Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yauheni Shastak | -- | 1861 | 2023-08-13 11:17:06 | | | |
| 2 | Sirius Huang | Meta information modification | 1861 | 2023-08-14 04:48:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shastak, Y.; Obermueller-Jevic, U.; Pelletier, W. Early Discoveries and Understanding of Vitamin E. Encyclopedia. Available online: https://encyclopedia.pub/entry/47992 (accessed on 07 March 2026).
Shastak Y, Obermueller-Jevic U, Pelletier W. Early Discoveries and Understanding of Vitamin E. Encyclopedia. Available at: https://encyclopedia.pub/entry/47992. Accessed March 07, 2026.
Shastak, Yauheni, Ute Obermueller-Jevic, Wolf Pelletier. "Early Discoveries and Understanding of Vitamin E" Encyclopedia, https://encyclopedia.pub/entry/47992 (accessed March 07, 2026).
Shastak, Y., Obermueller-Jevic, U., & Pelletier, W. (2023, August 13). Early Discoveries and Understanding of Vitamin E. In Encyclopedia. https://encyclopedia.pub/entry/47992
Shastak, Yauheni, et al. "Early Discoveries and Understanding of Vitamin E." Encyclopedia. Web. 13 August, 2023.
Copy Citation
Vitamin E, consisting of four tocopherols and four tocotrienols, with α-tocopherol as the most biologically active form, has a significant history in scientific research. It was first identified in the 1920s for its role in preventing neonatal mortality in rats. Over time, its chemical structure was elucidated, and its importance in the immune system, skin health, anti-inflammatory properties, and hormonal balance was revealed.
vitamin E
history
animal nutrition
production
activity
1. Introduction
Vitamin E plays a critical role in animal nutrition by serving as a potent lipid-soluble antioxidant as well as contributing to anti-inflammation, immune function, and gene expression regulation. As an antioxidant, it protects cell membranes and other lipid-containing structures from oxidative damage caused by free radicals [1]. Thereby, vitamin E is the major chain-breaking antioxidant inhibiting lipid peroxidation, a physiological function that is not provided by other dietary or endogenous antioxidants [2]. This makes it crucial for preserving cell integrity, particularly in tissues that are susceptible to oxidative stress, such as the liver, lungs, and muscles [3].
The significance of vitamin E in animal nutrition cannot be overestimated, as it has been recognized as an indispensable micronutrient for optimal health, growth, and development in livestock [4]. Throughout the past century, numerous studies and advancements have been made in understanding the crucial role vitamin E plays in livestock production. A deficiency of vitamin E can impair immune responses and increase the susceptibility of animals to infectious diseases. Furthermore, hypovitaminosis E has been linked to reduced reproductive performance in animals, including decreased fertility rates and increased embryonic mortality [5].
For livestock, optimizing vitamin E status is particularly important for animal health and production. In dairy cattle, supplementation with vitamin E has been demonstrated to enhance milk yield and lower the occurrence of mastitis [6][7][8]. In poultry, it has been associated with better growth rates, egg production, and hatchability [9][10]. Likewise, in swine, vitamin E supplementation has been proven to enhance meat quality, reduce stress, and increase growth rates [11][12][13][14].
To ensure that animals receive adequate amounts of vitamin E, it is common practice to add this micronutrient as synthetic dl-α-tocopheryl acetate to animal feeds. However, determining the optimal level of vitamin E supplementation can be challenging, as the requirements for this nutrient can vary depending on the species, age, and health status of the animal, as well as other factors [15].
2. Early Discoveries and Understanding of Vitamin E
Last year marked the 100th anniversary of the discovery of vitamin E in 1922, which was made by Herbert McLean Evans, an embryologist and endocrinologist, and his co-worker Kathrine Julia Scott Bishop, a medical physician and trained anatomist, while working at Berkeley University in California/USA. The two scientists observed that female rats fed on a purified diet had good growth and development and stayed healthy, but could not reproduce, as the embryos died and were resorbed after some 10 days of gravidity. However, when the semi-synthetic diet was supplemented with fresh green leaves of lettuce or dried alfalfa meal, a sudden restoration of fertility in previously sterile rats could be observed [16]. At first, the researchers believed that vitamin C, which had already been discovered at that time and was known not to be essential for growth, was necessary for pregnancy. However, they quickly realized that only the fat-soluble components of the leaves had led to a good result. After testing the hydro- and lipophilic extracts of various wheat by-products from a nearby flour mill, the two scientists discovered a new fat-soluble dietary lipophilic compound that causes sterility in rats when lacking in the feed [16].
The unknown dietary substance was initially called factor X, but it was soon renamed vitamin E by Barnett Sure [17] and Herbert Evans [18]. Evans and Bishop later demonstrated that male rats with diets lacking the new fat-soluble vitamin E also experienced sterility [18], leading to the vitamin’s subsequent designation as the “anti-sterility vitamin”. In the same year, Evans and his co-worker George Burr prepared a potent concentrate of vitamin E by saponification of wheat germ oil, which proved to possess high biological potency [19]. Wheat germ oil-based concentrates were used in many further experiments on vitamin E and served as a source for the development of the first commercial vitamin E products in the 1930s.
In 1936, Evans and his co-workers isolated two compounds with vitamin E activity from wheat germ oil, for which they proposed the names α-tocopherol and ß-tocopherol [20]. Soon afterward, a third active factor, γ-tocopherol, was found in cottonseed oil by Evans’ working group [21], and in 1947, a fourth tocopherol, named δ-tocopherol, was isolated from soybean oil [22]. In 1936, Evans and his co-workers suggested the nomenclature α-tocopherol, the childbirth-bearing alcohol, for the new compound based on the Greek terms “tokos” for childbirth, “phero” for to bear, and “-ol” indicating an alcohol. This designation was proposed by George Miller Calhoun, a classical philologist and professor of Greek at the University of California [20].
Therefore, the discovery of vitamin E by Evans and Bishop in 1922 [16] resulted in the identification and isolation of several tocopherols. Their dedication and contributions to the study of vitamin E will continue to be celebrated and studied for years to come.
3. Vitamin E’s Chemical Structure and Biological Activity
The chemical structure of vitamin E was elucidated by the German chemist Erhard Fernholz in 1938 [23] while working in the USA. Fernholz proposed a structural formula that regarded α-tocopherol as a substituted 6-hydrocarbon with a long aliphatic sidechain attached to a pyran ring (Figure 1). Prior to this, in 1937, Fernholz [24] had studied the thermal decomposition of α-tocopherol and formed durohydro quinone and an aliphatic hydrocarbon. Shortly after Fernholz’s proposal, the Swiss chemist Paul Karrer achieved the chemical synthesis of α-tocopherol for the first time [25][26]. Karrer condensed trimethyl hydroquinone with phytol bromide derived from natural phytol, using zinc chloride as a catalyst. However, Karrer was not sure at that time regarding the chemical structure of the molecule he synthesized. He tended to assume a coumaran ring instead of the proposed chroman ring by Fernholz.
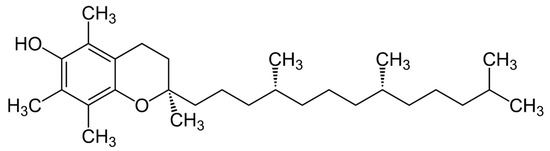
Figure 1. Structural formula for α-tocopherol.
The first semi-synthetic tocopherol synthesized by Karrer consisted of two different stereoisomers and was initially called dl-α-tocopherol or 2-ambo-α-tocopherol. Shortly after the first synthesis of α-tocopherol, Bergel and co-workers of Lister-Institute in London, UK, and Lee Irvin Smith and co-workers of the University of Minnesota, Minneapolis, MN, USA, accomplished the synthesis of α-tocopherol as well [27][28].
The biological activity of the synthesized compound in the common rat resorption-gestation test was confirmed by Otto Isler [29], who accomplished an analog synthesis of vitamin E with Paul Karrer simultaneously.
Finally, the chroman ring as a constituent of α-tocopherol was confirmed with the help of UV spectra and other comparative model tests by Walter John at Göttingen University in Germany [30][31]. Furthermore, Walter John validated the chemical structure of α-tocopherol proposed by Fernholz and isolated ß-tocopherol simultaneously. John showed that ß-tocopherol differs from α-tocopherol only by one methyl group less at the chroman ring. He published more than 24 papers and book chapters on vitamin E-related topics in his short scientific career between 1937 and 1942.
In conclusion, the discovery of vitamin E’s chemical structure by Fernholz and the synthesis of α-tocopherol by Karrer were significant milestones for this essential micronutrient. Walter John’s confirmation of the chroman ring in α-tocopherol and work on synthesizing vitamin E derivatives contributed to scientific understanding, though his work is largely unrecognized outside of German journals.
4. The Discovery of Vitamin E’s Unique Physiological Function as Chain-Breaking Antioxidant and the Antioxidant Network
In 1924, Henry Albright Mattill, a biochemist from the University of Iowa in Iowa City, IA, USA, conducted a study on the effects of milk consumption on reproduction. Along with his colleagues, he observed that rats became sterile when lard was added to their milk-based regimen. This led them to conclude that the fat content of a diet, in addition to vitamin E, affects reproduction. They proposed the hypothesis that the requirement for vitamin E increases with the amount of fat in the nutritional intake [32].
Three years later, in 1927, Mattill [33] reported another finding: The destruction of vitamin E in the presence of fat, particularly unsaturated fats. Building on this discovery, Mattill delved into further research on the autoxidation of fats. In collaboration with Marian Cummings, he put forward the idea that the oxidation of vitamin E could potentially safeguard other substances, such as vitamin A, from oxidation. They suggested that vitamin E possesses “antioxidant activity” and posited that its physiological role may lie in its ability to counteract oxidation [34]. It is worth noting that these early studies demonstrated the physiological consequences of the absence of antioxidant protection in lipids, namely, the sterility of rats.
A significant breakthrough in understanding the antioxidant role of vitamin E came from the research conducted by Aloys Tappel, a food biochemist at the University of California Davis/USA, during the 1950s. Alongside his colleagues, Tappel demonstrated that vitamin E effectively inhibits lipid peroxidation in living organisms. Through experiments conducted on isolated mitochondria and vitamin E-deficient animals, they observed elevated levels of lipid peroxidation in the liver, resulting in compromised mitochondrial stability [35][36].
In the early 1980s, Graham Burton and Kathrin Ingold, researchers from the National Research Council of Canada, conducted chemical investigations into the antioxidant properties of vitamin E and other phenolic compounds. They elucidated the chemical structure of α-tocopherol, which proved to be optimal for scavenging peroxyl radicals due to its hydroxylated chromanol ring with significant methylation. Furthermore, they noted that α-tocopherol possesses ideal characteristics for in vivo localization alongside lipids, thanks to its phytyl side chain [37]. Based on their findings, Burton and Ingold proposed that the primary, if not sole, function of α-tocopherol in living organisms is to act as an antioxidant. They even presented a reaction scheme for α-tocopherol in Figure 2 [38]. Subsequently, through studies involving individuals deficient in vitamin E, Burton and his colleagues demonstrated that α-tocopherol serves as the predominant chain-breaking antioxidant in vivo [2].
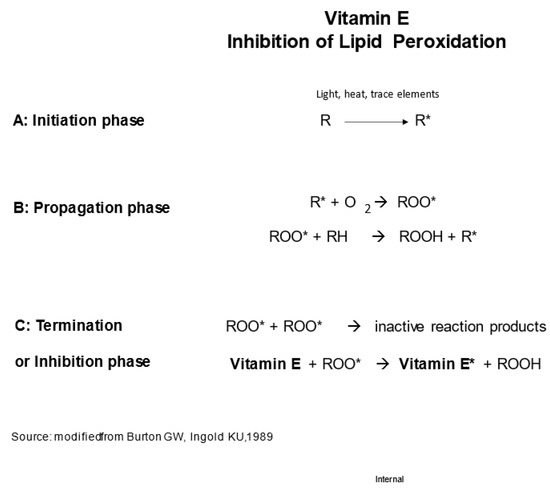
Figure 2. Inhibition of lipid peroxidation by vitamin E [38]. In the first step, the initiation phase, a fatty acid radical (RO*) is produced upon exposure of a fatty acid to light, heat, or trace elements. In the second step, the propagation phase, RO* reacts with oxygen to form a highly reactive peroxyl radical (ROO*), which oxidizes an adjacent fatty acid, leading to a chain reaction. Ultimately, the chain reaction comes to an end when ROO* radicals react with each other, the termination phase, or when a chain-breaking antioxidant such as vitamin E reacts with ROO*, the inhibition phase.
Finally, Lester Packer, a molecular and cell biologist from the University of California (Berkeley, CA, USA), made a significant observation regarding the combat against oxidative stress in cells. He recognized the importance of multiple antioxidants working together in what he referred to as “the antioxidant network” (Figure 3). Packer’s findings revealed that vitamin E and other antioxidants undergo oxidation but are subsequently recycled, forming a highly effective and precise defense system that adapts to oxidative stress [39][40][41].
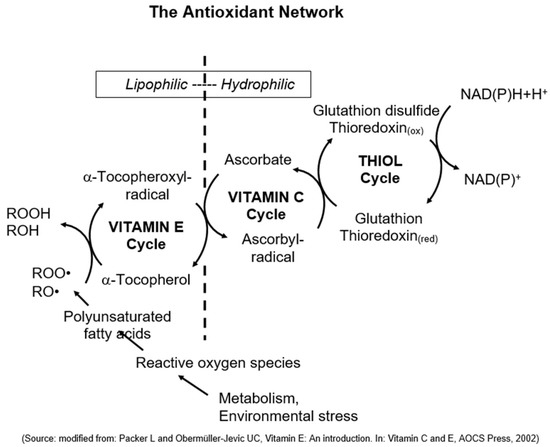
Figure 3. The antioxidant network (modified from Packer and Obermüller-Jevic, 2002 [41]). In cells, several antioxidants are present in both lipophilic and hydrophilic compartments. Lipid peroxides and other radicals are scavenged and reduced by vitamin E, leading to the formation of vitamin E radicals. In a subsequent chain reaction, vitamin E gets recycled by vitamin C and other antioxidants.
References
- Doğru Pekiner, B. Vitamin E as an antioxidant. J. Fac. Pharm. Ankara 2003, 32, 243–267.
- Burton, G.W.; Joyce, A.; Ingold, K.U. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet 1982, 2, 327.
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15.
- Idamokoro, E.M.; Falowo, A.B.; Oyeagu, C.E.; Afolayan, A.J. Multifunctional activity of vitamin E in animal and animal products: A review. Anim. Sci. J. 2020, 91, e13352.
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant Properties of Selenium and Vitamin E Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants 2021, 10, 1555.
- Hogan, J.S.; Weiss, W.P.; Smith, K.L. Role of Vitamin E and Selenium in Host Defense Against Mastitis. J. Dairy Sci. 1993, 76, 2795–2803.
- Cusack, P.; McMeniman, N.; Rabiee, A.; Lean, I. Assessment of the effects of supplementation with vitamin E on health and production of feedlot cattle using meta-analysis. Prev. Vet. Med. 2009, 88, 229–246.
- Chandra, G.; Aggarwal, A.; Singh, A.K.; Kumar, M.; Upadhyay, R.C. Effect of vitamin E and zinc supplementation on energy metabolites, lipid peroxidation, and milk production in peripartum sahiwal cows. Asian-Australas. J. Anim. Sci. 2013, 26, 1569–1576.
- Rengaraj, D.; Hong, Y.H. Effects of dietary vitamin E on fertility functions in poultry species. Int. J. Mol. Sci. 2015, 16, 9910–9921.
- Surai, P.F.; Fisinin, V.I.; Karadas, F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016, 2, 1–11.
- Cheah, K.S.; Cheah, A.M.; Krausgrill, D.I. Effect of dietary supplementation of vitamin E on pig meat quality. Meat Sci. 1995, 39, 255–264.
- Corino, C.; Oriani, G.; Pantaleo, L.; Pastorelli, G.; Salvatori, G. Influence of dietary vitamin E supplementation on “heavy” pig carcass characteristics, meat quality, and vitamin E status. J. Anim. Sci. 1999, 77, 1755–1761.
- Lu, T.; Harper, A.F.; Zhao, J.; Estienne, M.J.; Dalloul, R.A. Supplementing antioxidants to pigs fed diets high in oxidants: I. Effects on growth performance, liver function, and oxidative status. J. Anim. Sci. 2014, 92, 5455–5463.
- Wang, D.; Dal Jang, Y.; Rentfrow, G.K.; Azain, M.J.; Lindemann, M.D. Effects of dietary vitamin E and fat supplementation in growing-finishing swine fed to a heavy slaughter weight of 150 kg: I. Growth performance, lean growth, organ size, carcass characteristics, primal cuts, and pork quality. J. Anim. Sci. 2022, 100, skac081.
- McDowell, L.R. Vitamin nutrition of livestock animals: Overview from vitamin discovery to today. Can. J. Anim. Sci. 2006, 86, 171–179.
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–665.
- Sure, B. Dietary requirements for reproduction. II. The existence of a specific vitamin for reproduction. J. Biol. Chem. 1924, 58, 693–703.
- Evans, H.M. Invariable occurrence of male sterility with dietaries lacking fat soluble vitamine E. Proc. Natl. Acad. Sci. USA 1925, 11, 373–377.
- Evans, H.M.; Burr, G.O. The anti-sterility fat soluble vitamin E. Proc. Natl. Acad. Sci. USA 1925, 11, 334–341.
- Evans, H.M.; Emerson, O.H.; Emerson, G.A. The isolation from wheat germ oil of an alcohol, α-tocopherol, having the properties of vitamin E. J. Biol. Chem. 1936, 113, 319–332.
- Emerson, O.H.; Emersox, G.A.; Mohammad, A.; Evans, H.M. The chemistry of vitamin E-tocopherols from various sources. J. Biol. Chem. 1937, 122, 99–107.
- Stern, M.H.; Robeson, C.D.; Weisler, L.; Baxter, J.G. δ-Tocopherol. I. Isolation from Soybean Oil and Properties. J. Am. Chem. Soc. 1947, 69, 869–874.
- Fernholz, E. On the constitution of α-tocopherol. J. Am. Chem. Soc. 1938, 60, 700–705.
- Fernholz, E. The thermal decomposition of alpha-tocopherol. J. Am. Chem. Soc. 1937, 59, 1154–1155.
- Karrer, P.; Fritzsehe, H.; Ringier, B.H.; Salomon, H. α-Tocopherol. Helv. Chim. Acta 1938, 21, 520–525.
- Karrer, P. Vitamin E und verwandte Verbindungen. Helv. Chim. Acta 1939, 22, 334–350.
- Bergel, F.; Jacob, A.; Todd, A.R.; Work, T.S. Vitamin E Synthesis of α-Tocopherol. Nature 1938, 142, 36.
- Smith, L.I.; Ungnade, H.E.; Prichard, W.W. The Chemistry of Vitamin E. I. The Structure and Synthesis of α-Tocopherol. Science 1938, 88, 37–38.
- Isler, O. Die Stabilisierung von d,l-α-Tocopherol. Helv. Chim. Acta 1938, 21, 1756–1759.
- John, W. Über das Cumo-tokopherol, einen neuen Faktor der Vitamin E-Gruppe. Z. Physiol. Chem. 1937, 250, 11–24.
- John, W. Zum Beweis der Chromanstruktur des α-Tokopherols. Naturwissenschaften 1938, 5, 21–22.
- Mattill, H.A.; Carman, J.S.; Clayton, M.M. The nutritive properties of milk. III. The effectiveness of the X substance in preventing sterility in rats on milk rations high in fat. J. Biol. Chem. 1924, 61, 729–740.
- Mattill, H.A. The oxidative destruction of vitamins A and E and the protective action of certain vegetable oils. J. Am. Med. Assoc. 1927, 89, 1505–1508.
- Cummings, M.J.; Mattill, H.A. The auto-oxidation of fats with reference to their destructive effect on vitamin E. J. Nutr. 1931, 3, 421–432.
- Tappel, A.L.; Zalkin, H. Inhibition of lipide peroxidation in mitochondria by vitamin E. Arch. Biochem. Biophys. 1959, 80, 333–336.
- Tappel, A.L.; Zalkin, H. Inhibition of lipid peroxidation in microsomes by vitamin E. Nature 1960, 4705, 35.
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules. 1. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477.
- Burton, G.W.; Ingold, K.U. Vitamin E as an in vitro and in vivo antioxidant. Ann. N. Y. Acad. Sci. 1989, 570, 7–22.
- Packer, L.; Smith, J.R. Extension of the lifespan of cultured normal human diploid cells by vitamin E: A reevaluation. Proc. Natl. Acad. Sci. USA 1977, 74, 1640–1641.
- Packer, L.; Landvik, S. Vitamin E: Introduction to biochemistry and health benefits. Ann. N. Y. Acad. Sci. 1989, 570, 1–6.
- Packer, L.; Obermüller-Jevic, U.C. Vitamin E: An introduction. In The Antioxidant Vitamins C and E; Packer, L., Traber, M.G., Kramer, K., Eds.; AOCS Press: Champaign, IL, USA, 2002; pp. 133–151.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
14 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





