Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, J. Venom Components of Imported Fire Ant Venom. Encyclopedia. Available online: https://encyclopedia.pub/entry/47910 (accessed on 07 February 2026).
Chen J. Venom Components of Imported Fire Ant Venom. Encyclopedia. Available at: https://encyclopedia.pub/entry/47910. Accessed February 07, 2026.
Chen, Jian. "Venom Components of Imported Fire Ant Venom" Encyclopedia, https://encyclopedia.pub/entry/47910 (accessed February 07, 2026).
Chen, J. (2023, August 10). Venom Components of Imported Fire Ant Venom. In Encyclopedia. https://encyclopedia.pub/entry/47910
Chen, Jian. "Venom Components of Imported Fire Ant Venom." Encyclopedia. Web. 10 August, 2023.
Copy Citation
In the United States, imported fire ants are often referred to as red imported fire ants, Solenopsis invicta Buren, black imported fire ants, S. richteri Forel, and their hybrid (S. invicta × S. richteri). Due to their aggressive stings and toxic venom, imported fire ants pose a significant threat to public health, agriculture, and ecosystem health.
venom alkaloids
venom proteins
predatory toxins
venom peptides
1. Venom Alkaloids
Extensive research has been conducted on the venom alkaloids of fire ants. Prior to the classification of red and black imported fire ants as distinct species, the first piperidine alkaloid, trans-2-methyl-6-n-undecylpiperidine, was identified in Solenopsis saevissima [1]. Subsequently, a series of 2-methyl-6-alkyl or alkenyl piperidine alkaloids were discovered in both S. invicta and S. richteri [2][3][4] (Figure 1). The alkyl or alkenyl side chains on position six of the piperidine ring can consist of nine, 11, 13, 15, or 17 carbons. To simplify the nomenclature, the carbon numbers are typically used to denote these alkaloids. For instance, C13 represents an alkaloid with a saturated 13-carbon side chain, while C13:1 represents an alkaloid with an unsaturated 13-carbon side chain containing one double bond. The absolute configuration of all the piperidine alkaloids in fire ants is consistently 2R,6R for trans-isomers and 2R,6S for cis-isomers [2]. These piperidine alkaloids were commonly named solenopsins, which were further categorized as solenopsin A, B, C, and D based on the length of the alkyl side chain on position six of the piperidine ring (A: C11, B: C13, C: C15, and D: C17).

Figure 1. The general structures of fire ant venom alkaloids. R: the alkyl or alkenyl side chain on position six of the piperidine, piperidene, or pyridine ring, which can consist of 9, 11, 13, 15, or 17 carbons.
Each species of fire ant has its own distinct piperidine alkaloid profile. In S. invicta workers, the dominant piperidine alkaloids are C13, C13:1, C15, and C15:1, while in S. richteri workers, the dominant alkaloids are C11, C11:1, C13, and C13:1. Interestingly, the piperidine alkaloid profile in the venom of hybrid imported fire ant workers appears to be a mixture resembling that of their parent species [5][6][7]. The double bonds on the side chains of the alkaloids are predominantly in the cis-configuration, but trans-isomers of 2-methyl-6-tridecenylpiperidine (C13:1) and 2-methyl-6-pentadecenylpiperidine (C15:1) have also been identified in the venom of S. invicta workers [8]. Both cis- and trans-isomers of piperidines are present, but trans-isomers are typically more abundant in workers. The major alkaloids found in the venom of female alates are cis- and trans-2-methyl-undecylpiperidine (cis- and trans-C11). The production of venom alkaloids in fire ants is influenced by age, body size, and season [9][10]. Workers of intermediate age produce more venom compared to old and young workers, and the ratio of saturated and unsaturated C13 and C15 alkaloids differs between minor and major workers [9]. In reproductive individuals, older alates have higher proportions of both cis- and trans-C13 piperidines than younger alates. After the mating flight, the newly mated queen exhibits a similar alkaloid profile to female alates, but the relative abundance of trans-2-methyl-undecylpiperidine (trans-C11) decreases as the colony develops (Figure 2). The venom alkaloid profile in S. invicta workers is also influenced by social form. Monogyne workers have higher C13:C13:1 and C15:C15:1 ratios compared to polygyne workers. However, polygyne workers have higher levels of unsaturated alkaloids regardless of the growth temperature, sampling seasons, or geographic location [11].
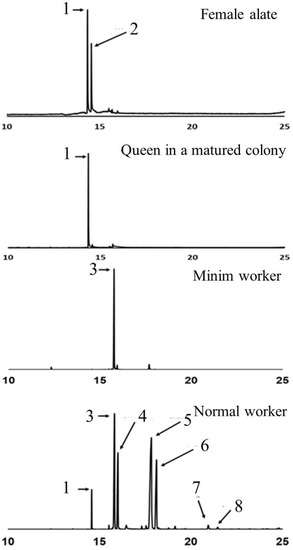
Figure 2. Chromatograms showing the major piperidine alkaloids in the venom of Solenopsis invicta female alates, the queen in a matured colony, the minim workers in the incipient colony, and normal workers in a matured colony, including 1: cis-C11, 2: trans-C11, 3: trans-C13:1, 4: trans-C13, 5: trans-C15:1, 6: trans-C15, 7: trans-C17:1, 8: trans-C17.
Almost 40 years after the identification of the first piperidine alkaloids, a series of related alkaloids called 2-methyl-6-alkyl (or alkenyl) piperidenes were characterized in the venom of both ant species and their hybrid [8][12][13][14] (Figure 1). These alkaloids include both Δ1,6 and Δ1,2 isomers of 2-methyl-6-alkyl or alkenyl piperidenes. Similar to piperidine alkaloids, the alkyl or alkenyl side chains on position six of the piperidene ring can have carbon chain lengths of 9, 11, 13, 15, and 17. Piperidene alkaloids are found in workers, female alates, and queens, but only 2-methyl-6-undecylpiperidene (both Δ1,2- and Δ1,6-C11) occurs in female alates and queens [15]. These alkaloids were identified much later than piperidine alkaloids likely due to their structural similarity, often co-eluting with piperidines on GC columns, and their lower abundance.
The nomenclature of piperidene alkaloids can be confusing. For instance, some compounds have been named piperideines, while others are called piperidienes. Different methods have been employed to specify the location of the double bond on the piperidine ring. For example, a compound such as 2-methyl-6-alkyl-6-piperidene may also be referred to as 2-methyl-6-alkyl-Δ1,6-piperidene, while 2-methyl-6-alkyl-1-piperidene could also be known as 2-methyl-6-alkyl-Δ1,2-piperidene. It is crucial to be aware of these different naming conventions when discussing and researching fire ant venom alkaloids and their related compounds. Clarity and consistency in the nomenclature will aid in the accurate understanding and communication of scientific information in this field.
The discovery of new alkaloid components in fire ant venom continues to this day. In 2019, pyridine alkaloids were detected in the venom of imported fire ants, including S. invicta, S. richteri, and the hybrid [16] (Figure 1). This discovery was made possible through the use of a unique technique called solid-phase microextraction (SPME) coupled with gas chromatography–mass spectrometry (GC–MS) and a modified thermal desorption process. The SPME fiber was loaded with venom secretion, and a series of consecutive GC–MS injections were performed, each with a partial desorption. This approach allowed for the identification of hidden pyridine alkaloid peaks that were previously masked by overlapping piperidine or piperidene alkaloid peaks. As a result, ten 2-methyl-6-alkyl (or alkenyl) pyridines were discovered for the first time in the venom of imported fire ants.
The role of minim workers in the establishment and development of a fire ant colony is important. However, the chemistry of their venom has received limited attention. Previous studies have reported that the major component in the venom of minim workers in S. invicta is the C13:1 piperidine alkaloid (Figure 2), and the presence of a piperidene alkaloid has been proposed [17]. However, there is currently no available information regarding the venom chemistry of minim workers in S. richteri and the hybrid imported fire ants. Furthermore, there is a lack of knowledge regarding the venom proteins in the venom of minim workers for both species and their hybrid. Further research is needed to explore the venom chemistry of minim workers in different fire ant species and gain a better understanding of their venom composition and potential functions.
Indeed, the biosynthesis of fire ant venom alkaloids remains a relatively unexplored area of research. While extensive studies have been conducted on the identification and characterization of venom alkaloids in fire ants, there is limited knowledge about the biosynthetic pathways responsible for their production. Only one publication focused on the biosynthesis of solenopsins in S. geminata has been reported thus far [18]. It was hypothesized that both cis- and trans-solenopsins are acetate derived, similar to other alkaloids found in insects, such as tetraponerine-8 and coccinelline. Solenopsins are biosynthesized first by the formation of long chain acid from the linear combination of acetate units, then followed by the loss of the carboxyl group, the introduction of an amino group, intramolecular cyclization, and a reduction in the imino group. This pathway was believed to be similar to the biosynthesis of the hemlock alkaloid coniine. Similar to how γ-coniceine serves as a precursor to coniine in hemlock, both Δ1,2 and Δ1,6 piperidenes are believed to serve as precursors to piperidine alkaloids in fire ants. The identification of 2-methyl-6-alkyl (or alkenyl) pyridine alkaloids in fire ants may add further complexity to the possible biosynthesis pathway of fire ant piperidine alkaloids, since the reduction from pyridine alkaloids can be another possible route for the biosynthesis of piperidine alkaloids.
Investigating the biosynthesis of venom alkaloids in fire ants could provide valuable insights into how these compounds are synthesized and regulated, shedding light on the mechanisms behind caste- and age-dependent profiles and social form-dependent variations in alkaloid composition. Understanding the biosynthetic pathways may also uncover new targets for intervention, offering potential new strategies for specific control measures in fire ants. Additionally, the genes involved in venom alkaloid biosynthesis could be heterologously expressed in microorganisms, enabling the production of alkaloids with a high yield and purity, which could facilitate further biological studies and introduce possibilities for their potential application in various domains, including the development of pesticides and antibiotics [19].
2. Venom Proteins
Systemic allergic reactions to fire ant stings are observed in approximately 2% of victims [20]. Extensive research has been dedicated to identifying the allergenic components present in fire ant venom. Using techniques such as gel filtration and high-performance cation exchange chromatography, four worker allergens have been isolated and characterized: Sol i 1, Sol i 2, Sol i 3, and Sol i 4 [21]. The properties and details of these allergens have been extensively investigated and reviewed [22][23][24][25][26][27][28][29][30]. Among the worker allergens, Sol i 2 and Sol i 3 are the major proteins found in S. invicta venom, while Sol i 1 and Sol i 4 are present in smaller amounts [21]. Sol i 2 and Sol i 4 are considered to be among the most potent allergens [23]. It is worth noting that workers and queens exhibit different sequence isoforms for these venom proteins. For instance, the major protein in fire ant worker venom is referred to as Sol i 2w, while in fire ant queen venom, it is denoted as Sol i 2q. These isoforms show a sequence identity of approximately 75.6% [21]. Regarding Sol i 4, several minor isoforms have been identified, including Sol i 4, Sol i 4.01, Sol i 4.02, and Sol i 4 q (Sol i 4 from the queen) [24][31]. These isoforms of Sol i 4 have been the subject of rigorous research and investigation. Two additional isoforms of Sol i 2, namely Sol i 2X1 and Sol i 2X2, are listed in the National Center for Biotechnology Information (NCBI) sequence database. These isoforms were derived from the genome sequence of S. invicta and can be identified by their respective accession numbers, XP_011156049 and XP_011156057 [32]. The study of fire ant venom allergens is crucial for understanding the mechanisms underlying allergic reactions and developing diagnostic tools and potential therapies for individuals who are hypersensitive to fire ant stings.
Since milked venom or whole abdomens were used for studying the venom proteins [21][24][31][33], the glandular origin of these proteins has been questioned since fire ants release both the content of the poison sac and the Dufour’s gland through the sting apparatus. The poison gland origin of these proteins was recently confirmed using imaging mass spectrometry [32].
The understanding of fire ant venom protein components has significantly improved through the extensive proteomic characterization conducted by dos Santos Pinto et al. [33] and Cai et al. [34]. In the dos Santos et al. study, 46 proteins were identified in the venom of S. invicta. These proteins included allergenic proteins, phospholipase A2, a growth factor, myotoxins, the phospholipase A2 inhibitor, thioredoxin peroxidase, neurotoxins, and the anemone cytolytic toxin. The most abundant proteins included a pseudechetoxin (PsTx)-like protein, a Scolopendra toxin-like protein, three different forms of venom Sol i 3, and venom Sol i 1. These 46 proteins were categorized into four groups: true venom components, housekeeping proteins, body muscle proteins, and proteins involved in chemical communication. The active but non-toxic venom components were further classified into three subgroups based on their potential functions: self-venom protection, colony asepsis, and chemical communication. The true toxins were classified into five other subgroups, including proteins influencing victim homeostasis, neurotoxins, proteins promoting venom diffusion, proteins causing tissue damage/inflammation, and allergens. In a recent study comparing the uniprot toxin database, Cai et al. [34] screened a total of 316 toxin-related unigenes and 47 proteins from a total of 33231 unigenes and 721 proteins and predicted the structure of calglandulin, venom Sol i 3, and the venom prothrombin activator hopsarin-D. They also found that S. invicta toxins contained phospholipase A2, one of the most prevalent proteins in bee toxins [35], which may have contributed to the cross-reactivity shown in the Sol i 1 protein of S. invicta and bees. They found calglandulin for the first time in S. invicta venom. This protein was associated with the secretion of toxins from the gland into the venom [36], indicating that calglandulin may play a role in the production of venom in S. invicta. In addition, a total of seven putative sequences in the transcriptome and three putative sequences in the proteome were identified as serine proteinase-like BMK-CBP in S. invicta venom, which has only been reported in Chinese red scorpion (Buthus martensii Karsch) venom [37]. They also investigated the structure of the venom prothrombin activator hopsarin-D, which served a similar function as mammalian coagulation Fxa [38].
The venom protein components in S. richteri can differ from those in S. invicta. While three homologous proteins were identified in S. richteri venom as Sol r 1, Sol r 2, and Sol r 3 [30], there is no equivalent of Sol i 4 in S. richteri venom. The hybrid imported fire ants possess unique venom proteins not found in their parent species. For instance, lateral flow immunoassays on the venom proteins revealed that hybrid imported ants from Tennessee contained Sol i 2, Sol r 2, as well as the proteins Solh2, Solh2Tr97, and Solr2A69 [39]. Solh2 and Solh2Tr97 were believed to be unique to the hybrid imported ants. Additionally, the venom proteins can have different sequence isoforms in different castes [21][24][31]. For example, Sol i 2 in worker venom and queen venom share only a 75.6% sequence identity, indicating potential caste-dependent venom functions in fire ants. Therefore, an extensive caste-differentiated proteomic characterization of venom in both species and their hybrid is necessary to fully comprehend the protein components in imported fire ant venoms.
3. Venom Peptides
With the significant advances in genomic, proteomics, and mass spectrometry techniques, numerous ant venom peptide toxins have been characterized [40][41][42][43][44][45]. However, the peptide components in fire ant venom have received minimum attention, which is likely due to the difficulty in obtaining a sufficient amount of fire ant venom that is free of alkaloids [33]. The existence of peptides in fire ant venom was clearly demonstrated in the first attempt of the proteomic characterization of fire ant venom. The MALDI-TOF MS spectrum of the whole fire ant venom showed a series of small proteins, or large peptides, which occurred at molecular weights smaller than 10 kDa [33]. More importantly, the presence of the atrial natriuretic peptide (ANP) was demonstrated in S. invicta venom, the first report of the ANP in Hymenoptera venom [33]. The ANP is a cardiac hormone that regulates the salt–water balance and blood pressure by stimulating renal salt, water excretion, and vasodilation [46].
References
- MacConnell, J.G.; Blum, M.S.; Fales, H.M. Alkaloid from fire ant venom: Identification and synthesis. Science 1970, 168, 840–841.
- Jones, T.H.; Blum, M.S.; Fales, H.M. Ant venom alkaloids from Solenopsis and Monomorium species. Recent developments. Tetrahedron 1982, 38, 1949–1958.
- Brand, J.M.; Blum, M.S.; Fales, H.M.; MacConnell, J.G. Fire ant venoms: Comparative analyses of alkaloidal components. Toxicon 1972, 10, 259–271.
- MacConnell, J.G.; Williams, R.N.; Brand, J.M.; Blum, M.S. New alkaloids in the venoms of fire ants. Ann. Entomol. Soc. Am. 1974, 67, 134–135.
- Vander Meer, R.K.; Lofgren, C.S.; Alvarez, F.M. Biochemical evidence for hybridization in fire ants. Florida Entomol. 1985, 68, 501–506.
- Ross, K.G.; Vander Meer, R.K.; Fletcher, D.J.C.; Vargo, E.L. Biochemical phenotypic and genetic studies of two introduced fire ants and their hybrid (Hymenoptera: Formicidae). Evolution 1987, 41, 280–293.
- Chen, L.; Hu, Q.B.; Fadamiro, H.Y. Reduction of venom alkaloids in Solenopsis richteri × Solenopsis invicta hybrid: An attempt to identify new alkaloidal components. J. Agric. Food Chem. 2010, 58, 11534–11542.
- Chen, L.; Fadamiro, H.Y. Re-investigation of venom chemistry of Solenopsis fire ants. II. Identification of novel alkaloids in S. invicta. Toxicon 2009, 53, 479–486.
- Deslippe, R.J.; Guo, Y.J. Venom alkaloids of fire ants in relation to worker size and age. Toxicon 2000, 38, 223–232.
- Haight, K.L.; Tschinkel, W.R. Patterns of venom synthesis and use in the fire ant, Solenopsis invicta. Toxicon 2003, 42, 673–682.
- Lai, L.C.; Huang, R.N.; Wu, W.J. Venom alkaloids of monogyne and polygyne forms of the red imported fire ant, Solenopsis invicta, in Taiwan. Insectes Soc. 2008, 55, 443–449.
- Chen, J.; Cantrell, C.L.; Shang, H.W.; Rojas, M.G. Piperideine alkaloids from the poison gland of the red imported fire ant (Hymenoptera: Formicidae). J. Agric. Food Chem. 2009, 57, 3128–3133.
- Chen, L.; Fadamiro, H.Y. Re-investigation of venom chemistry of Solenopsis fire ants. I. Identification of novel alkaloids in S. richteri. Toxicon 2009, 53, 469–478.
- Chen, J.; Shang, H.; Jin, X. Interspecific variation of delta1,6-piperideines in imported fire ants. Toxicon 2010, 55, 1181–1187.
- Chen, L.; Lu, Y.Y.; Hu, Q.B.; Fadamiro, H.Y. Similarity in venom alkaloid chemistry of alate queens of imported fire ants: Implication for hybridization between Solenopsis richteri and S. invicta in the Southern United States. Chem. Biodivers. 2012, 9, 702–713.
- Chen, J.; Zhao, Y.; Li, X.-C.; Zhao, J.-H. Pyridine alkaloids in the venom of imported fire ants. J. Agric. Food Chem. 2019, 67, 11388–11395.
- Bosworth, J.; Vander Meer, R.K. Colony founding minims: A new Solenopsis invicta caste. In Proceedings of the 1984 Imported Fire Ant Conference, Gainesville, FL, USA, 27–28 March 1984; pp. 92–105.
- Leclercq, S.; Braekman, J.C.; Daloze, D.; Pasteels, J.M.; Van der Meer, R.K. Biosynthesis of the solenopsins, venom alkaloids of the fire ants. Naturwissenschaften 1996, 83, 222–225.
- Fox, E.G.P. Venom Toxins of Fire Ants. In Venom Genomics and Proteomics; Gopalakrishnakone, P., Calvete, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 149–167.
- Stafford, C.T.; Hutto, L.S.; Rhoades, R.B.; Thompson, W.O.; Impson, L.K. Imported fire ant as a health hazard. South. Med. J. 1989, 82, 1515–1519.
- Hoffman, D.R.; Dove, D.E.; Jacobson, R.S. Allergens in Hymenoptera venom XX. Isolation of four allergens from imported fire ant (Solenopsis invicta) venom. J. Allergy Clin. Immunol. 1988, 82 Pt 1, 818–827.
- Chen, J.; Shang, H. Advances in research on the venom chemistry of imported fire ants. In Recent Advances in Entomological Research: From Molecular Biology to Pest Management; Liu, T., Kang, L., Eds.; Higher Education Press: Berlin, Germany; Springer: Berlin/Heidelberg, Germany, 2011; pp. 417–433.
- Hoffman, D.R. Allergens in Hymenoptera venom XXV. The amino acid sequences of antigen 5 molecules and the structural basis of antigenic cross-reactivity. J. Allergy Clin. Immunol. 1993, 92, 707–716.
- Hoffman, D.R. Allergens in Hymenoptera venom XXIV: The amino acid sequences of imported fire ant venom allergens Sol i II, Sol i III, and Sol i IV. J. Allergy Clin. Immunol. 1993, 91, 71–78.
- Hoffman, D.R.; Sakell, R.H.; Schmidt, M. Sol i 1, the phospholipase allergen of imported fire ant venom. J. Allergy Clin. Immunol. 2005, 115, 611–616.
- Hoffman, D.R. Ant venoms. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 342–346.
- Hoffman, D.R. Fire ant venom allergy. Allergy 1995, 50, 535–544.
- Schmidt, M.; McConnell, T.J.; Hoffman, D.R. Production of a recombinant imported fire ant venom allergen, Sol i 2, in native and immunoreactive form. J. Allergy Clin. Immunol. 1996, 98, 82–88.
- Padavattan, S.; Schmidt, M.; Hoffman, D.R.; Marković-Housley, Z. Crystal structure of the major allergen from fire ant venom, Sol i 3. J. Mol. Biol. 2008, 383, 178–185.
- Hoffman, D.R.; Smith, A.M.; Schmidt, M.; Moffitt, J.E.; Guralnick, M. Allergens in Hymenoptera venom. XXII. Comparison of venoms from two species of imported fire ants, Solenopsis invicta and richteri. J. Allergy Clin. Immunol. 1990, 85, 988–996.
- Lockwood, S.A.; HaghiPour-Peasley, J.; Hoffman, D.R.; Deslippe, R.J. Identification, expression, and immuno-reactivity of Sol i 2 & Sol i 4 venom proteins of queen red imported fire ants, Solenopsis invicta Buren (Hymenoptera: Formicidae). Toxicon 2012, 60, 752–759.
- Das, T.; Alabi, I.; Colley, M.; Yan, F.; Griffith, W.; Bach, S.; Weintraub, S.T.; Renthal, R. Major venom proteins of the fire ant Solenopsis invicta: Insights into possible pheromone-binding function from mass spectrometric analysis. Insect Mol. Biol. 2018, 27, 505–511.
- Dos Santos Pinto, J.R.; Fox, E.G.P.; Saidemberg, D.M.; Santos, L.D.; da Silva Menegasso, A.R.; Costa-Manso, E.; Machado, E.A.; Bueno, O.C.; Palma, M.S. Proteomic view of the venom from the fire ant Solenopsis invicta buren. J. Proteome Res. 2012, 11, 4643–4653.
- Cai, L.; Yang, F.; Wang, Y.; Yang, J.; Zhu, Y.; Ma, X.; Höfer, J.; Wang, Y.; Ma, Y.; Xiao, L. A combined protein toxin screening based on the transcriptome and proteome of Solenopsis invicta. Proteome Sci. 2022, 20, 15.
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee venom: An updating review of its bioactive molecules and its health applications. Nutrients 2020, 12, 3360.
- St Pierre, L.; Woods, R.; Earl, S.; Masci, P.P.; Lavin, M.F. Identification and analysis of venom gland-specific genes from the coastal taipan (Oxyuranus scutellatus) and related species. Cell. Mol. Life Sci. 2005, 62, 2679–2693.
- Gao, R.; Zhang, Y.; Gopalakrishnakone, P. Purification and N-terminal sequence of a serine proteinase-like protein (BMK-CBP) from the venom of the Chinese scorpion (Buthus martensii Karsch). Toxicon 2008, 52, 348–353.
- Rao, V.S.; Joseph, J.S.; Kini, R.M. Group D prothrombin activators from snake venom are structural homologues of mammalian blood coagulation factor Xa. Biochem. J. 2003, 369, 635–642.
- Valles, S.M.; Oliver, J.B.; Addesso, K.M.; Perera, O.P. Unique venom proteins from Solenopsis invicta × Solenopsis richteri hybrid fire ants. Toxicon X 2021, 9–10, 100065.
- Eagles, D.A.; Saez, N.J.; Krishnarjuna, B.; Bradford, J.J.; Chin, Y.K.; Starobova, H.; Mueller, A.; Reichelt, M.E.; Undheim, E.A.B.; Norton, R.S.; et al. A peptide toxin in ant venom mimics vertebrate EGF-like hormones to cause long-lasting hypersensitivity in mammals. Proc. Natl. Acad. Sci. USA 2022, 119, e2112630119.
- Wanandy, T.; Gueven, N.; Davies, N.W.; Brown, S.G.; Wiese, M.D. Pilosulins: A review of the structure and mode of action of venom peptides from an Australian ant Myrmecia pilosula. Toxicon 2015, 98, 54–61.
- Rádis-Baptista, G.; Dodou, H.V.; Prieto-da-Silva, Á.R.; Zaharenko, A.J.; Kazuma, K.; Nihei, K.I.; Inagaki, H.; Mori-Yasumoto, K.; Konno, K. Comprehensive analysis of peptides and low molecular weight components of the giant ant Dinoponera quadriceps venom. Biol. Chem. 2020, 401, 945–954.
- Pluzhnikov, K.A.; Kozlov, S.A.; Vassilevski, A.A.; Vorontsova, O.V.; Feofanov, A.V.; Grishin, E.V. Linear antimicrobial peptides from Ectatomma quadridens ant venom. Biochimie 2014, 107 Pt B, 211–215.
- Rifflet, A.; Gavalda, S.; Téné, N.; Orivel, J.; Leprince, J.; Guilhaudis, L.; Génin, E.; Vétillard, A.; Treilhou, M. Identification and characterization of a novel antimicrobial peptide from the venom of the ant Tetramorium bicarinatum. Peptides 2012, 38, 363–370.
- Kazuma, K.; Masuko, K.; Konno, K.; Inagaki, H. Combined venom gland transcriptomic and venom peptidomic analysis of the predatory ant Odontomachus monticola. Toxins 2017, 9, 323.
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019, 111, 18–25.
More
Information
Subjects:
Entomology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
11 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




