Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md Faheem Haider | -- | 3489 | 2023-08-14 05:04:42 | | | |
| 2 | Camila Xu | Meta information modification | 3489 | 2023-08-14 08:31:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Haider, T.; Adnan, M.; Akhter, M.H.; Afzal, O.; Altamimi, A.S.A.; Ahmad, I.; Alossaimi, M.A.; Jaremko, M.; Emwas, A.; Haider, M.F. Vesicular Nanoformulations for Skin Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/48016 (accessed on 09 February 2026).
Haider T, Adnan M, Akhter MH, Afzal O, Altamimi ASA, Ahmad I, et al. Vesicular Nanoformulations for Skin Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/48016. Accessed February 09, 2026.
Haider, Tanweer, Mohammad Adnan, Md. Habban Akhter, Obaid Afzal, Abdulmalik S. A. Altamimi, Irfan Ahmad, Manal A. Alossaimi, Mariusz Jaremko, Abdul-Hamid Emwas, Md. Faheem Haider. "Vesicular Nanoformulations for Skin Cancer" Encyclopedia, https://encyclopedia.pub/entry/48016 (accessed February 09, 2026).
Haider, T., Adnan, M., Akhter, M.H., Afzal, O., Altamimi, A.S.A., Ahmad, I., Alossaimi, M.A., Jaremko, M., Emwas, A., & Haider, M.F. (2023, August 14). Vesicular Nanoformulations for Skin Cancer. In Encyclopedia. https://encyclopedia.pub/entry/48016
Haider, Tanweer, et al. "Vesicular Nanoformulations for Skin Cancer." Encyclopedia. Web. 14 August, 2023.
Copy Citation
Skin cancer can be classified into melanomas from melanocytes and nonmelanoma skin cancer (NMSC) from the epidermally-derived cell. The vesicular nanocarrier system is one of the most preferred delivery systems and is helpful in immunology, membrane biology, diagnostics, and, most recently, genetic engineering.
skin cancer
nanoformulations
skin permeation
cutaneous squamous cell carcinoma
1. Liposomes
Dr. Alec D Bangham FRS, a British hematologist, initially described liposomes in 1961 (published 1964) at the Babraham Institute in Cambridge. Liposome comes from two Greek words: ‘Lipos’, which means fat, and ‘Soma’, which means body [1]. Liposomes are colloidal or microparticulate carriers with a 0.05–5.0 µm diameter. Drugs with a wide range of lipophilicity can be contained in liposomes within the phospholipid bilayer, the entrapped aqueous volume, or at the bilayer interface [2]. Single or more lipid bilayers are generated via hydrophilic and hydrophobic interactions with the aqueous phase in liposomes. In addition, Phosphatidylcholine (PC) and Dipalmitoyl PC can be utilized to make liposomes [3][4]. Various liposome types are available and depicted in Figure 1 for different diseases, including skin cancer. The formation of bilayers depends upon the ingredients utilized in the formulation. The types of each vesicle are unique in their way [5][6].
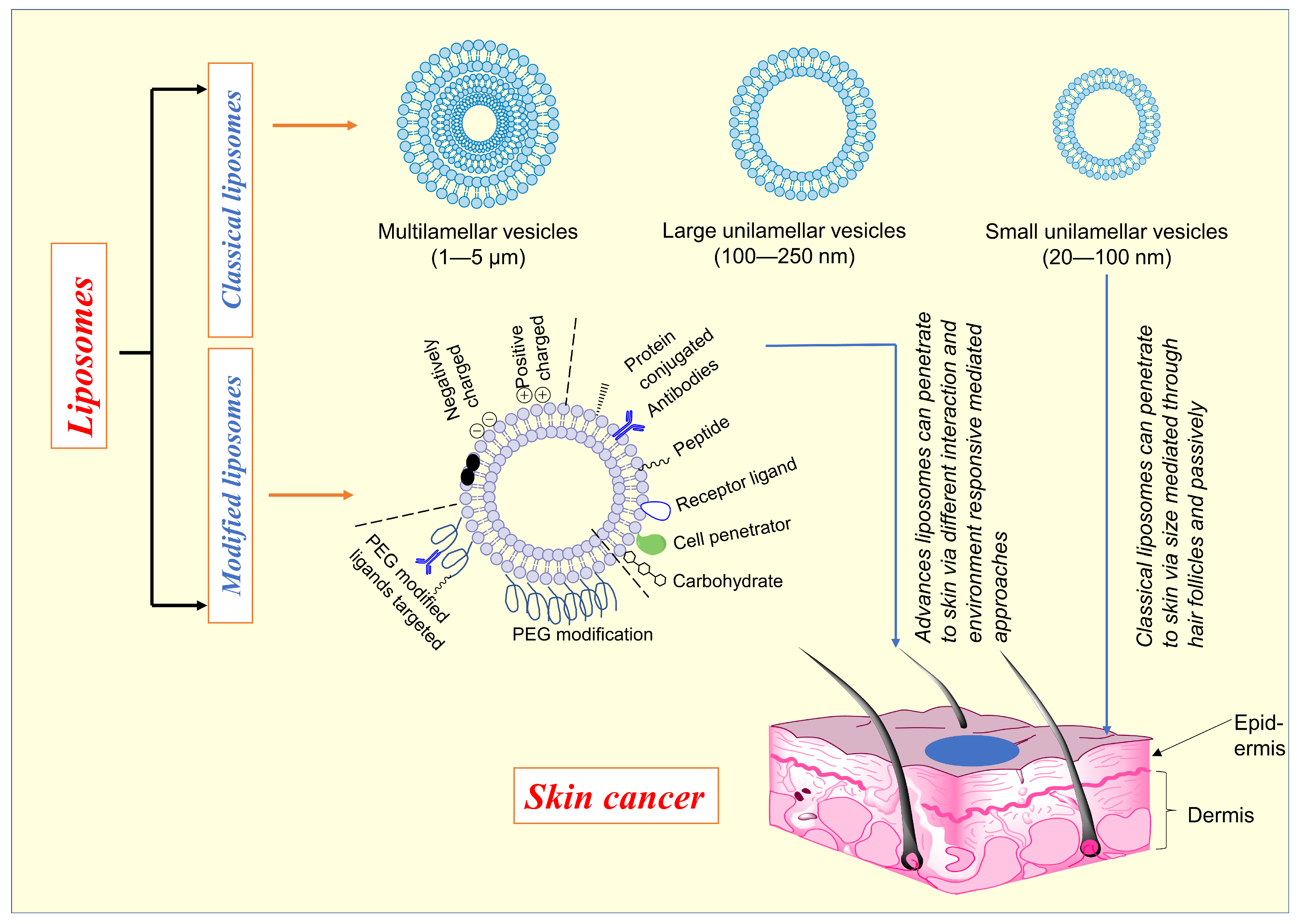
Figure 1. Depicts the various types of liposomes and the mechanism of penetration in skin cancer.
Liposomes are a well-established technology platform with many clinical applications [5]. Upon topical application, liposome uptake by the stratum corneum is most significant for positively charged liposomes, least for negatively charged liposomes, and least for neutral liposomes, implying that the electrostatic adsorption is the initial interaction between the corneal surface and liposomes [7]. The liposomal formulation might be enhanced pharmacokinetics, lower organ toxicity, and the potential to increase tumor absorption [8][9][10]. Various types of liposomes (Figure 1) were studied to improve skin penetration, controlled drug released, accumulation of drugs at specific sites, etc., to enhance the cytotoxic activity against skin cancer.
Endothelial growth factor receptors (EGFRs) are overexpressed in SCC, and responsible for poor prognosis and malignancy. EGFRs can be used to target the treatment of cancer. Petrilli et al. [7] have prepared the liposomal system loaded with 5-FU and co-administered with anti-EGFR (cetuximab) antibodies for targeting EGFRs. The uptake of EGFR-targeted 5-FU loaded liposomes showed about 3.5 times more uptake than control non-EGFR targeted liposomes. The antitumor efficiency of EGFRs-targeted showed more than 60% higher than the control liposomes when administrated subcutaneously. Another study by Singh [11] developed a liposomal system for dual targeting. Prepared liposomes meant for the targeting of AKT and COX-2. Liposomes were prepared and loaded with the combination of Dox and celecoxib and evaluated the cytotoxic efficacy in skin cancer and found that cancer cell viability inhibition was more than 99% at low concentrations, compared to alone. Multiple targeting might be a better treatment option for skin cancer treatment. The charge of tumor cells is more negative than normal cells [12][13]. One of the approaches to target skin cancer cells to cancer surface charged derived nanoformulations to enhance the cytotoxic effect of drug carriers. Jose et al. [14] used the cell surface charge to improve the cellular uptake of liposomes by using anti-STAT3 siRNA DOTAP-based cationic liposomes of curcumin. The cell viability of the co-delivery of curcumin and STAT3 siRNA using cationic liposomes in B16F10 mouse melanoma cells was inhibited considerably compared to either liposomal curcumin or STAT3 siRNA alone. The cationic liposomes are efficient drug delivery for the delivery of drugs or siRNA to the cancer cells for the treatment of skin cancer. The pH of the tumor microenvironment is acidic in comparison to the normal tissue environment [15][16]. pH-triggered drug delivery for delivery of drug to the tumor cells might be another approach. Lee and Nu [17] developed the pH-gradient anthocyanin-loaded liposomes for enhanced skin and improved cellular uptake. The antioxidant effect and skin permeation of formulation have significantly increased in this approach. The concentration of ROS was decreased by this approach, which later enhanced the cytotoxic effect in skin cancer. Under oxidative stress conditions, ROS production is significantly increased, leading to the oxidation of cellular proteins, lipids, and ultimately DNA, resulting in lethal lesions in cells that aid cancer development [15]. Another approach to enhance the penetration of drugs through the skin barrier and cellular uptake in skin cancer is deformable liposomes. Liposomes with edge activators increase skin permeability by reducing the stiffness of the bilayer structure and causing it to deform [18]. Marwah et al. [19] developed the deformable liposomal formulation using the tween 80 (edge-activator) and loaded with epigallocatechin gallatein (has antineoplastic properties) and evaluated the cellular uptake and cytotoxicity on HDFa and HaCat cells. The cell viability inhibition of formulation was found to be significantly below 100 µM and high cellular uptake in cancer cells. In a similar study, El-Kayal et al. [20] developed permeation enhancer-containing vesicles loaded with epigallocatechin-3-gallate for skin cancer treatment. The vesicles showed good inhibitory action against the A431 cells and reduced tumor size in the mice model. In another study, Sharma et al. [21], developed the C-type lectin receptor targeted nanoliposomes conjugated with mannose for the cross-presentation of ovalbumin as a model antigen. According to the findings, nanoliposomes dramatically increased antigen intake and cross-presentation to elicit CD8+ cell-mediated cellular immunity.
Liposomes are important drug delivery carriers for treating cancers, including skin cancer. Due to the penetration of skin barriers, classical liposomes have limitations in skin cancer treatment. However, the gradual improvement in liposomes, such as deformation liposomes, surface-modified liposomes, dual-targeted liposomes, etc., has better skin layer penetration and cytotoxic effects.
2. Niosomes
Niosomes are microscopic lamellar structures ranging in size from 10 to 1000 nanometers. Surfactants are non-immunogenic, biodegradable, and biocompatible makeup niosomes [22]. The two main components employed in forming niosomes are cholesterol and nonionic surfactants. Surfactants are essential in creating niosomes, whereas cholesterol provides stiffness and appropriate shape. For the manufacture of niosomes, nonionic surfactants such as spans (20, 40, 60, 85 and 80), tweens (20, 40, 60 and 80), and Brij (30, 35, 52, 58, 72 and 76) are commonly utilized [23][24]. Niosomes have amphiphilic characteristics, allowing hydrophilic medications to be entrapped in the core cavity and hydrophobic pharmaceuticals to be entrapped in the nonpolar area of the bilayer [25]. The alkyl chain length affects the surfactant’s hydrophilic-lipophilic balance (HLB) value (the lower the HLB value, the lower the entrapment efficiency). The studies found the most efficient entrapment between tween 20 and span 60 [26]. L’Oréal was the first company (in the cosmetics sector) to develop and patent nonionic surfactant niosomes [27]. Nonionic surfactants are nonpolar and polar sections with high interfacial tension that commonly form bilayers when hydrated or upon hydration [28]. Ether Injection, Hand Shaking, and Reverse Phase Evaporation are the most common methods for making niosomes [29]. Deformable niosomes are a mixture of nonionic surfactants, ethanol, and water. These tiny vesicles can readily pass through the pores of the stratum corneum, causing an increase in penetration effectiveness [30][31]. Table 1 indicates a comparison between niosomes and liposomes [32].
Table 1. Comparison between niosomes and liposomes [32].
| Niosomes | Liposomes |
|---|---|
| Less expensive than liposomes | More expensive than niosomes |
| Nonionic surfactants are stable | Phospholipids may undergo oxiditive degradation |
| The surface charge may present on niosomes | The neutral charge may be due to phospholipid |
| The particular method requires the purification, storage, and handling of phospholipids | Comparatively, no particular method requires |
Its key features are the increased permeability of niosomes through the SC and the ability to reach the desired site of action [33]. Both hydrophilic and lipophilic drugs can be added to vesicular systems containing nonionic surfactants without causing toxicity. Resveratrol has poor bioavailability, low water solubility, chemical instability, and restricted skin permeability. The niosomal hydrogel system of resveratrol increases the permeation and deposition in the skin, enhancing the therapeutic action of resveratrol [34]. Chermahini et al. [35] developed the 5-FU-loaded for skin cancer, and results suggested that niosome-encapsulated fluorouracil showed significantly higher anticancer activity of the niosomal formulation when compared to other treatments with niosomes, which might be due to the enhanced skin permeability of niosomes. In a similar study by Paolino et al. [36], the study showed that 5-FU loaded alpha,omega-hexadecyl-bis-(1-aza-18-crown-6) and span 80 containing the niosomal system has several folds more cytotoxic effects (in SKEML-28-cells) than the control; it might be due to the enhanced permeation enhancement of niosomes. Topical use of niosomes may increase the residence time of the drug in the stratum corneum and epidermis while reducing the systemic absorption of the drug [35]. Pawar et al. [37] developed the N-lauryl glucosamine conjugated Doxorubicin (Dox)-loaded nanoniosomes to target specific drug delivery for the treatment of cancer. The results suggested that conjugated nanoniosomes have more cytotoxicity efficacy against cancer and are less toxic against normal cells than non-conjugated systems. Shah et al. [38] prepared a niosomal gel containing the antioxidant Gamma oryzanol. The permeation through the skin was enhanced and might be an improved option for skin cancer treatment.
3. Transferosomes
Gregor Cevc coined the term “transferosome” in 1991. The name “Transfero” comes from the Latin word “transfero,” which means “to carry across,” and the Greek word “soma”, which means “body” [39]. Transferosome comprises one inner aqueous compartment and is enclosed by a lipid bilayer with an edge activator. Transferosome (a vesicle) has both self-regulating and self-optimizing properties. Transferosomes are elastic and can deform, squeeze and cross the skin’s stratum corneum. Edge activators fluidize or solubilize the skin’s lipids, enhancing skin permeation [40]. Transferosomes have higher entrapment efficiency, flux, and deposition when compared to liposomes and niosomes [41]. Various types of ingredients used in the formulation of transferosomes and their role are given in Table 2.
Table 2. Various ingredients of transferosomes and their role, along with examples.
| Ingredients | Role | Example |
|---|---|---|
| Phospholipid | Vesicle forming unit | Phosphatidylcholine, dipalmitoyl phosphatidyl choline |
| Edge activators (surface active agents) | Enhance flexibility | Tween 20, span 80, sodium deoxycholate, sodium cholate |
| Alcohol | Solvents | Methanol, ethanol |
| Buffers | Hydration medium | Phosphate saline (pH 6.4) |
Transferosomes, also known as deformable vesicles, have increased drug delivery to the skin. Enhanced amounts of both small and large therapeutic agents are delivered into and through the skin using transferosomes [42]. Jangdey et al. [43] developed ultra-flexible lipid vesicles such as an apigenin-loaded transfersomal system for the enhanced skin delivery of skin cancer treatments. The result suggested that the permeation of the drug through the skin via transferosomal formulation was significantly higher (p < 0.05) than that of the marketed product. The permeation of cytotoxic moieties in the tranferosomal system can improve skin cancer treatment. Another investigation by Sivarajakumar et al. [44] prepared the paclitaxel-loaded transfersomal vesicular systems to enhance the delivery of drugs at the site of skin cancer cells by improving the permeation through the skin. The results suggested that optimized transfersomes had a flux of 6.68 ± 0.46 and a percent drug retention of (0.79 ± 0.05) through the skin of mice. In another investigation, Jangdey et al. [45] developed the concanavalin-A conjugated nanotransfersomal gel of apigenin for enhanced targeted delivery of UV-induced malignant melanoma, which binds directly to the melanocytes gel layer in UVB-induced skin carcinoma. According to the findings, the cytotoxicity of concanavalin-A conjugated nanotransfersomal gel against A375 in a concentration range of 0.4–2.0 mg/mL, but less toxicity toward HaCaT cells. According to these investigations, transferosomes can increase cytotoxic efficacy by enhancing drug penetration at the site of action and cellular uptake.
4. Ethosomes
Ethanolic liposomes are also called ethosomes. Ethosomes are noninvasive delivery vehicles that allow medications to be delivered deep into the skin’s layers and/or the circulatory system. These soft, pliable vesicles are designed to distribute active substances more effectively [46]. Ethosomes comprise phospholipids, ethanol (higher concentration), and water. Ethosomes range in size from tens of nanometers (nm) to microns (µ) and permeate the skin layers more quickly, and transdermal flux is substantially higher [47]. Touitou named the term ethosomes, and high concentration of ethanol (20–50%) is the main reason for better skin permeation. Ethosomal formulation can disrupt the lipid bilayer structure of the skin and penetrate the stratum corneum (which possesses a very compact structure) [48]. Ethosomes enhance the lipid fluidity of the cell membrane and reduce the density of multi-layered lipids of the cell membrane, where it binds to skin lipids and release drugs into the deeper layers of the skin [49].
Ethosomes may be divided into the classical and binary ethosomes. Classical ethosomes show improved skin penetration and stability profiles compared to classic liposomes. The molecular weights of pharmaceuticals trapped in classical ethosomes have increased, ranging from 130.077 Da to 24 kDa [50]. Binary ethosomes were first introduced by Zhou. These were created by mixing another alcohol with the traditional ethosomes. Propylene glycol (PG) and isopropyl alcohol (IPA) are the most often employed alcohols in binary ethosomes [51][52].
Several studies have demonstrated the superiority of an ethosomal carrier over other nanocarriers, implying that it has a significant impact on drug delivery systems. Table 3 shows additives for the formulation of ethosomes and their functions [53]. The systematic presentation of the mechanism of action of ethosomes is given in Figure 2. Ethosomes, when applied topically, disrupt the skin, increase the lipid fluidity of the skin, and promote penetration across the skin. Later they fused with the skin and released the drug gradually [54].
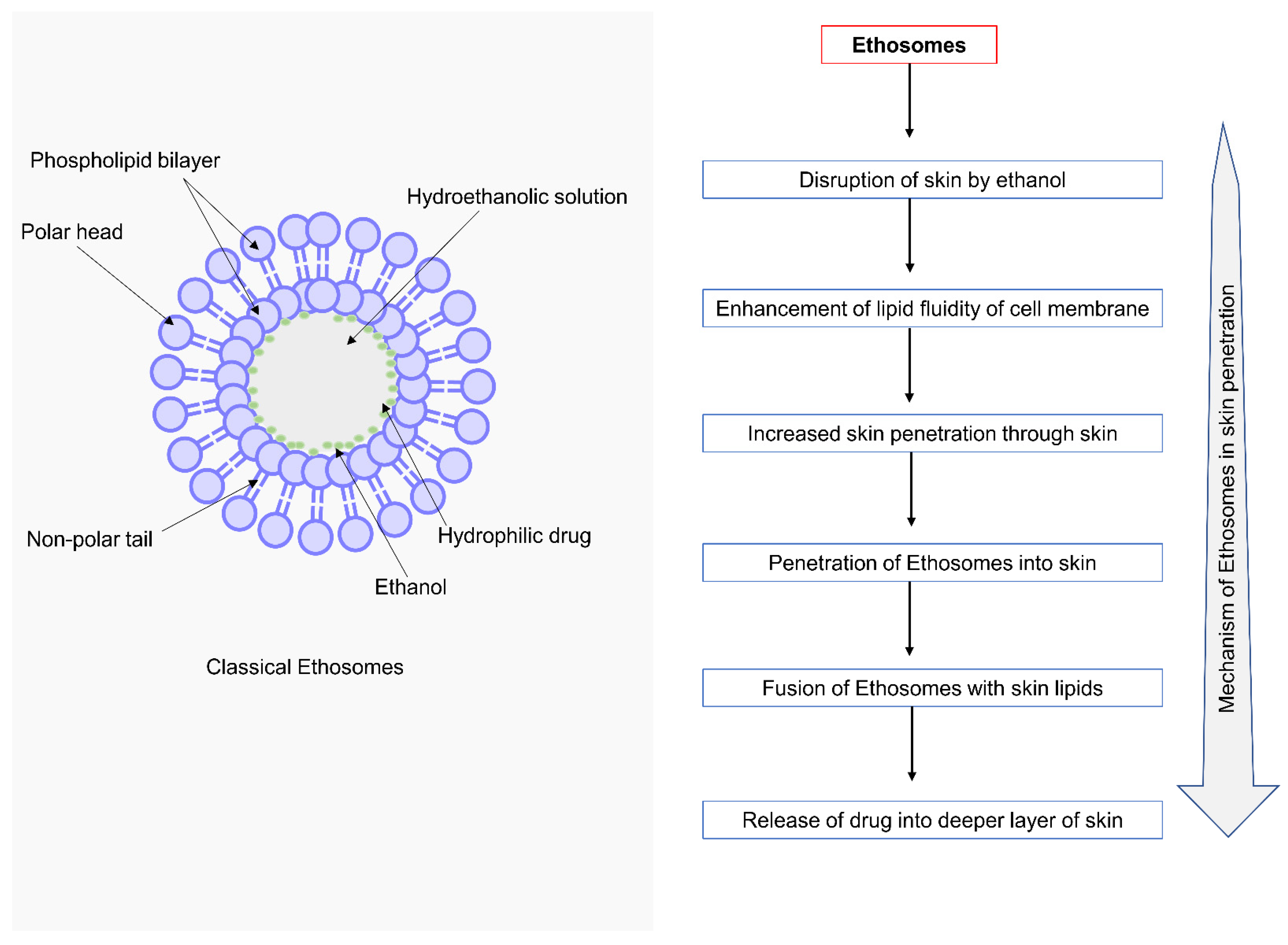
Figure 2. Schematic representation of the classical ethosomes and mechanism of action ethosomes.
Table 3. Various ingredients with their use in formulating ethosomes.
| Class | Concentration (%) | Example | Uses |
|---|---|---|---|
| Phospholipids | 0.5–10 | Phospholipon 90G, 90H, 80H, Lipoid S100, S75, S75–3, E80 Dipalmityl phosphatidylcholine, Distearyl phosphatidylcholine |
Vesicle forming unit |
| Edge activators/surfactant or permeation enhancer |
10–50 of the totals phospholipid concentration |
Tween 60, 80, 20 Span 80, 60, 40, 20 Cremophor RH-40 SPACE (skin penetrating and cell entering peptide) Oleic acid, Sodium cholate, Deoxy sodium cholate, Dimethyl sulfoxide |
Increase the skin permeability or act as a penetration enhancer |
| Alcohol | 20–50 | Ethanol, Isopropyl alcohol | For providing the softness for vesicle membrane As a penetration enhancer |
| Glycol | Propylene glycol (p.G.) Transcutol RTM | Permeation enhancer | |
| Cholesterol | 0.1–1 | Cholesterol | Gives stability and rigidity to vesicle |
| Dye | q.s. | Rhodamine-123 Rhodamine red Fluorescence Isothiocyanate (FITC) 6-Carboxy fluorescence |
Characterization study |
| Vehicle | q.s. | Carbopol, etc. | Ac as gel former |
An investigation by Gamal et al. [55] prepared the sonidegib-loaded ethosomes and later incorporated them in the gel to effectively treat skin cancer. Ethosomal formulation of sonidegib depicts a mean size of (199.53 ± 4.51 nm), steady-state flux (5.58 ± 0.08 µg/cm2/h), and entrapment efficiency of (87.5 ± 2.5); this exhibits that ethosomes possess higher entrapment efficiency and the other physiochemical properties are acceptable. The antitumor efficacy of ethosomal formulation showed significantly higher relative anti-tumor activity and 3.18 times bioavailability than the oral sonidegib drug. Another study by Peram et al. [54][56] prepared the curcumin-loaded ethosomes. The optimized curcumin-loaded ethosomes significantly reduced (p < 0.05) the cell viability of A375 cells compared to free curcumin. It could be attributed to the persistent release of curcumin from ethosomes, resulting in continued drug exposure to tumor cells and more significant anticancer activity. Mousa et al. [57] prepared the metformin-loaded ethosomes for skin cancer treatment. Results showed the high permeation efficiency of ethosomes through the skin and higher antitumor efficiency than the pure drug. The small-sized ethosomes penetrate deeper into the skin layer, later causing improved antitumor efficacy.
The formulations are a few examples of ethosomes, which have small-sized, better skin layer penetration and significantly high anticancer activity. The ethosomal formulation gives hope for a better treatment strategy for skin cancer treatment.
5. Transethosomes
Transethosomes are vesicles with an irregular shape, and the size lies between 40 nm and 200 nm depending upon the size of the drug. Transethosomes are a type of UDV (ultra-deformable vesicle) and a novel lipid vesicle that is flexible and deformable. UDVs were developed at the beginning of the 1990s and could deliver the drug into the epidermis or skin’s dermis and deep circulation [58]. Transethosomes (TELs) consist of phospholipids, ethanol, water, and edge activators (surfactants) or permeation enhancers (e.g., oleic acid). The edge activators (surfactants) are single-chain surfactants that provide flexibility by destabilizing the vesicle’s lipid bilayer, which in turn reduces interfacial tension and augments its structure deformability [56]. When substantial levels of ethanol (about 30%) are combined with edge activators, a synergy is created that allows transethosomes to enter and disperse deep into the epidermis. Lipid bilayer rearrangement and ethanol aid in enhancing the solubility of lipophilic medicines and disturbing the SC. As a result, transethosome can carry medications deep into the dermal layers or even the systemic circulation [59]. Moolakkadath et al. [60] developed a fisetin-loaded transethosome delivery system for nonmelanoma skin cancer. They optimized the formulation using the Box–Behnken design and found that the optimized formulation had nano-range vesicle size (74.21 ± 2.65 nm) possessing good entrapment efficiency (68.31 ± 1.48%) and good flux (4.13 ± 0.17 mg/cm2/h) for fisetin dermal delivery. These formulations showed high penetration through the skin, providing a better treatment strategy for skin cancer treatment. Abdulbaqi et al. [59] concluded that the permeation of transethosomal gel has superior permeation properties than non-transethosomal gel. Another study by Abdulbaqi et al. [59] developed colchicine-loaded transethosomes to enhance skin penetration. The findings found that transethosomal gel has better stability at refrigerated conditions (4 °C ± 2 °C) and high skin permeation efficiency. The study concluded that transethosomal gels are potent carriers for the transdermal delivery of colchicine.
Transethosomal drug delivery is one of the important drug delivery systems for the delivery of drugs or other active moieties from the skin due to their better skin Lipid Nanoparticles (LNPs).
Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLC) are lipid nanoparticles that are very stable; well tolerated and protect medications from degradation while ensuring a consistent release over time [61][62].
6. Solid Lipid Nanoparticles (SLNs)
SLNs are a colloidal system with sizes ranging from 50 to 1000 nm. A high-pressure homogenization process combines biodegradable and biocompatible solid lipids, emulsifiers, and water. Triglycerides, glycerides, and other lipids commonly employ waxes and fatty acids [63]. SLNs form a monolayer on the skin, creating an occlusive effect that increases water retention inside the skin [64]. Tupal et al. [63] prepared and optimized the formulations of Dox-loaded solid lipid nanoparticles and optimized showed a mean particle size of 92 nm and entrapment efficiency of 86% following a 40-day study period, both the low and high doses of Dox-loaded SLNs were found to produce significantly better results than free Dox formulation (p < 0.05). In terms of tumor volume and weight assessments, there was no statistical difference between the low and high doses of Dox-SLNs (p > 0.05) [65]. Studies by Kaur et al. [66][67] prepared the curcumin-loaded SLNs for evaluating the anticancer activity, stability, permeation, and pharmacokinetics parameters. The results suggested that SLNs have high permeation, stability, bioavailability, and anti-cancer effects. The incorporation of SLNs in gels or patches can improve the treatment of skin diseases, including skin cancer. In this prospect, a study was performed by Sabir et al. [68]. They tested this approach, prepared the curcumin-loaded solid lipid nanoparticles, and incorporated them into the patches for transdermal delivery. The prepared particles showed a mean particle size of 170 ± 2 nm with an entrapment efficiency of 90 ± 3.5% (w/w). The permeation efficiency of SLNs incorporated patches has about 6.5 folds permeation than the non-patches SLNs. In another study by Gonçalves et al., which prepared the cutaneous SLNs and loaded multiple natural compounds such as naringenin, nordihydroguaiaretic acid (NDGA), and kaempferol and found that the SLNs had a mean particle size of 200 nm with high drug entrapment efficiency. Formulations were evaluated against human keratinocytes (HaCaT) for anti-cancer activity. The formulation reduced the ROS, produced due the oxidative stress and provides significant anticancer activity. In another study by Banerjee et al. [69] Tyr-3-octreotide modified SLNs loaded with Ptx were prepared for specific targeting for the highly expressed somatostatin receptors present on the melanoma cells to enhance the treatment of the same. The results of the study showed that Tyr-3-octreotide exhibits remarkable anti-melanoma activities without any observable toxicity. Several pieces of research showed that SLNs permeate through the skin, penetrate the deeper layers, and reach target sites. These formulations might be effective carriers for skin cancer treatment.
7. Nanostructured Lipid Carriers (NLCs)
NLCs are made up of solid and liquid lipids and have a crystalline structure that is not perfect. The lipid is either encased in a solid lipid matrix or is found on the surfactant layer [70]. The solid lipid component imparts properties for controlled drug release, whereas the liquid phase with lower water content provides substantial drug loading [71]. Furthermore, enhanced drug loading in NLCs is supported by increased distances between the fatty acid chains and the unstructured crystal [72]. They are significantly more appropriate for medication formulation than SLNs. NLCs are simple to make and are produced using pressure homogenization, nanoemulsion, or aqueous dispersion procedures. [73][74].
Moradi et al. [75] developed the NLCs containing Tretinoin as an active moiety to improve skin uptake and reduce the side effects. The findings demonstrated that a prolonged release profile maintains tretinoin penetration and absorption while promoting skin tolerability. Iqbal et al. [76] also prepared the silymarin-loaded NLCs and later incorporated them in the gel to treat skin cancer against the B16 melanoma cell line. According to the results, the group treated with the silymarin-NLC gel appeared to have significantly higher levels of superoxide dismutase, catalase, and glutathione and significantly lower levels of IL-1α and TNF-α. The antitumor effect of silymarin-NLC gel was also evaluated, and the results showed a significant (p < 0.05) reduction in tumors. Another study by Gundogdu et al. [77] developed the Imatinib (tyrosine kinase enzyme inhibitor)-loaded NLCs for cancer treatment. Besides the physiochemical properties of the formulation, the anticancer effect was evaluated against the CRL-1739 cell line. The formulation showed 23.61 µM of IC50 and induction of apoptosis in the cancer cells. The cytotoxicity efficiency of the formulation was significantly high in cells.
References
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9.
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Schaeffer, H.E.; Krohn, D.L. Liposomes in topical drug delivery. Investig. Ophthalmol. Vis. Sci. 1982, 22, 220–227.
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002, 4, 95.
- Haider, T.; Dubey, S.; Kanwar, I.L.; Pandey, V.; Soni, V. Preparation, Optimization and in vitro Studies of Spectrin Decorated Liposomes: A Promising Strategy for Cancer Treatment: Spectrin anchored liposomes for treatment of cancer. Trends Pept. Protein Sci. 2021, 6, e6.
- Petrilli, R.; Eloy, J.O.; Saggioro, F.P.; Chesca, D.L.; de Souza, M.C.; Dias, M.V.; Luis, L.; Lee, R.J.; Lopez, R.F. Skin cancer treatment effectiveness is improved by iontophoresis of EGFR-targeted liposomes containing 5-FU compared with subcutaneous injection. J. Control. Release 2018, 283, 151–162.
- Adler-Moore, J.P.; Chiang, S.-M.; Satorius, A.; Guerra, D.; McAndrews, B.; McManus, E.J.; Proffitt, R.T. Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome). J. Antimicrob. Chemother. 1991, 28, 63–71.
- Yarosh, D.; Alas, L.G.; Yee, V.; Oberyszyn, A.; Kibitel, J.T.; Mitchell, D.; Rosenstein, R.; Spinowitz, A.; Citron, M. Pyrimidine dimer removal enhanced by DNA repair liposomes reduces the incidence of UV skin cancer in mice. Cancer Res. 1992, 52, 4227–4231.
- Bayoumi, M.; Arafa, M.G.; Nasr, M.; Sammour, O.A. Nobiletin-loaded composite penetration enhancer vesicles restore the normal miRNA expression and the chief defence antioxidant levels in skin cancer. Sci. Rep. 2021, 11, 20197.
- Singh, S. Liposome encapsulation of doxorubicin and celecoxib in combination inhibits progression of human skin cancer cells. Int. J. Nanomed. 2018, 13 (Suppl. S1), 11–13.
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898.
- Haider, T.; Soni, V. Response surface methodology and artificial neural network-based modeling and optimization of phosphatidylserine targeted nanocarriers for effective treatment of cancer: In vitro and in silico studies. J. Drug Deliv. Sci. Technol. 2022, 75, 103663.
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective Skin Cancer Treatment by Topical Co-delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes. AAPS PharmSciTech 2018, 19, 166–175.
- Haider, T.; Tiwari, R.; Vyas, S.P.; Soni, V. Molecular determinants as therapeutic targets in cancer chemotherapy: An update. Pharmacol. Ther. 2019, 200, 85–109.
- Haider, T.; Sandha, K.K.; Soni, V.; Gupta, P.N. Recent advances in tumor microenvironment associated therapeutic strategies and evaluation models. Mater. Sci. Eng. C 2020, 116, 111229.
- Lee, C.; Na, K. Anthocyanin-Loaded Liposomes Prepared by the pH-Gradient Loading Method to Enhance the Anthocyanin Stability, Antioxidation Effect and Skin Permeability. Macromol. Res. 2020, 28, 289–297.
- Castañeda-Reyes, E.D.; Perea-Flores, M.D.J.; Davila-Ortiz, G.; Lee, Y.; de Mejia, E.G. Development, Characterization and Use of Liposomes as Amphipathic Transporters of Bioactive Compounds for Melanoma Treatment and Reduction of Skin Inflammation: A Review. Int. J. Nanomed. 2020, 15, 7627–7650.
- Marwah, M.; Perrie, Y.; Badhan, R.K.S.; Lowry, D. Intracellular uptake of EGCG-loaded deformable controlled release liposomes for skin cancer. J. Liposome Res. 2020, 30, 136–149.
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (-)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: A comprehensive experimental study with preclinical investigation. Eur. J. Pharm. Sci. 2019, 137, 104972.
- Sharma, R.; Mody, N.; Kushwah, V.; Jain, S.; Vyas, S.P. C-Type lectin receptor(s)-targeted nanoliposomes: An intelligent approach for effective cancer immunotherapy. Nanomedicine 2017, 12, 1945–1959.
- Haider, M.; Kanoujia, J.; Tripathi, C.B.; Arya, M.; Kaithwas, G.; Saraf, S.A. Pioglitazone loaded vesicular carriers for anti-diabetic activity: Development and optimization as per central composite design. J. Pharm. Sci. Pharmacol. 2015, 2, 11–20.
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971.
- Sarker, A.; Shimu, I.J.; Alam, S.A.A. Niosome: As dermal drug delivery tool. IOSR J. Pharm. Biol. Sci. 2015, 10, 73–79.
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581.
- Hamishehkar, H.; Rahimpour, Y.; Kouhsoltani, M. Niosomes as a propitious carrier for topical drug delivery. Expert Opin. Drug Deliv. 2013, 10, 261–272.
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39.
- Jothy, M.A.; Shanmuganathan, S. An overview on niosome as carrier in dermal drug delivery. J. Pharm. Sci. Res. 2015, 7, 923.
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36.
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380.
- Xu, Y.-Q.; Chen, W.-R.; Tsosie, J.K.; Xie, X.; Li, P.; Wan, J.-B.; He, C.-W.; Chen, M.-W. Niosome Encapsulation of Curcumin: Characterization and Cytotoxic Effect on Ovarian Cancer Cells. J. Nanomater. 2016, 2016, 6365295.
- Yeo, P.L.; Lim, C.L.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Niosomes: A review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2017, 11, 301–314.
- Shinu, P.; Nair, A.B.; Kumari, B.; Jacob, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Venugopala, K.N.; Attimarad, M.; Nagaraja, S. Recent Advances and Appropriate use of Niosomes for the Treatment of Skin Cancer. Indian J. Pharm. Educ. Res. 2022, 56, 924–937.
- Sharma, P.K.S.P.; Saxena, P.; Jaswanth, A.; Chalamaiah, M.; Tekade, K.R.; Balasubramaniam, A. Novel Encapsulation of Lycopene in Niosomes and Assessment of its Anticancer Activity. J. Bioequiv. Bioavailab. 2016, 8, 224–232.
- Chermahini, S.H.; Najafi, R.B. Niosome encapsulated fluorouracil as drug delivery system to basal-cell skin Cancer. J. Nanosci. Nanomed 2019, 3, 1–4.
- Paolino, D.; Cosco, D.; Muzzalupo, R.; Trapasso, E.; Picci, N.; Fresta, M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int. J. Pharm. 2008, 353, 233–242.
- Pawar, S.; Shevalkar, G.; Vavia, P. Glucosamine-anchored doxorubicin-loaded targeted nano-niosomes: Pharmacokinetic, toxicity and pharmacodynamic evaluation. J. Drug Target. 2016, 24, 730–743.
- Shah, H.S.; Gotecha, A.; Jetha, D.; Rajput, A.; Bariya, A.; Panchal, S.; Butani, S. Gamma oryzanol niosomal gel for skin cancer: Formulation and optimization using quality by design (QbD) approach. AAPS Open 2021, 7, 9.
- Chauhan, P.; Tyagi, B.K. Herbal novel drug delivery systems and transfersomes. J. Drug Deliv. Ther. 2018, 8, 162–168.
- Benson, H.A.E. Transfersomes for transdermal drug delivery. Expert Opin. Drug Deliv. 2006, 3, 727–737.
- Kumar, N.; Dubey, A.; Mishra, A.; Tiwari, P. Ethosomes: A Novel Approach in Transdermal Drug Delivery System. Int. J. Pharm. Life Sci. 2020, 11, 6598–6608.
- Manosroi, A.; Jantrawut, P.; Khositsuntiwong, N.; Manosroi, W.; Manosroi, J. Novel Elastic Nanovesicles for Cosmeceutical and Pharmaceutical Applications. Chiang Mai J. Sci. 2009, 36, 166–178.
- Jangdey, M.S.; Gupta, A.; Saraf, S.; Saraf, S. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: In vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1452–1462.
- Raahulan, S.; Sanapalli, B.K.R.; Karri, V.V.S.R. Veera Venkata Satyanarayana Reddy, Paclitaxel loaded transfersomal vesicular drug delivery for the treatment of melanoma skin cancers. Int. J. Res. Pharm. Sci. 2019, 10, 2891–2897.
- Jangdey, M.S.; Kaur, C.D.; Saraf, S. Efficacy of Concanavalin-A conjugated nanotransfersomal gel of apigenin for enhanced targeted delivery of UV induced skin malignant melanoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 904–916.
- Aute, P.P.; Kamble, M.S.; Chaudhari, P.D.; Bhosale, A.V. A comprehensive review on ethosomes. Int. J. Res. Dev. Pharm. Life Sci. 2012, 2, 218–224.
- Parashar, T.; Sachan, R.; Singh, V.; Singh, G.; Tyagi, S.; Patel, C.; Gupta, A. Ethosomes: A recent vesicle of transdermal drug delivery system. Int. J. Res. Dev. Pharm. Life Sci. 2013, 2, 285–292.
- Hariharanb, S.; Justinc, A. Topical delivery of drugs using ethosomes: A review. Indian Drugs 2019, 56, 7.
- Zhang, Z.; Wo, Y.; Zhang, Y.; Wang, D.; He, R.; Chen, H.; Cui, D. In vitro study of ethosome penetration in human skin and hypertrophic scar tissue. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1026–1033.
- Zhang, J.-P.; Wei, Y.-H.; Zhou, Y.; Li, Y.-Q.; Wu, X.-A. Ethosomes, binary ethosomes and transfersomes of terbinafine hydrochloride: A comparative study. Arch. Pharmacal Res. 2012, 35, 109–117.
- Ismail, T.A.; Shehata, T.M.; Mohamed, D.I.; Elsewedy, H.S.; Soliman, W.E. Quality by Design for Development, Optimization and Characterization of Brucine Ethosomal Gel for Skin Cancer Delivery. Molecules 2021, 26, 3454.
- Nainwal, N.; Jawla, S.; Singh, R.; Saharan, V.A. Transdermal applications of ethosomes—A detailed review. J. Liposome Res. 2019, 29, 103–113.
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as A Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2020, 12, 6.
- Peram, M.R.; Jalalpure, S.; Kumbar, V.; Patil, S.; Joshi, S.; Bhat, K.; Diwan, P. Factorial design based curcumin ethosomal nanocarriers for the skin cancer delivery: In Vitro evaluation. J. Liposome Res. 2019, 29, 291–311.
- Gamal, A.; Saeed, H.; El-Ela, F.I.A.; Salem, H.F. Improving the Antitumor Activity and Bioavailability of Sonidegib for the Treatment of Skin Cancer. Pharmaceutics 2021, 13, 1560.
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 755–765.
- Mousa, I.A.; Hammady, T.M.; Gad, S.; Zaitone, S.A.; El-Sherbiny, M.; Sayed, O.M. Formulation and Characterization of Metformin-Loaded Ethosomes for Topical Application to Experimentally Induced Skin Cancer in Mice. Pharmaceuticals 2022, 15, 657.
- Garg, V.; Singh, H.; Bhatia, A.; Raza, K.; Singh, S.K.; Singh, B.; Beg, S. Systematic Development of Transethosomal Gel System of Piroxicam: Formulation Optimization, In Vitro Evaluation, and Ex Vivo Assessment. AAPS PharmSciTech 2017, 18, 58–71.
- Abdulbaqi, I.M.; Darwis, Y.; Assi, R.A.; Khan, N.A.K. Transethosomal gels as carriers for the transdermal delivery of colchicine: Statistical optimization, characterization, and ex vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 795–813.
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019, 560, 78–91.
- Dua, J.S.; Rana, P.A.C.; Bhandari, D.A.K. Liposome: Methods of preparation and applications. Int. J. Pharm. Stud. Res. 2012, 3, 14–20.
- Šturm, L.; Ulrih, N.P. Basic Methods for Preparation of Liposomes and Studying Their Interactions with Different Compounds, with the Emphasis on Polyphenols. Int. J. Mol. Sci. 2021, 22, 6547.
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016, 33, 372–380.
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259.
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical nanostructured lipid carrier gel of quercetin and resveratrol: Formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int. J. Pharm. 2020, 587, 119705.
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879.
- Rohit, B.; Pal, K.I. A method to prepare solid lipid nanoparticles with improved entrapment efficiency of hydrophilic drugs. Curr. Nanosci. 2013, 9, 211–220.
- Sabir, F.; Qindeel, M.; Rehman, A.U.; Ahmad, N.M.; Khan, G.M.; Csoka, I.; Ahmed, N. An efficient approach for development and optimisation of curcumin-loaded solid lipid nanoparticles’ patch for transdermal delivery. J. Microencapsul. 2021, 38, 233–248.
- Banerjee, I.; De, M.; Dey, G.; Bharti, R.; Chattopadhyay, S.; Ali, N.; Chakrabarti, P.; Reis, R.L.; Kundu, S.C.; Mandal, M. A peptide-modified solid lipid nanoparticle formulation of paclitaxel modulates immunity and outperforms dacarbazine in a murine melanoma model. Biomater. Sci. 2019, 7, 1161–1178.
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530.
- Gu, Y.; Yang, M.; Tang, X.; Wang, T.; Yang, D.; Zhai, G.; Liu, J. Lipid nanoparticles loading triptolide for transdermal delivery: Mechanisms of penetration enhancement and transport properties. J. Nanobiotechnol. 2018, 16, 68.
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 1 (Suppl. S54), S131–S155.
- Torchilin, V.P. Micellar Nanocarriers: Pharmaceutical Perspectives. Pharm. Res. 2007, 24, 1–16.
- Agrawal, R.D.; Tatode, A.A.; Rarokar, N.R.; Umekar, M.J. Polymeric micelle as a nanocarrier for delivery of therapeutic agents: A comprehensive review. J. Drug Deliv. Ther. 2020, 10, 191–195.
- Moradi, A.; Farboud, E.S.; Nasrollahi, S.A.; Kashani, M.N.; Dinarvand, R. Tretinoin loaded nanostructured lipid carrier (NLC): Safe and effective drug delivery system. Nanosci. Nanotechnol.-Asia 2017, 7, 221–229.
- Iqbal, B.; Ali, J.; Ganguli, M.; Mishra, S.; Baboota, S. Silymarin-loaded nanostructured lipid carrier gel for the treatment of skin cancer. Nanomedicine 2019, 14, 1077–1093.
- Gundogdu, E.; Demir, E.-S.; Ekinci, M.; Ozgenc, E.; Ilem-Ozdemir, D.; Senyigit, Z.; Gonzalez-Alvarez, I.; Bermejo, M. An Innovative Formulation Based on Nanostructured Lipid Carriers for Imatinib Delivery: Pre-Formulation, Cellular Uptake and Cytotoxicity Studies. Nanomaterials 2022, 12, 250.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
14 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





