubility and diffusivity of solutes, allowing for efficient extraction of various compounds. scH2O is especially effective at extracting polar and ionic compounds due to its high polarity in the supercritical state. One of the notable features of scH2O is its unique ability to undergo ionization, resulting in the generation of reactive hydroxyl radicals. These radicals can promote the degradation of organic pollutants or complex molecules, making scH2O a potential solvent for waste treatment and environmental remediation applications [36][37].
Furthermore, scH2O offers benefits in terms of selectivity. The solubility of different compounds in scH2O varies with temperature and pressure, allowing for the selective extraction of specific target compounds from complex matrices. This selectivity can be fine-tuned by adjusting the extraction conditions, offering control over the extraction process. Organic pollutants, natural products, and bioactive compounds can all be extracted using supercritical water. Various heat-sensitive substances, including essential oils and phenolic compounds, have been extracted using this method [38][39].
4.3. Supercritical Ethanol (scEtOH)
When ethanol is heated and compressed beyond its critical temperature (243 °C) and critical pressure (6.38 MPa), it changes phase and takes on characteristics of both a gas and a liquid, giving rise to the term “supercritical ethanol” (scEtOH). In this state, ethanol has enhanced solvating power and can efficiently extract various compounds from different matrices. Supercritical ethanol has a higher solvating power compared to subcritical ethanol due to its increased density and diffusion coefficient. This facilitates the removal of polar and non-polar compounds, expanding its usefulness. Ethanol is known for its ability to extract a variety of bioactive compounds, including natural products, pharmaceuticals, and flavors. In its supercritical state, ethanol can further enhance its extraction capabilities, improving the extraction efficiency and selectivity [40][41].
Extracting essential oils, bioactive compounds from plant material, and other compounds from natural products are just some of the many applications of supercritical ethanol in the extraction process. The specific conditions of temperature and pressure can be adjusted to optimize the extraction process and target specific compounds of interest [42][43]. While the use of supercritical ethanol as an extraction solvent is not as extensively documented as supercritical carbon dioxide, it shows potential for various applications and can be further explored in research and development [44].
4.4. Supercritical Propane (scC3H8)
Propane that has been subjected to conditions above its critical temperature (96.7 °C) and critical pressure (42.5 bar) is said to be supercritical, or scC3H8 for short. In this state, propane can possess unique solvating properties and may be explored as an extraction solvent in certain applications. Propane is known for its non-polarity and low critical temperature, which may limit its solvating power compared to other supercritical fluids. However, it can still potentially be utilized for the extraction of non-polar compounds and lipophilic substances [45][46]. The use of supercritical propane as an extraction solvent may have applications in specific industries or processes where its solvating properties and unique characteristics can provide advantages over other solvents. Supercritical propane shows promise as an extraction solvent, but there is a lack of data on its use so far; more study is needed to fully understand its capabilities.
4.5. Supercritical Nitrogen (scN2)
Supercritical nitrogen (scN2) is nitrogen that has been subjected to conditions above its critical temperature (−147.1 °C) and critical pressure (33.5 bar), at which it becomes a hybrid between a gas and a liquid. In this state, nitrogen may possess unique solvating properties and can potentially be used as an extraction solvent in specific applications. Nitrogen is an inert gas and generally considered nonpolar, which may limit its solvating power for polar compounds compared to other supercritical fluids. However, it can still potentially be used for the extraction of nonpolar or low-polarity compounds [47][48].
The use of supercritical nitrogen as an extraction solvent may have applications in certain industries or processes where its inertness and specific solvating properties can provide advantages over other solvents. However, it is important to note that the literature on supercritical nitrogen as an extraction solvent is limited, and further research and development would be necessary to explore its full potential.
5. Deep Eutectic Solvents
Solvents that fall into the category of “deep eutectic solvents” (DES) are eutectic mixtures of two or more components, typically a hydrogen bond donor (HBD) and an acceptor (HBA). These solvents exhibit unique properties that make them attractive alternatives to conventional organic solvents. DES can be composed of bio-based or non-bio-based components, and they possess characteristics such as low toxicity, low volatility, high thermal stability, and tunable physical properties. The formation of DES occurs when the mixture of HBD and HBA components forms a eutectic system, characterized by a lower melting point than the individual components alone. The hydrogen bond interactions between the components contribute to the unique properties of DES, including their ability to dissolve a wide range of solutes.
Many different fields, such as catalysis, extraction, electrochemistry, and separation processes, have taken an interest in DES because of their potential utility. These solvents offer advantages such as high solubility for polar and non-polar compounds, tunable properties through the selection of different HBD-HBA combinations, and their ability to serve as reaction media for a variety of chemical reactions. The mixtures of choline chloride and urea, glycerol, and ethylene glycol are all examples of DES. Applications utilizing these DES have included biomass pretreatment, metal extraction, and organic synthesis [8][9][10].
5.1. Choline Chloride–Urea
Choline chloride–urea DES is made by reacting the quaternary ammonium salt choline chloride with the hydrogen bond acceptor urea. This DES exhibits a unique eutectic behavior, characterized by a lower melting point compared to the individual components. Choline chloride–urea DES has been extensively studied for its excellent solubility power, which makes it effective against a wide variety of organic and inorganic compounds in need of dissolution. It has been explored in various applications, such as biomass processing, metal extraction, and catalysis. In biomass pretreatment, choline chloride–urea DES has been shown to effectively dissolve cellulose, aiding in the extraction of valuable components [49][50].
5.2. Choline Chloride–Glycerol
When choline chloride and the hydrogen bond donor glycerol are mixed together, they form choline chloride–glycerol DES. This DES has many positive characteristics that make it an attractive choice, such as its low volatility, high thermal stability, and high solvating power. Choline chloride–glycerol DES has been widely investigated for its potential applications in extraction processes, synthesis of nanoparticles, and organic transformations. Because of its versatility as a solvent, it has been put to use in the environmentally friendly extraction of natural products such as bioactive compounds from plants [51].
5.3. Choline Chloride–Ethylene Glycol
The reaction between choline chloride and ethylene glycol produces choline chloride–ethylene glycol DES. The viscosity, solubility, and thermal stability of this DES are just a few of its remarkable characteristics. Choline chloride–ethylene glycol DES has been investigated for various applications, including solvent extraction, catalysis, and biomass processing. Its potential as a solvent for removing valuable compounds from plants such as essential oils and bioactive molecules has been demonstrated. The ability of choline chloride–ethylene glycol DES to dissolve and extract target compounds efficiently makes it a promising solvent in green extraction processes [52].
The overall production flow of chemical reactions for DES production has been summarized in Figure 4. The choline chloride salt (HBA) contributes a hydrogen ion, which binds with the hydrogen of the hydroxyl group (HBD) in the DES to form a hydrogen bond.
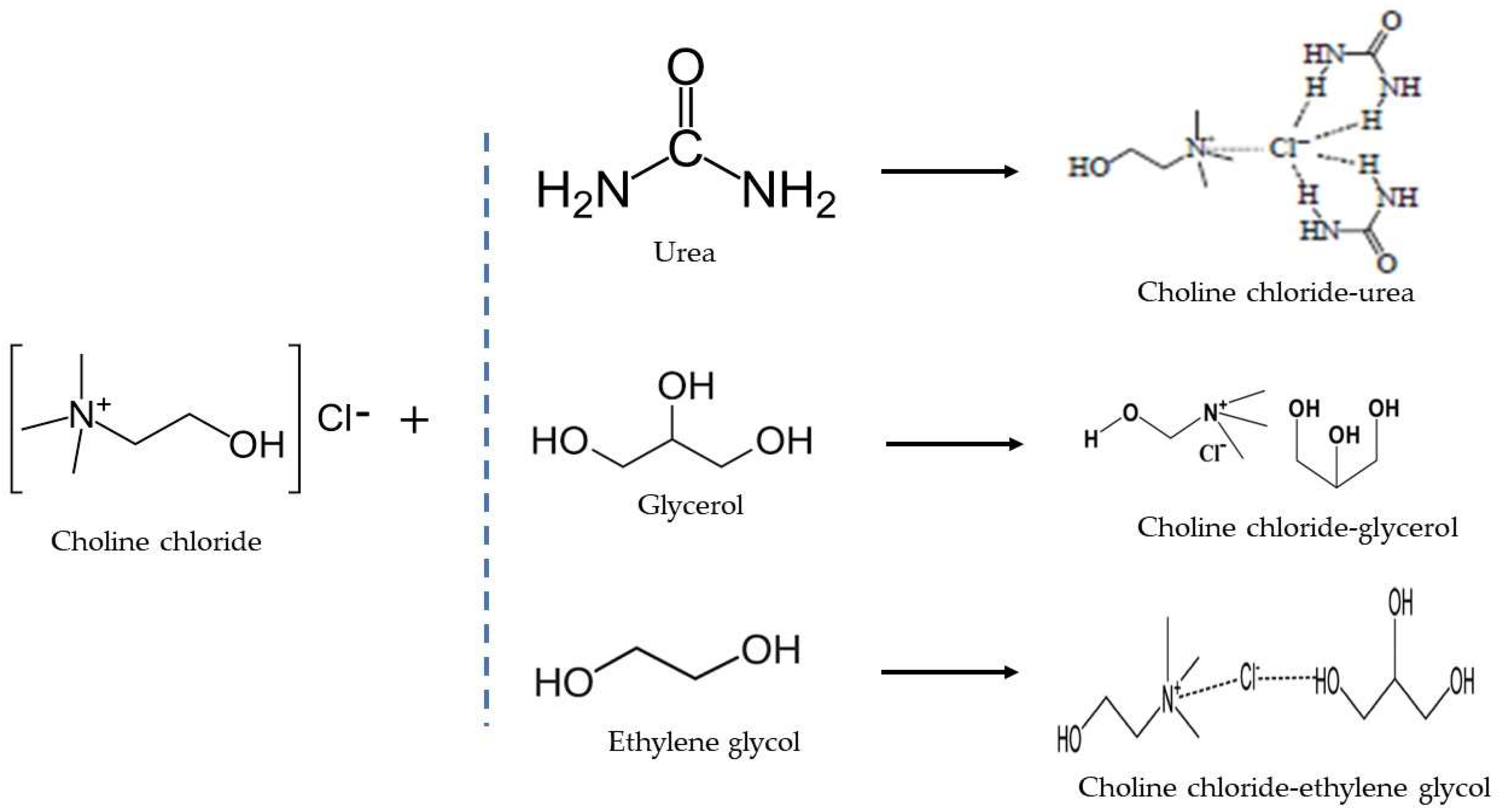
Figure 4. Overall Reactions mechanism for DES Production.
6. Properties of Green Solvents
Green solvents, as opposed to traditional solvents, are safer for human health and the environment because they are made from renewable resources. The unique properties and characteristics of these solvents make them desirable for a wide range of uses. Green solvents’ key properties and characteristics are illustrated and explained in Figure 5 [49][53][54][55].
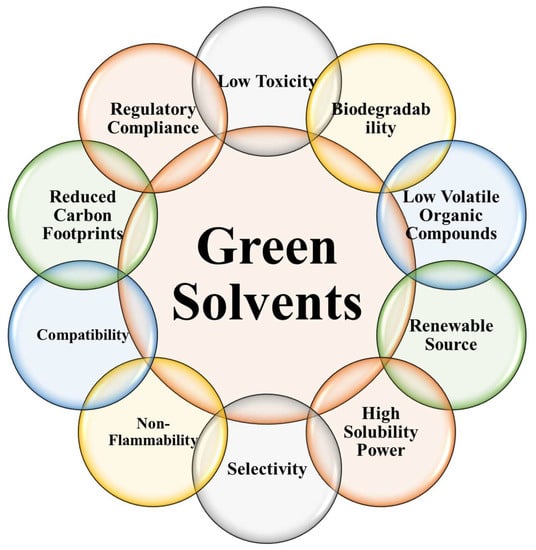
Figure 5. Key properties of the green solvents.
Renewable Source: Renewable plant materials, biomass, or food waste are the building blocks of green solvents. They are more environmentally friendly than solvents made from fossil fuels, which deplete natural resources and increase greenhouse gas production.
Low Toxicity: In comparison to traditional solvents, green solvents are safer to use. They pose less of a threat to human health and the environment because of their lower volatility and lower levels of hazardous components.
Biodegradability: Green solvents are biodegradable, meaning they can be broken down by microbes or enzymes in the environment. This feature lessens their ability to persist in ecosystems and the damage they do to groundwater, surface water, and atmospheric chemistry.
Low VOCs Content: Chemical compounds that can evaporate into the air are called VOCs. Cleaner indoor and outdoor air, less smog, and fewer health risks are all benefits of using green solvents because of their typically low VOC content.
High Solubility Power: Many environmentally friendly solvents have high solubility and can be used to dissolve a wide variety of organic and inorganic compounds. Because of this quality, they can be used in a wide range of contexts, such as extraction, synthesis, and formulation.
Selectivity: Some environmentally friendly solvents can be selective toward a given compound or group of compounds, allowing for more precise extraction and separation. This selectivity can improve process efficiency by zeroing in on useful components and eliminating unnecessary purification steps.
Non-Flammability: In comparison to traditional solvents, many eco-friendly options are less flammable. This quality makes handling, storage, and transport less likely to result in fires.
Compatibility: Most environmentally friendly solvents work well with a wide variety of substrates, from metals to plastics to even natural fibers. Because of this compatibility, they can be used in many different fields without posing a threat to machinery or degrading raw materials.
Reduced Carbon Footprint: Green solvents help cut down on pollution and carbon dioxide emissions. Carbon dioxide (CO2) emissions from their production and use are lower than those of traditional solvents, lending support to climate change mitigation efforts.
Regulatory Compliance: The standards for health, safety, and environmental protection that go into making green solvents are rigorous. To guarantee their usefulness in particular contexts and their conformity with applicable rules, they are put through extensive testing and evaluation.
Some of the physiochemical properties of green solvents are listed in detail in Table 1. Green solvents are a more sustainable and environmentally friendly alternative to traditional solvents due to their unique properties and characteristics.
Table 1. Physicochemical Properties of Green solvents.
| Solvents | Molar Mass (g.mol−1) | Solubility in Water | Boiling Point (°C) | Melting Point (°C) | Density (g/mL) | Vapor Pressure | Viscosity | Appearance | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Water | 18 | 100 | 0 | 1.000 at 3.98 °C | 23.8 mmHg at 25 °C | 0.8949 mPa·s at 25 °C | colorless | [56][57] | |
| Ethanol | 46.07 | 106 mg/mL at 25 °C | 78.24 | −114.4 | 0.7893 at 20 °C | 10 kPa at 29.2 °C | 1.074 mPa·s at 25 °C | colorless | [58][59] |
| EA | 88.1 | 8.3 g/100 mL at 20 °C | 77.1 | −83.6 | 0.9006 at 20 °C 0.8945 at 25 °C |
73.0 Torr at 20 °C | 0.45 cP at 20 °C | colorless | [60][61] |
| Diethyl ether | 74.12 | 6.05 g/100 mL at 25 °C | 34.55 | −116.3 | 0.7133 at 20 °C 0.7076 at 25 °C |
442 Torr at 20 °C | 0.24 cP at 20 °C | colorless | [62][63] |
| MTBE ** | 88.2 | 4.2 g/100 mL at 20 °C | 55 | −109 | 0.74 at 25 °C | 27 kPa at 20 °C | 0.36 cP at 25 °C | colorless | [64] |
| MeTHF * | 86.13 | 15 g/100 mL at 25 °C | 80.2 | −136 | 0.854 at 25 °C | 3.6 kPa at 20 °C 34.5 kPa at 50 °C |
4 mPa·s at 25 °C | colorless | [65][66] |
| DME ++ | 46.07 | 71 g/L at 20 °C | −24 | −141 | 0.735 (liquid, at −25 °C) | 592.8 kPa at 25 °C | colorless gas | [67][68] | |
| Cyclohexane | 84.161 | immiscible | 80.7 | 6.47 | 0.7739, liquid at 25 °C | 78 mmHg at 20 °C | 1.02 cP at 17 °C | colorless | [69][70] |
| Benzene | 78.114 | immiscible | 80.5 | 5.5 | 0.8765 at 20 °C | 12.7 kPa at 25 °C | 0.6076 cP at 25 °C | colorless | [71] |
| Carbon disulfide | 76.13 | slightly soluble | 46.5 | −111.5 | 1.266 at 25 °C | 48.1 kPa at 25 °C | 0.363 cP at 20 °C | colorless | [72] |
| Glycerol | 92.094 | miscible | 290 | 17.9 | 1.26 at 25 °C | 0.40 Pa at 50 °C | 1.412 Pa·s at 20 °C | colorless | [73][74] |
| GVL | 100.116 | 100 mg/mL | 205 | −31 | 1.0546 at 20 °C | 3.5 kPa at 80 °C | colorless | [75][76] | |
| CPME + | 1.1 g/100 g at 23 °C | 106 | −140 | 0.86 at 25 °C | 59.9 hPa at 25 °C | 0.57 cP at 20 °C | colorless | [77][78] | |
| D-limonene | 136.238 | insoluble | 176 | −74.35 | 0.844 at 25 °C | 190 Pa at 20 °C | 0.8462 mPa·s at 25 °C | colorless | [79][80] |
| p-cymene | 134.222 | insoluble | 177 | −68 | 0.86 at 25 °C | 1.5 mmHg at 20 °C | 0.81–7.1 mm2/s | colorless | [81] |
| DMC | 90.078 | 13.9 g/100 mL at 25 °C | 90 | 2–4 | 1.069 at 25 °C | 18 mmHg at 21.1 °C | 0.625 cP at 20 °C | colorless | [82] |
| Ethyl lactate | 118.131 | miscible | 154.2 | −25 | 1.0328 at 20 °C | 1.16 mmHg at 25 °C | 0.0261 cP at 20 °C | colorless | [83] |
* Methyltetrahydrofuran, ** Methyl tert-Butyl Ether, ++ Dimethyl ether, + Cyclopentyl methyl ether.
References
- Warner, P.T.A.J.C. Green Chemistry: Theory and Practice. CiNii Books. 1998. Available online: https://www.amazon.com/Green-Chemistry-Practice-Paul-Anastas/dp/0198506988 (accessed on 8 July 2023).
- Dicks, A.P.; Hent, A. Green Chemistry Metrics: A Guide to Determining and Evaluating Process Greenness; Springer: Berlin/Heidelberg, Germany, 2014; Available online: https://link.springer.com/book/10.1007/978-3-319-10500-0 (accessed on 8 July 2023).
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2017, 6, 32–48.
- Erythropel, H.C.; Zimmerman, J.B.; De Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.B.I.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.; Tu, Q.; et al. The Green ChemisTREE: 20 Years after Taking Root with the 12 Principles. Green Chem. 2018, 20, 1929–1961.
- Pereira, C.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl Lactate as a Solvent: Properties, Applications and Production Processes—A Review. Green Chem. 2011, 13, 2658.
- Kumar, S.; Jain, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Kim, K.-H. Green Synthesis of Metal–Organic Frameworks: A State-of-the-Art Review of Potential Environmental and Medical Applications. Coord. Chem. Rev. 2020, 420, 213407.
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.B.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Smith, E.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.; Looi, C.Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol. Adv. 2017, 35, 105–134.
- Abdullah, M.Z.; Hussein, H.A.; Alshajrawi, O.M.S. Ethyl Lactate as a Green Solvent in the Pharmaceutical Industry. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 185–194.
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total Lipid Extraction of Food Using D-Limonene as an Alternative to n-Hexane. Chromatographia 2008, 68, 311–313.
- Soszka, E.; Jȩdrzejczyk, M.; Keller, N.; Ruppert, A.M. High Yield Production of 2-Methyltetrahydrofuran Biofuel with Reusable Ni-Co Catalysts. Fuel 2023, 332, 126118.
- Raj, T.; Chandrasekhar, K.; Banu, J.R.; Rene, E.R.; Aslam, M.; Kim, S.H. Synthesis of γ-Valerolactone (GVL) and Their Applications for Lignocellulosic Deconstruction for Sustainable Green Biorefineries. Fuel 2021, 303, 121333.
- Pyo, S.-H.; Park, J.-W.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl Carbonate as a Green Chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66.
- Lim, J.H.; Chua, L.S.; Mustaffa, A.A. Ionic Liquids as Green Solvent and Their Applications in Bioactive Compounds Extraction from Plants. Process Biochem. 2022, 122, 292–306.
- Da Silva, R.V.; Rocha-Santos, T.; Duarte, A.C. Supercritical Fluid Extraction of Bioactive Compounds. Trends Anal. Chem. 2016, 76, 40–51.
- Talibi, M.; Hellier, P.; Ladommatos, N. Investigating the Combustion and Emissions Characteristics of Biomass-Derived Platform Fuels as Gasoline Extenders in a Single Cylinder Spark-Ignition Engine; SAE International: Beijing, China, 2017.
- Teoh, K.-S.; Melchiorre, M.; Kreth, F.A.; Bothe, A.; Köps, L.; Ruffo, F.; Balducci, A. Γ-Valerolactone as Sustainable and Low-Toxic Solvent for Electrical Double Layer Capacitors. ChemSusChem 2022, 16, e202201845.
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl Carbonate: A Versatile Reagent for a Sustainable Valorization of Renewables. Green Chem. 2018, 20, 288–322.
- O’Neill, M.; Sankar, M.; Hintermair, U. Sustainable Synthesis of Dimethyl- and Diethyl Carbonate from CO2 in Batch and Continuous Flow—Lessons from Thermodynamics and the Importance of Catalyst Stability. ACS Sustain. Chem. Eng. 2022, 10, 5243–5257.
- Choi, Y.D.; Verpoorte, R. Green Solvents for the Extraction of Bioactive Compounds from Natural Products Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Food Sci. 2019, 26, 87–93.
- Zhao, H.; Xia, S.; Ma, P. Use of Ionic Liquids as ‘Green’ Solvents for Extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096.
- Kong, X.-J.; Li, J.-R. An Overview of Metal–Organic Frameworks for Green Chemical Engineering. Engineering 2021, 7, 1115–1139.
- Souza, M.M.V.M.; Santos, M.; Sumere, B.R.; Da Silva, L.C.; Cunha, D.; Martínez, J.; Barbero, G.F. Isolation of Gallic Acid, Caffeine and Flavonols from Black Tea by on-Line Coupling of Pressurized Liquid Extraction with an Adsorbent for the Production of Functional Bakery Products. Lebensm.-Wiss. Technol. 2020, 117, 108661.
- Patra, A.; Abdullah, S.; Pradhan, R.C. Review on the Extraction of Bioactive Compounds and Characterization of Fruit Industry By-Products. Bioresour. Bioprocess. 2022, 9, 14.
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic Assisted Aqueous Extraction of Catechin and Gallic Acid from Syzygium Cumini Seed Kernel and Evaluation of Total Phenolic, Flavonoid Contents and Antioxidant Activity. Chem. Eng. Process. 2020, 149, 107841.
- Da Silva, L.C.; Souza, M.M.V.M.; Sumere, B.R.; Silva, L.F.; Da Cunha, D.T.; Barbero, G.F.; Bezerra, R.M.N.; Martínez, J. Simultaneous Extraction and Separation of Bioactive Compounds from Apple Pomace Using Pressurized Liquids Coupled On-Line with Solid-Phase Extraction. Food Chem. 2020, 318, 126450.
- Ravikumar, H.; Chua, B.L.; Mah, S.H.; Chow, Y.H. An Insight into Extraction, Isolation, Identification and Quantification of Bioactive Compounds from Crataegus monogyna Plant Extract. Rev. Agric. Sci. J.-STAGE 2022, 10, 304–327.
- Muhamad, I.I.; Hassan, N.A.; Mamat, S.S.; Nawi, N.M.; Rashid, W.; Tan, N.A.H. Extraction Technologies and Solvents of Phytocompounds from Plant Materials: Physicochemical Characterization and Identification of Ingredients and Bioactive Compounds from Plant Extract Using Various Instrumentations. In Ingredients Extraction by Physicochemical Methods in Food; Academic Press: London, UK, 2017; pp. 523–560.
- Alternative Solvents for Natural Products Extraction; Springer Nature: Berlin/Heidelberg, Germany, 2014; Available online: https://link.springer.com/book/10.1007/978-3-662-43628-8 (accessed on 8 July 2023).
- Chemat, F.; Strube, J. Green Extraction of Natural Products: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2016; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9783527676828 (accessed on 8 July 2023).
- Zhou, F.; Hearne, Z.; Li, C.-J. Water—the Greenest Solvent Overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123.
- McHugh, M.; Krukonis, V. Supercritical Fluid Extraction: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2013; Available online: https://www.sciencedirect.com/book/9780080518176/supercritical-fluid-extraction (accessed on 8 July 2023).
- Ray, A.; Dubey, K.; Marathe, S.J.; Singhal, R. Supercritical Fluid Extraction of Bioactives from Fruit Waste and Its Therapeutic Potential. Food Biosci. 2023, 52, 102418.
- Chen, Z.; Chen, H.; Xu, Y.; Hu, M.; Hu, Z.-T.; Wang, J.; Pan, Z. Reactor for Biomass Conversion and Waste Treatment in Supercritical Water: A Review. Renew. Sustain. Energy Rev. 2023, 171, 113031.
- Koschinsky, A.; Garbe-Schönberg, D.; Sander, S.G.; Schmidt, K.; Gennerich, H.-H.; Strauss, H. Hydrothermal Venting at Pressure-Temperature Conditions above the Critical Point of Seawater, 5 °S on the Mid-Atlantic Ridge. Geology 2008, 36, 615.
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I.; et al. Hydrogen Donating Capacity of Water in Catalytic and Non-Catalytic Aquathermolysis of Extra-Heavy Oil: Deuterium Tracing Study. Fuel 2021, 283, 118957.
- Zhao, J.; Xu, D.; Hao, B.; Guo, S.; Guo, Y.; Wang, S. Chemical Reactions of Organic Compounds in Supercritical Water Gasification and Oxidation. Water Res. 2021, 190, 116634.
- Subramani, V.B.; Atanda, L.; Doherty, W.O.S.; Rackemann, D.W.; Moghaddam, L. Co-Liquefaction of Cotton Gin Trash and Low-Density Polyethylene Wastes via Supercritical Ethanolysis for Hydrocarbon-Rich Oil. Energy Convers. Manag. 2023, 290, 117216.
- Wang, X.; Xu, W.; Zhang, D.; Li, X.; Shi, J. Structural Characteristics–Reactivity Relationships for Catalytic Depolymerization of Lignin into Aromatic Compounds: A Review. Int. J. Mol. Sci. 2023, 24, 8330.
- Brand, S.; Hardi, F.; Kim, J.; Suh, D.I. Effect of Heating Rate on Biomass Liquefaction: Differences between Subcritical Water and Supercritical Ethanol. Energy 2014, 68, 420–427.
- Furusjö, E.; Akalin, M.; Karagöz, S. Experimental Design for Extraction of Bio-Oils from Flax Seeds under Supercritical Ethanol Conditions. Clean Technol. Environ. Policy 2015, 18, 461–471.
- Zeb, H.; Choi, J.; Kim, Y.; Kim, J. A New Role of Supercritical Ethanol in Macroalgae Liquefaction (Saccharina japonica): Understanding Ethanol Participation, Yield, and Energy Efficiency. Energy 2017, 118, 116–126.
- Kuo, T.M.; Gardner, H. Lipid Biotechnology; CRC Press: Boca Raton, FL, USA, 2002; Available online: https://www.amazon.com/Lipid-Biotechnology-Tsung-Min-Kuo/dp/0824706196 (accessed on 8 July 2023).
- King, J.W. Supercritical Fluid Technology for Lipid Extraction, Fractionation, and Reactions. In Lipid Biotechnology; Taylor and Francis: Abingdon, UK, 2002; pp. 656–680.
- Sun, X.; Li, X.; Tan, X.; Zheng, W.; Zhu, G.; Cai, J.; Zhang, Y.-Y. Pyrolysis of Heavy Oil in Supercritical Multi-Thermal Fluid: An Effective Recovery Agent for Heavy Oils. J. Pet. Sci. Eng. 2021, 196, 107784.
- Ma, C. Injection Production Process of Fluids Produced by Supercritical Water. Oxidation. Patent CN102322248A, 18 January 2021.
- Prabhune, A.; Dey, R. Green and Sustainable Solvents of the Future: Deep Eutectic Solvents. J. Mol. Liq. 2023, 379, 121676.
- Sharma, V.; Tsai, M.-L.; Chen, C.-T.A.; Sun, P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C. Deep Eutectic Solvents as Promising Pretreatment Agents for Sustainable Lignocellulosic Biorefineries: A Review. Bioresour. Technol. 2022, 360, 127631.
- Atilhan, M.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644.
- El-Deen, A.K.; Shimizu, K. Deep Eutectic Solvents as Promising Green Solvents in Dispersive Liquid–Liquid Microextraction Based on Solidification of Floating Organic Droplet: Recent Applications, Challenges and Future Perspectives. Molecules 2021, 26, 7406.
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800.
- Welton, T. Solvents and Sustainable Chemistry. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150502.
- Oklu, N.K.; Matsinha, L.C.; Makhubela, B.C.E. Bio-Solvents: Synthesis, Industrial Production and Applications. In Solvents, Ionic Liquids and Solvent Effects; IntechOpen: London, UK, 2020.
- PubChem Water. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/962 (accessed on 8 July 2023).
- The Drive to Make Things Happen. Available online: https://vrchemistry.chem.ox.ac.uk/potential/text/solutions3.htm (accessed on 8 July 2023).
- PubChem Hazardous Substances Data Bank (HSDB): 82. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/82#section=FDA-Requirements-(Complete) (accessed on 8 July 2023).
- Sierra-Amor, R.I. CRC Handbook of Laboratory Safety, 5th ed.; Keith Furr, A., Ed.; CRC Press LCC: Boca Raton, FL, USA, 2000; p. 774. ISBN 0-8493-2523-4.
- Vedantu Ethyl Acetate. VEDANTU. 2022. Available online: https://www.vedantu.com/chemistry/ethyl-acetate (accessed on 8 July 2023).
- LSU Ethyl Acetate Solvent Properties. Available online: https://macro.lsu.edu/howto/solvents/ethylacetate.htm (accessed on 8 July 2023).
- LSU Ethyl Ether Solvent Properties. Available online: https://macro.lsu.edu/howto/solvents/ether.htm (accessed on 8 July 2023).
- PubChem Diethyl Ether. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diethyl-Ether#section=Computed-Descriptors (accessed on 8 July 2023).
- ICSC 1164—Methyl Tert-Butyl Ether. Available online: https://inchem.org/documents/icsc/icsc/eics1164.htm (accessed on 8 July 2023).
- GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=510639&lang=en (accessed on 8 July 2023).
- Pace, V.; Holzer, W.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R. 2-Methyltetrahydrofuran. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–6.
- GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=025460&lang=en (accessed on 8 July 2023).
- Dimethylether. Available online: https://web.archive.org/web/20211106032850/https://encyclopedia.airliquide.com/dimethylether (accessed on 8 July 2023).
- PubChem Cyclohexane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyclohexane#section=Structures (accessed on 8 July 2023).
- DCCEEW Cyclohexane. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/cyclohexane (accessed on 8 July 2023).
- Admin Benzene (C6H6)—Definition, Discovery, Structure, Resonance, Aromaticity & Uses of Benzene (C6H6). BYJUS 2023. Available online: https://byjus.com/chemistry/benzene/ (accessed on 8 July 2023).
- DCCEEW Carbon Disulfide. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/carbon-disulfide#:~:text=Pure%20carbon%20disulfide%20is%20a,at%200.016%20to%200.42%20ppm (accessed on 8 July 2023).
- Worldofchemicals. Glycerol: Properties, Production and Uses. 2017. Available online: worldofchemicals.com (accessed on 8 July 2023).
- Wikipedia. Wikipedia Contributors National Institute for Occupational Safety and Health. 2023. Available online: https://en.wikipedia.org/wiki/National_Institute_for_Occupational_Safety_and_Health (accessed on 8 July 2023).
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green Chem. 2008, 10, 238–242.
- Baird, Z.S.; Uusi-Kyyny, P.; Pokki, J.-P.; Pedegert, E.; Alopaeus, V. Vapor Pressures, Densities, and PC-SAFT Parameters for 11 Bio-Compounds. Int. J. Thermophys. 2019, 40, 102.
- 5614-37-9 CAS | Cyclopentyl Methyl Ether (CPME) | High Purity Solvents | Article No. 03139. Available online: https://www.lobachemie.com/High-Purity-Solvents-03139/CYCLOPENTYL-METHYL-ETHER-CPME-CASNO-5614-37-9.aspx (accessed on 8 July 2023).
- Cyclopentyl Methyl Ether (CPME) | Specialty Solvents | Zeon Corporation. Available online: https://www.zeon.co.jp/en/business/enterprise/special/solvent-cpme/ (accessed on 8 July 2023).
- Gov, N.O. of R. and R.U. D-LIMONENE | CAMEO Chemicals | NOAA. Available online: https://cameochemicals.noaa.gov/chemical/20568 (accessed on 8 July 2023).
- Sonu, K.S.; Mann, B.; Sharma, R.; Kumar, R.; Singh, R. Physico-Chemical and Antimicrobial Properties of d-Limonene Oil Nanoemulsion Stabilized by Whey Protein–Maltodextrin Conjugates. J. Food Sci. Technol. 2018, 55, 2749–2757.
- P-Cymene | 99-87-6. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB9262508.htm (accessed on 8 July 2023).
- Dimethyl Carbonate | 616-38-6. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8853983.htm (accessed on 8 July 2023).
- Ethyl L(-)-Lactate. Available online: https://www.chembk.com/en/chem/Ethyl%20L(-)-lactate (accessed on 8 July 2023).
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
16.4K
Revisions:
3 times
(View History)
Update Date:
16 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





