
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlos Daniel Varela-Chinchilla | -- | 2412 | 2023-08-09 20:52:40 | | | |
| 2 | Peter Tang | Meta information modification | 2412 | 2023-08-10 03:07:48 | | | | |
| 3 | Peter Tang | -1 word(s) | 2411 | 2023-08-10 03:08:27 | | |
Video Upload Options
Cancer is considered one of the most threatening diseases worldwide. Diet could be one of the factors that can be enhanced to comprehensively address a cancer patient’s condition. Unfortunately, most molecules capable of targeting cancer cells are found in uncommon food sources. Among them, depsipeptides have emerged as one of the most reliable choices for cancer treatment. These cyclic amino acid oligomers, with one or more subunits replaced by a hydroxylated carboxylic acid resulting in one lactone bond in a core ring, have broadly proven their cancer-targeting efficacy, some even reaching clinical trials and being commercialized as “anticancer” drugs.
1. Introduction
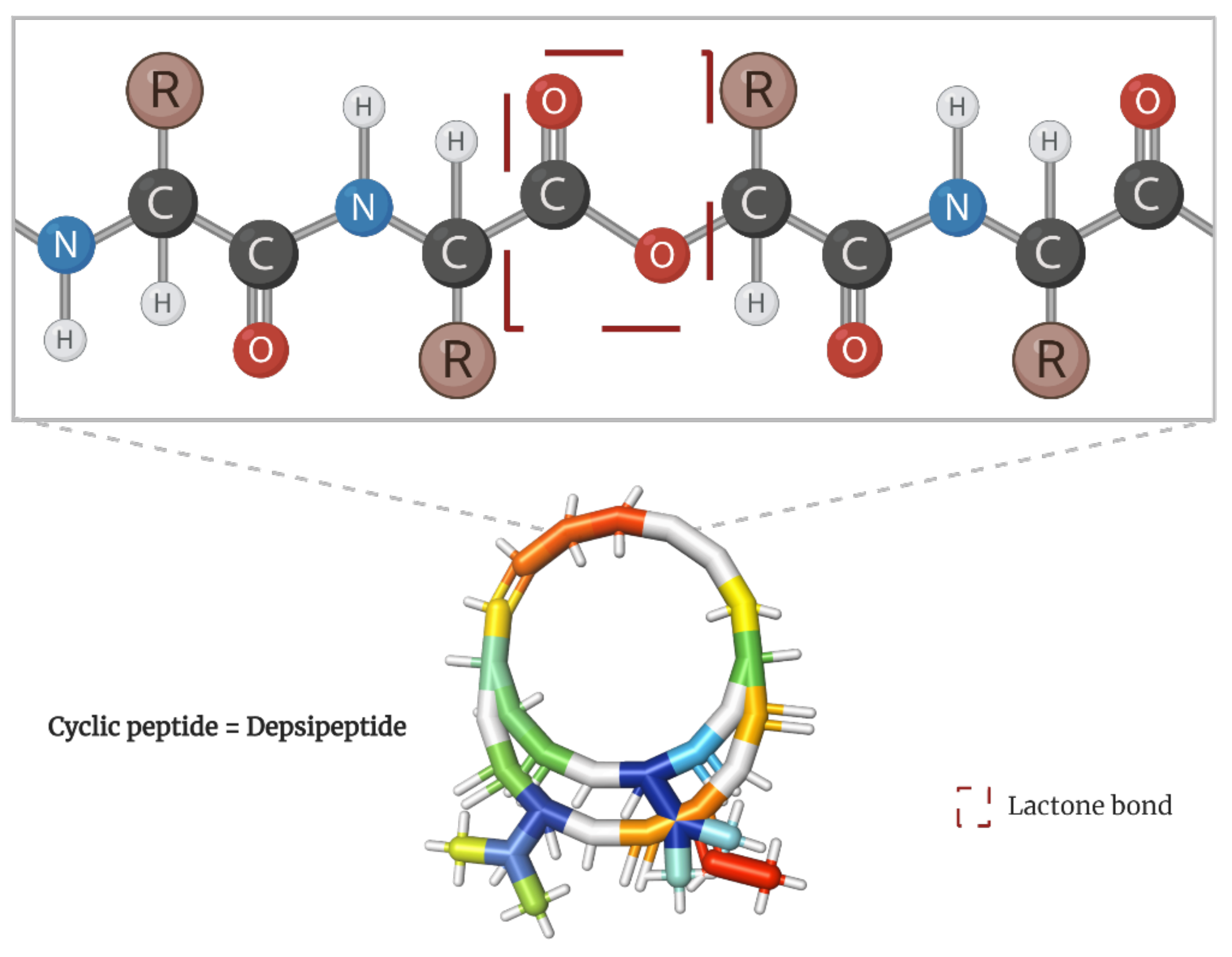
2. Depsipeptides with a Recognized Mechanism of Targeting Cancer Cells
2.1. Depsipeptides Inducing Apoptosis in Tumor Cells
|
Key |
Depsipeptide |
IUPAC Condensed Formula |
Molecular Weight (g/mol) |
Ref. |
|---|---|---|---|---|
|
a |
Apratoxin |
Cyclo[N(Me)Ala-N(Me)Ile-Pro-Unk-Tyr(Me)] |
840.1 |
[31] |
|
b |
Aurilide |
Cyclo[N(Me)Ala-Unk-Val-D-N(Me)Leu-Sar-Val] |
834.1 |
[21] |
|
c |
Beauvericin |
Cyclo[D-Oval-N(Me)Phe-D-Oval-N(Me)Phe-D-Oval-N(Me)Phe] |
783.9 |
[32] |
|
d |
Coibamide |
N(Me2)Val-Oval-N(Me)Ser(Me)-N(Me)Leu-N(Me)Thr(1)-N(Me)Ser(Me)-N(Me)Ile-Ala-N(Me)Leu-Tyr(Me)-N(Me)Ala-(1) |
1287.6 |
[33] |
|
e |
Dehydrodidemnin |
Pyruvoyl-Pro-D-N(Me)Leu-D-Thr(1)-Unk-Leu-Pro-DL-N(Me)Tyr(Me)-(1) |
1110.3 |
[34] |
|
f |
Enniatin |
Cyclo[DL-Oval-DL-N(Me)xiIle-DL-Oval-DL-N(Me)xiIle-DL-Oval-DL-N(Me)xiIle] |
681.9 |
[35] |
|
g |
Grassypeptolide |
Cyclo[D-N(Me)Leu-D-aThr-Unk-N(Me)Val-Pro-Unk] |
1102.4 |
[36] |
|
h |
Hantupeptin |
Cyclo[N(Me)Ile-Ophe-Pro-N(Me)Val-Unk-Val] |
740.9 |
[37] |
|
i |
Lagunamide |
Cyclo[Ala-D-N(Me)Phe-Sar-aIle-N(Me)Ala-Unk] |
842.1 |
[38] |
|
j |
Tiahuramide |
Cyclo[N(Me)Ile-Unk-Val-N(Me)Val-Ophe-Pro] |
736.9 |
[39] |
2.2. Depsipeptides Inducing Autophagy in Tumor Cells
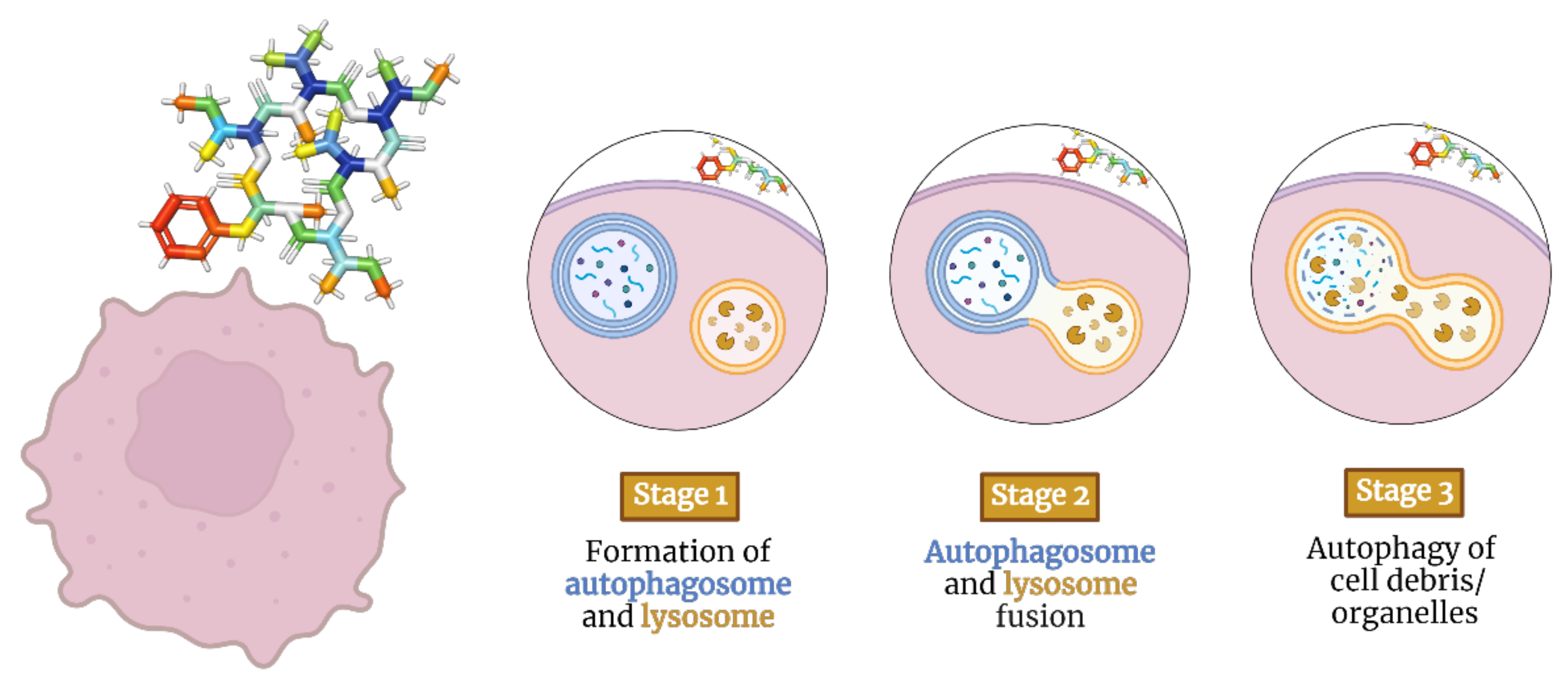
Beauvenniatins
|
Depsipeptide |
IUPAC Condensed Formula |
Molecular Weight (g/mol) |
Ref. |
|---|---|---|---|
|
Beauvenniatin |
Cyclo[D-OaIle-N(Me)Phe-D-OaIle-N(Me)Val-D-OaIle-N(Me)Val] |
729.9 |
[45] |
2.3. Depsipeptides Inhibiting Elastases
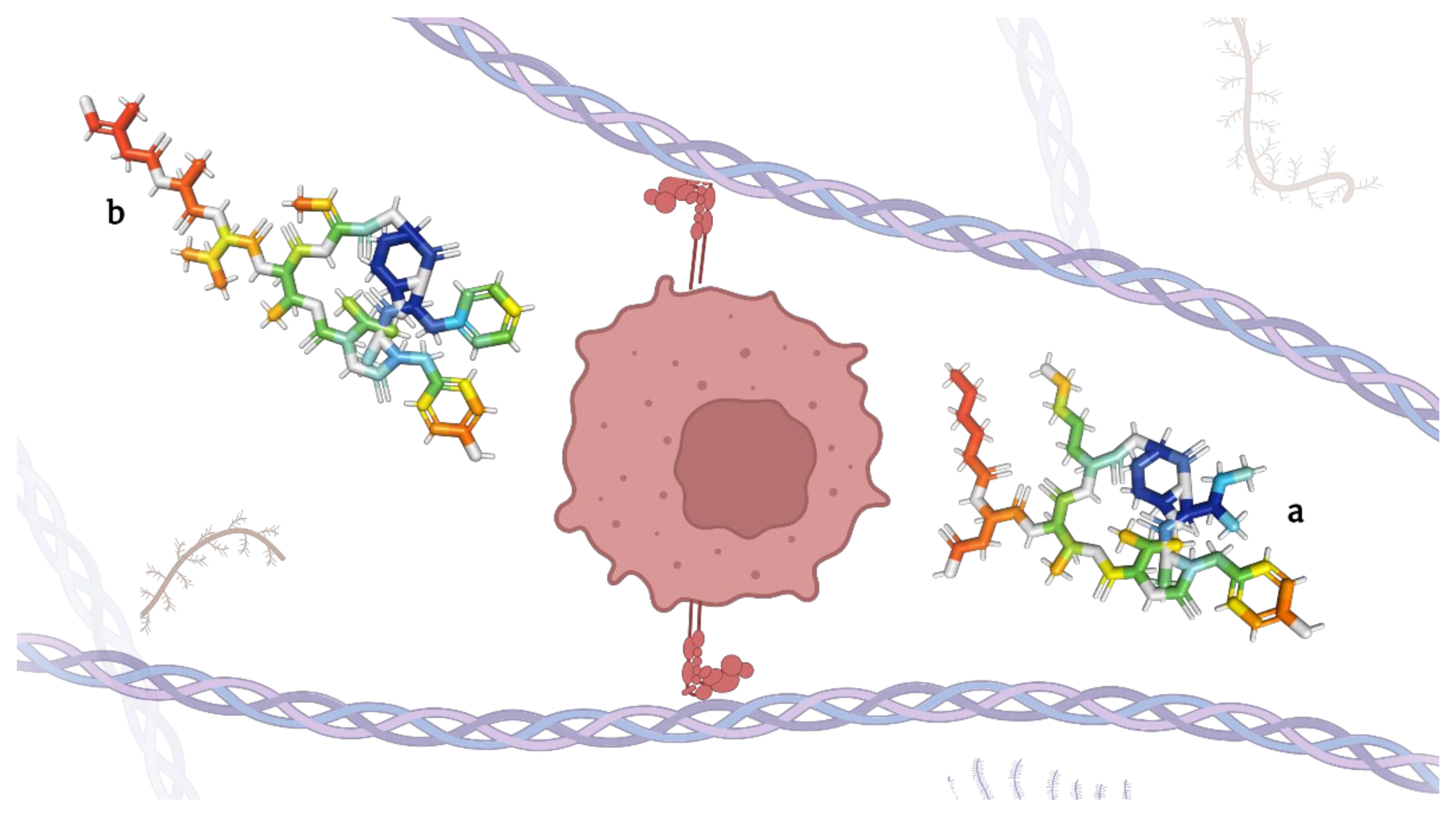
|
Key |
Depsipeptide |
IUPAC Formula |
Molecular Weight (g/mol) |
Ref. |
|---|---|---|---|---|
|
a |
Micropeptins |
(3S)-4-[[(2S,5S,8S,11R,12S,15S,18S,21R)-15-(4-aminobutyl)-2-[(2S)-butan-2-yl]-21-hydroxy-5-[(4-hydroxyphenyl)methyl]-4,11-dimethyl-3,6,9,13,16,22-hexaoxo-8-propan-2-yl-10-oxa-1,4,7,14,17-pentazabiCyclo [16.3.1]docosan-12-yl]amino]-3-(hexanoylamino)-4-oxobutanoic acid |
945.1 |
[51] |
|
b |
Tutuilamides |
(2S)-N-[(2S,5S,8S,11R,12S,15Z,18S,21R)-2-benzyl-15-ethylidene-21-hydroxy-5-[(4-hydroxyphenyl)methyl]-4,11-dimethyl-3,6,9,13,16,22-hexaoxo-8-propan-2-yl-10-oxa-1,4,7,14,17-pentazabiCyclo [16.3.1]docosan-12-yl]-2-[[(2S)-2-[[I-4-chloro-3-methylbut-3-enoyl]amino]propanoyl]amino]-3-methylbutanamide |
1007.6 |
[52] |
2.4. Depsipeptides Inhibiting Histone Deacetylases
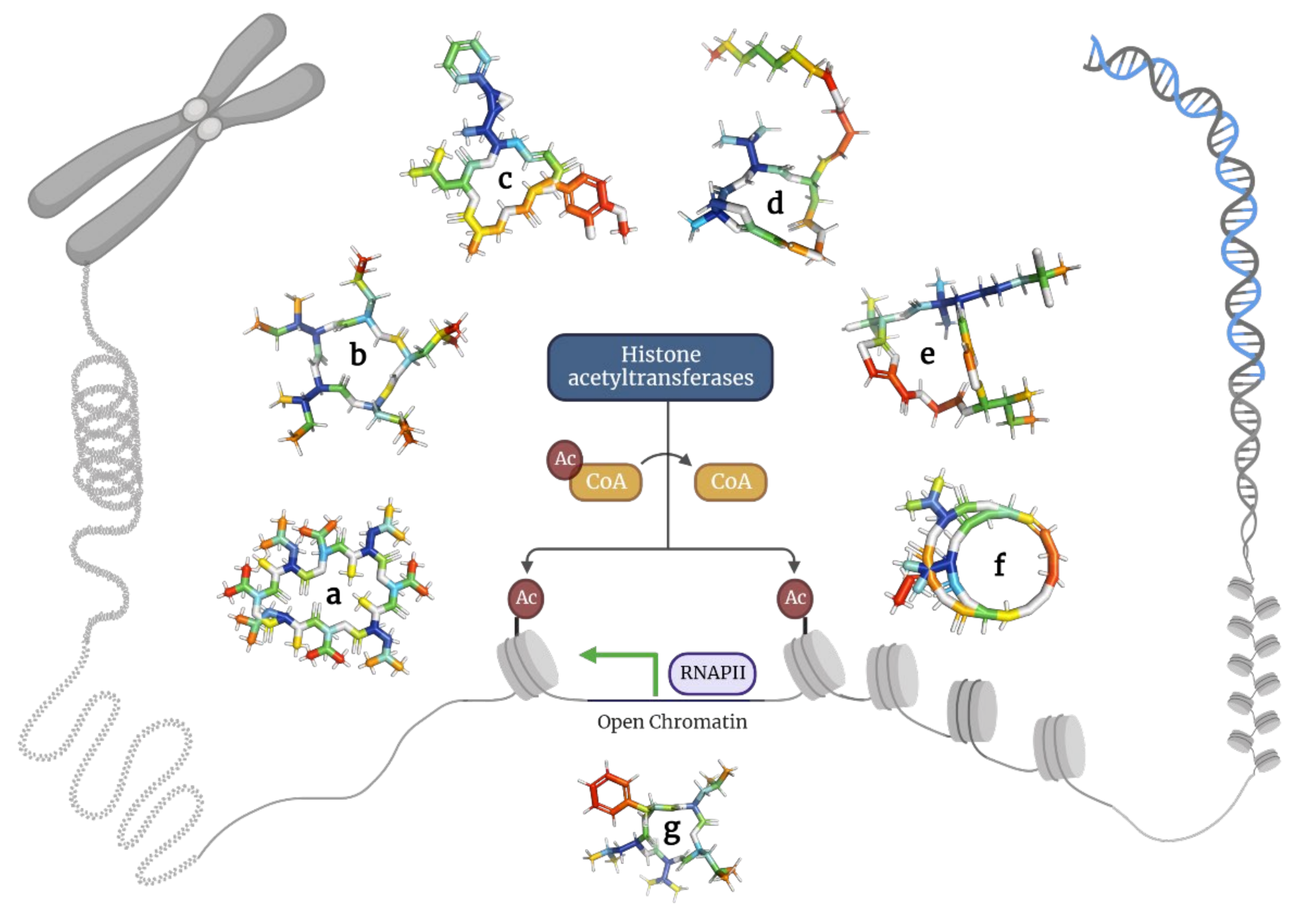
|
Key |
Depsipeptide |
IUPAC Formula |
Molecular Weight (g/mol) |
Ref. |
|---|---|---|---|---|
|
a |
Bassianolide |
Cyclo[N(Me)Leu-D-Oval-N(Me)Leu-D-Oval-N(Me)Leu-D-Oval-N(Me)Leu-D-Oval] |
909.2 |
[55] |
|
b |
Clavatustide |
Cyclo [2Abz-2Abz-D-Ophe-N(Et)Gly] |
471.5 |
[56] |
|
c |
Cryptophycin |
10-[(3-chloro-4-methoxyphenyl)methyl]-6-methyl-3-(2-methylpropyl)-16-[1-(3-phenyloxiran-2-yl)ethyl]-1,4-dioxa-8,11-diazacyclohexadec-13-ene-2,5,9,12-tetrone |
655.2 |
[57] |
|
d |
Largazole |
S-[I-4-[(5R,8S,11S)-5-Me-6,9,13-trioxo-8-propan-2-yl-10-oxa-3,17-dithia-7,14,19,20-tetrazatriCyclo [14.2.1.12,5]icosa-1(18),2(20),16(19)-trien-11-yl]but-3-enyl] octanethioate |
622.9 |
[58] |
|
e |
Lyngbyabellin |
(7S,14S,18S)-7-[(2S)-butan-2-yl]-14-(4,4-dichloropentyl)-18-(2-hydroxypropan-2-yl)-15,15-dimethyl-13,17-dioxa-9,20-dithia-3,6,22,23-tetrazatriCyclo [17.2.1.18,11]tricosa-1(21),8(23),10,19(22)-tetraene-2,5,12,16-tetrone |
691.7 |
[18] |
|
f |
Romidepsin |
7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabiCyclo [8.7.6]tricos-16-ene-3,6,9,19,22-pentone |
540.7 |
[59] |
|
g |
Sansalvamide |
Cyclo[L-leucyl-N-oxa-L-leucyl-L-valyl-L-leucyl-L-phenylalanyl] |
586.8 |
[60] |
2.5. Depsipeptides Disrupting Microfilaments

|
Key |
Depsipeptide |
IUPAC Formula |
Molecular Weight (g/mol) |
Ref. |
|---|---|---|---|---|
|
a |
Desmethoxymajusculamide C |
Cyclo[Ala-Unk-Ala-Unk-Gly-N(Me)Ile-Gly-N(Me)Val-N(Me)Phe] |
955.2 |
[64] |
|
b |
Dolastatin |
N(Me2)Val-Val-Unk |
785.1 |
[65] |
|
c |
Miuraenamide |
(3E,15E)-6-[(3-bromo-4-hydroxyphenyl)methyl]-3-[methoxy(phenyl)methylidene]-7,9,16,19-tetramethyl-1-oxa-4,7,10-triazacyclononadec-15-ene-2,5,8,11-tetrone |
684.6 |
[66] |
|
d |
Nobilamide |
Propionyl-D-Phe-D-Leu-Phe-D-aThr-Val-Ala-Abu(2,3-dehydro)-OH |
836.0 |
[67] |
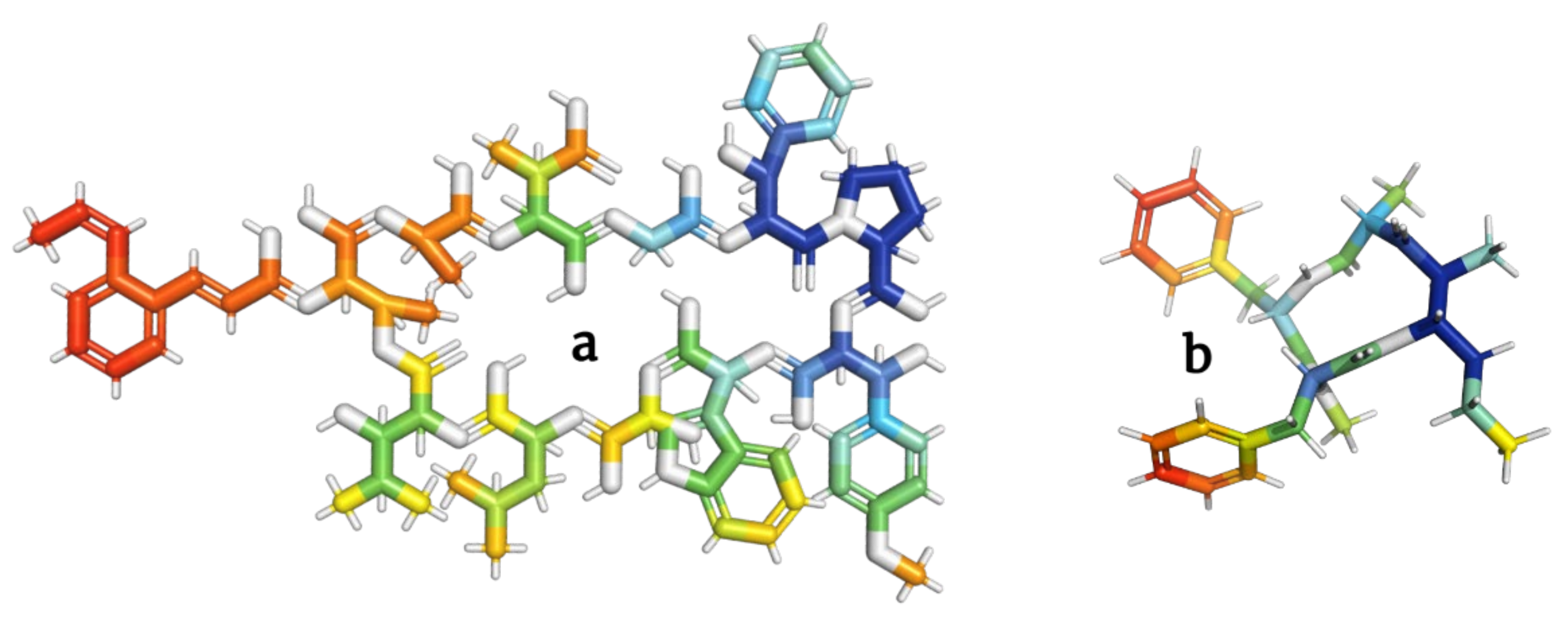
2.6. Depsipeptides Inhibiting Cell Growth
|
Key |
Depsipeptide |
IUPAC Formula |
Molecular Weight (g/mol) |
Ref. |
|---|---|---|---|---|
|
a |
Skyllamycins |
N-[I-3-[2-[(Z)-1-Propenyl]phenyl]propenoyl]-Cyclo[L-Thr*-L-Ala-[(3S)-3-Me-L-Asp-]-Gly-[(βS)-β-hydroxy-L-Phe-]-L-Pro-[(βS)-β-hydroxy-O-Me-L-Tyr-]-D-Trp-[(2S)-2-hydroxy-Gly-]-D-Leu-[(3S)-3-hydroxy-D-Leu-]-] |
1483.6 |
[74] |
|
b |
Stereocalpin |
Cyclo[Phe-N(Me)Phe-Unk] |
492.6 |
[75] |
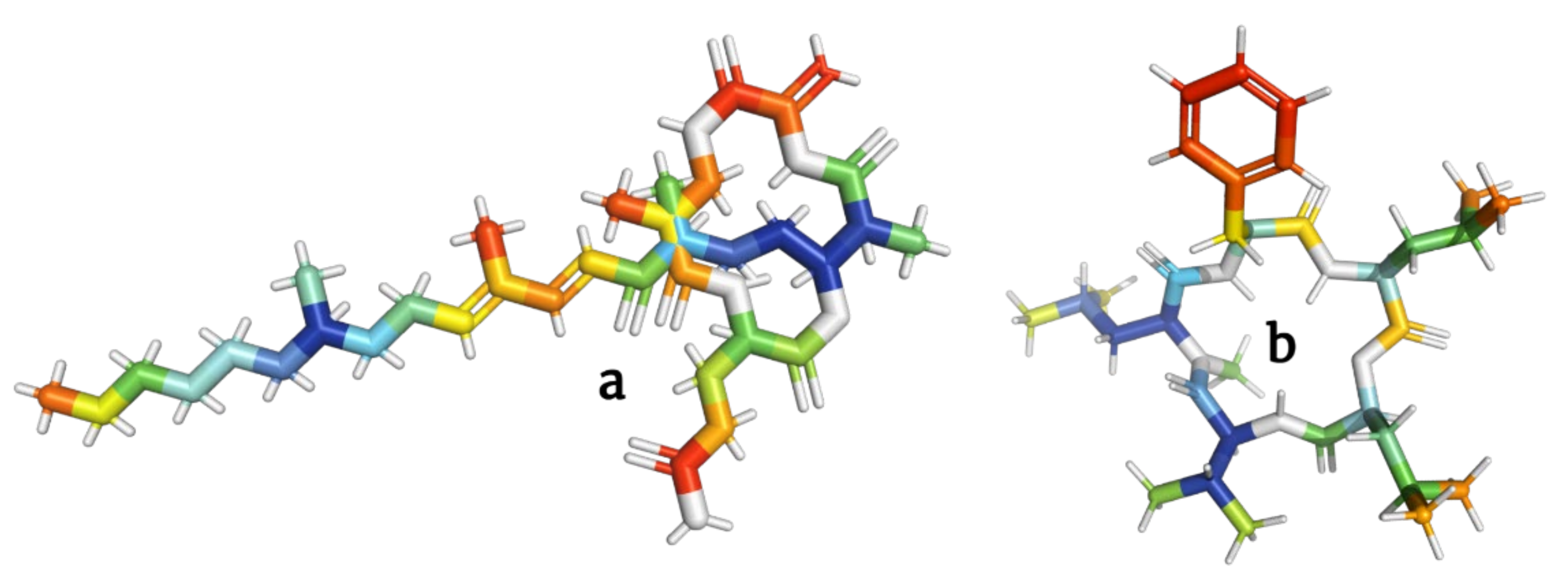
2.7. Depsipeptides Inhibiting Topoisomerases
|
Key |
Depsipeptide |
IUPAC Formula |
Molecular Weight (g/mol) |
Ref. |
|---|---|---|---|---|
|
a |
Fusaristatin A |
3-[6,13-dimethyl-10-methylidene-2,5,9,12-tetraoxo-14-[(5E,7E)-3,7,11-trimethyl-4-oxoheptadeca-5,7-dienyl]-1-oxa-4,8,11-triazacyclotetradec-3-yl]propanamide |
658.9 |
[79] |
|
b |
N-methylsansalvamide |
Cyclo[Leu-Oleu-Val-N(Me)Leu-Phe] |
600.8 |
[28] |
2.8. Oncosis

|
Depsipeptide |
IUPAC Formula |
Molecular Weight (g/mol) |
Ref. |
|---|---|---|---|
|
Kahalalide F |
L-Val, N-(5-Me-1-oxohexyl)-D-valyl-l-threonyl-l-valyl-D-valyl-D-prolyl-l-ornithyl-D-alloisoleucyl-D-allothreonyl-D-alloisoleucyl-D-valyl-l-phenylalanyl-(2z)-2-amino-2-butenoyl-, (13->8)-lactone |
1477.9 |
[83] |
3. Current Methods for Purification and Synthesis of Depsipeptides
3.1. Proved Methods for Purifying Depsipeptides
3.2. Synthesis of Depsipeptides
4. Recognized Disadvantages of Depsipeptides
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030.
- World Health Organization. Global Health Estimates for 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; WHO: Geneva, Switzerland, 2020.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Pilleron, S.; Sarfati, D.; Janssen-Heijnen, M.; Vignat, J.; Ferlay, J.; Bray, F.; Soerjomataram, I. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int. J. Cancer 2019, 144, 49–58.
- Pilleron, S.; Soto-Perez-de-Celis, E.; Vignat, J.; Ferlay, J.; Soerjomataram, I.; Bray, F.; Sarfati, D. Estimated global cancer incidence in the oldest adults in 2018 and projections to 2050. Int. J. Cancer 2021, 148, 601–608.
- Trinidad-Calderón, P.A.; López-Castillo, L.-M.; Gallegos-Martínez, S.; Trujillo-de-Santiago, G.; García-Lara, S.; Álvarez, M.M. nurP28, a New-to-Nature Zein-Derived Peptide, Enhances the Therapeutic Effect of Docetaxel in Breast Cancer Monolayers and Spheroids. Molecules 2022, 27, 2824.
- Serna-Thome, G.; Castro-Eguiluz, D.; Fuchs-Tarlovsky, V.; Sanchez-Lopez, M.; Delgado-Olivares, L.; Coronel-Martinez, J.; Molina-Trinidad, E.M.; De La Torre, M.; Cetina-Perez, L. Use of functional foods and oral supplements as adjuvants in cancer treatment. Rev. Investig. Clin. 2018, 70, 136–146.
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K.; Malik, J.; Mandal, S.K.; Kilpatrick, K.L.; Das, G.; Kerry, R.G.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476.
- Sharma, P.; Kaur, H.; Kehinde, B.A.; Chhikara, N.; Sharma, D.; Panghal, A. Food-Derived Anticancer Peptides: A Review. Int. J. Pept. Res. Ther. 2021, 27, 55–70.
- Zhang, Q.T.; Liu, Z.D.; Wang, Z.; Wang, T.; Wang, N.; Wang, N.; Zhang, B.; Zhao, Y.F. Recent advances in small peptides of marine origin in cancer therapy. Mar. Drugs 2021, 19, 115.
- Zhang, J.N.; Xia, Y.X.; Zhang, H.J. Natural cyclopeptides as anticancer agents in the last 20 years. Int. J. Mol. Sci. 2021, 22, 3973.
- Adrover-Castellano, M.L.; Schmidt, J.J.; Sherman, D.H. Biosynthetic Cyclization Catalysts for the Assembly of Peptide and Polyketide Natural Products. ChemCatChem 2021, 13, 2095–2116.
- Taevernier, L.; Wynendaele, E.; Gevaert, B.; Spiegeleer, B. Chemical Classification of Cyclic Depsipeptides. Curr. Protein Pept. Sci. 2017, 18, 425–452.
- Alonzo, D.A.; Schmeing, T.M. Biosynthesis of depsipeptides, or Depsi: The peptides with varied generations. Protein Sci. 2020, 29, 2316–2347.
- Rangel, M.; Santana, C.; Pinheiro, A.; Anjos, L.; Barth, T.; Júnior, O.; Fontes, W.; Castro, M. Marine Depsipeptides as Promising Pharmacotherapeutic Agents. Curr. Protein Pept. Sci. 2016, 18, 72–91.
- Lee, Y.; Phat, C.; Hong, S.C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 2017, 95, 94–105.
- Pavlicevic, M.; Maestri, E.; Marmiroli, M. Marine Bioactive Peptides—An Overview of Generation, Structure and Application with a Focus on Food Sources. Mar. Drugs 2020, 18, 424.
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491.
- Kang, H.; Choi, M.-C.; Seo, C.; Park, Y. Therapeutic Properties and Biological Benefits of Marine-Derived Anticancer Peptides. Int. J. Mol. Sci. 2018, 19, 919.
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592.
- Urbańska, K.; Orzechowski, A. Unappreciated role of LDHA and LDHB to control apoptosis and autophagy in tumor cells. Int. J. Mol. Sci. 2019, 20, 2085.
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619.
- Giampazolias, E.; Tait, S.W.G. Mitochondria and the hallmarks of cancer. FEBS J. 2016, 283, 803–814.
- Sharma, A.; Boise, L.H.; Shanmugam, M. Cancer metabolism and the evasion of apoptotic cell death. Cancers 2019, 11, 1144.
- Moschos, M.M.; Dettoraki, M.; Androudi, S.; Kalogeropoulos, D.; Lavaris, A.; Garmpis, N.; Damaskos, C.; Garmpi, A.; Tsatsos, M. The role of histone deacetylase inhibitors in uveal melanoma: Current evidence. Anticancer Res. 2018, 38, 3817–3824.
- Lee, H.S.; Phat, C.; Choi, S.U.; Lee, C. Synergistic effect of a novel cyclic pentadepsipeptide, neoN-methylsansalvamide, and paclitaxel on human multidrug resistance cancer cell lines. Anticancer Drugs 2013, 24, 455–460.
- Wan, X.; Serrill, J.D.; Humphreys, I.R.; Tan, M.; McPhail, K.L.; Ganley, I.G.; Ishmael, J.E. ATG5 promotes death signaling in response to the cyclic depsipeptides coibamide a and apratoxin A. Mar. Drugs 2018, 16, 77.
- Okada, K.; Hakata, S.; Terashima, J.; Gamou, T.; Habano, W.; Ozawa, S. Combination of the histone deacetylase inhibitor depsipeptide and 5-fluorouracil upregulates major histocompatibility complex class II and p21 genes and activates caspase-3/7 in human colon cancer HCT-116 cells. Oncol. Rep. 2016, 36, 1875–1885.
- Cai, W.; Ratnayake, R.; Gerber, M.H.; Chen, Q.-Y.; Yu, Y.; Derendorf, H.; Trevino, J.G.; Luesch, H. Development of apratoxin S10 (Apra S10) as an anti-pancreatic cancer agent and its preliminary evaluation in an orthotopic patient-derived xenograft (PDX) model. Investig. New Drugs 2019, 37, 364–374.
- Caloni, F.; Fossati, P.; Anadón, A.; Bertero, A. Beauvericin: The beauty and the beast. Environ. Toxicol. Pharmacol. 2020, 75, 103349.
- He, W.; Qiu, H.-B.; Chen, Y.-J.; Xi, J.; Yao, Z.-J. Total synthesis of proposed structure of coibamide A, a highly N- and O-methylated cytotoxic marine cyclodepsipeptide. Tetrahedron Lett. 2014, 55, 6109–6112.
- Zuo, W.; Kwok, H.F. Development of marine-derived compounds for cancer therapy. Mar. Drugs 2021, 19, 342.
- Taevernier, L.; Veryser, L.; Roche, N.; Peremans, K.; Burvenich, C.; Delesalle, C.; De Spiegeleer, B. Human skin permeation of emerging mycotoxins (beauvericin and enniatins). J. Expo. Sci. Environ. Epidemiol. 2016, 26, 277–287.
- Kwan, J.C.; Rocca, J.R.; Abboud, K.A.; Paul, V.J.; Luesch, H. Total structure determination of grassypetolide, a new marine cyanobacterical cytotoxin. Org. Lett. 2008, 10, 789–792.
- Xue, Y.; Zhao, P.; Quan, C.; Zhao, Z.; Gao, W.; Li, J.; Zu, X.; Fu, D.; Feng, S.; Bai, X.; et al. Cyanobacteria-derived peptide antibiotics discovered since 2000. Peptides 2018, 107, 17–24.
- Huang, W.; Ren, R.G.; Dong, H.Q.; Wei, B.G.; Lin, G.Q. Diverse synthesis of marine cyclic depsipeptide lagunamide A and its analogues. J. Org. Chem. 2013, 78, 10747–10762.
- Levert, A.; Alvariño, R.; Bornancin, L.; Abou Mansour, E.; Burja, A.M.; Genevière, A.M.; Bonnard, I.; Alonso, E.; Botana, L.; Banaigs, B. Structures and Activities of Tiahuramides A-C, Cyclic Depsipeptides from a Tahitian Collection of the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2018, 81, 1301–1310.
- Bialik, S.; Dasari, S.K.; Kimchi, A. Autophagy-dependent cell death—Where, how and why a cell eats itself to death. J. Cell Sci. 2018, 131, jcs215152.
- Zhang, L.; Wang, Y.; Huang, W.; Wei, Y.; Jiang, Z.; Kong, L.; Wu, A.A.; Hu, Z.; Huang, H.; Xu, Q.; et al. Biosynthesis and Chemical Diversification of Verucopeptin Leads to Structural and Functional Versatility. Org. Lett. 2020, 22, 4366–4371.
- Kitagaki, J.; Shi, G.; Miyauchi, S.; Murakami, S.; Yang, Y. Cyclic depsipeptides as potential cancer therapeutics. Anticancer Drugs 2015, 26, 259–271.
- Liu, J.; Zhu, X.; Kim, S.J.; Zhang, W. Antimycin-type depsipeptides: Discovery, biosynthesis, chemical synthesis, and bioactivities. Nat. Prod. Rep. 2016, 33, 1146–1165.
- Urbaniak, M.; Stępień, Ł.; Uhlig, S. Evidence for naturally produced beauvericins containing N-Methyl-Tyrosine in Hypocreales Fungi. Toxins 2019, 11, 182.
- Urbaniak, M.; Waskiewicz, A.; Stepien, Ł. Fusarium cyclodepsipeptide mycotoxins: Chemistry, biosynthesis, and occurrence. Toxins 2020, 12, 765.
- Tian, J.; Han, J.J.; Zhang, X.; He, L.W.; Zhang, Y.J.; Bao, L.; Liu, H.W. New Cyclohexadepsipeptides from an Entomogenous Fungus Fusarium proliferatum and Their Cytotoxicity and Autophagy-Inducing Activity. Chem. Biodivers. 2016, 13, 852–860.
- Bunyapaiboonsri, T.; Vongvilai, P.; Auncharoen, P.; Isaka, M. Cyclohexadepsipeptides from the Filamentous Fungus Acremonium sp. BCC 2629. Helv. Chim. Acta 2012, 95, 963–972.
- Marinaccio, L.; Stefanucci, A.; Scioli, G.; Della Valle, A.; Zengin, G.; Cichelli, A.; Mollica, A. Peptide Human Neutrophil Elastase Inhibitors from Natural Sources: An Overview. Int. J. Mol. Sci. 2022, 23, 2924.
- Al-Awadhi, F.H.; Paul, V.J.; Luesch, H. Structural Diversity and Anticancer Activity of Marine-Derived Elastase Inhibitors: Key Features and Mechanisms Mediating the Antimetastatic Effects in Invasive Breast Cancer. ChemBioChem 2018, 19, 815–825.
- Chen, Q.-Y.; Luo, D.; Seabra, G.M.; Luesch, H. Ahp-Cyclodepsipeptides as tunable inhibitors of human neutrophil elastase and kallikrein 7: Total synthesis of tutuilamide A, serine protease selectivity profile and comparison with lyngbyastatin 7. Bioorg. Med. Chem. 2020, 28, 115756.
- Geada, P.; Gkelis, S.; Teixeira, J.; Vasconcelos, V.; Vicente, A.A.; Fernandes, B. Cyanobacterial Toxins as a High Value-Added Product; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081010273.
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria and microalgae bioactive compounds in skin-ageing: Potential to restore extracellular matrix filling and overcome hyperpigmentation. J. Enzyme Inhib. Med. Chem. 2021, 36, 1829–1838.
- Zhang, H.; Shang, Y.P.; Chen, H.Y.; Li, J. Histone deacetylases function as novel potential therapeutic targets for cancer. Hepatol. Res. 2017, 47, 149–159.
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Dimitroulis, D.; Spartalis, E.; Margonis, G.A.; Schizas, D.; Deskou, I.; Doula, C.; Magkouti, E.; et al. Targeting histone deacetylases in malignant melanoma: A future therapeutic agent or just great expectations? Anticancer Res. 2017, 37, 5355–5362.
- Mun, B.; Park, Y.J.; Sung, G.H.; Lee, Y.; Kim, K.H. Synthesis and antitumor activity of (−)-bassianolide in MDA-MB 231 breast cancer cells through cell cycle arrest. Bioorg. Chem. 2016, 69, 64–70.
- Chettu, S.K.; Madhu, R.B.; Raolji, G.B.; Babu, K.R.; Rao, N.S.K.; Gopalakrishnan, S.; Ismail, A.; Reddy, G.B.; Shafi, S. First total synthesis of cyclodepsipeptides clavatustide A and B and their enantiomers. RSC Adv. 2016, 6, 61555–61565.
- Schmidt, J.J.; Khatri, Y.; Brody, S.I.; Zhu, C.; Pietraszkiewicz, H.; Valeriote, F.A.; Sherman, D.H. A versatile chemoenzymatic synthesis for the discovery of potent cryptophycin analogs. ACS Chem. Biol. 2020, 15, 524–532.
- Poli, G.; Di Fabio, R.; Ferrante, L.; Summa, V.; Botta, M. Largazole Analogues as Histone Deacetylase Inhibitors and Anticancer Agents: An Overview of Structure–Activity Relationships. ChemMedChem 2017, 12, 1917–1926.
- Smolewski, P.; Robak, T. The discovery and development of romidepsin for the treatment of T-cell lymphoma. Expert Opin. Drug Discov. 2017, 12, 859–873.
- Romans-Fuertes, P.; Sondergaard, T.E.; Sandmann, M.I.H.; Wollenberg, R.D.; Nielsen, K.F.; Hansen, F.T.; Giese, H.; Brodersen, D.E.; Sørensen, J.L. Identification of the non-ribosomal peptide synthetase responsible for biosynthesis of the potential anti-cancer drug sansalvamide in FusariumFusarium solani. Curr. Genet. 2016, 62, 799–807.
- Kigoshi, H.; Kita, M. Antitumor Effects of Sea Hare-Derived Compounds in Cancer. In Handbook of Anticancer Drugs from Marine Origin; Springer International Publishing: Cham, Switzerland, 2015; pp. 701–739. ISBN 9783319071459.
- Tan, L.T.; Gupta, D.K. Molecular Targets of Anticancer Agents from Filamentous Marine Cyanobacteria. In Handbook of Anticancer Drugs from Marine Origin; Springer International Publishing: Cham, Switzerland, 2015; pp. 571–592. ISBN 9783319071459.
- Mandal, S.; Rath, J. Anticancer Drug Development from Cyanobacteria. In Extremophilic Cyanobacteria for Novel Drug Development. SpringerBriefs in Pharmaceutical Science & Drug Development; Springer: Cham, Switzerland, 2015; pp. 63–78.
- Śliżewska, A.; Żymańczyk-Duda, E. Cyanobacteria as valuable tool in biotechnology. Catalysts 2021, 11, 1259.
- Zhou, W.; Nie, X.D.; Zhang, Y.; Si, C.M.; Zhou, Z.; Sun, X.; Wei, B.G. A practical approach to asymmetric synthesis of dolastatin 10. Org. Biomol. Chem. 2017, 15, 6119–6131.
- Tost, M.; Andler, O.; Kazmaier, U. A Matteson Homologation-Based Synthesis of Doliculide and Derivatives. Eur. J. Org. Chem. 2021, 2021, 6459–6471.
- Yamashita, T.; Matoba, H.; Kuranaga, T.; Inoue, M. Total syntheses of nobilamides B and D: Application of traceless Staudinger ligation. Tetrahedron 2014, 70, 7746–7752.
- Dominguez-Brauer, C.; Thu, K.L.; Mason, J.M.; Blaser, H.; Bray, M.R.; Mak, T.W. Targeting Mitosis in Cancer: Emerging Strategies. Mol. Cell 2015, 60, 524–536.
- Haschka, M.; Karbon, G.; Fava, L.L.; Villunger, A. Perturbing mitosis for anti-cancer therapy: Is cell death the only answer? EMBO Rep. 2018, 19, e45440.
- Mehta, A.; Soni, V.K.; Shukla, D.; Vishvakarma, N.K. Cyanobacteria: A Potential Source of Anticancer Drugs; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128193112.
- Ahmed, S.; Khan, H.; Fakhri, S.; Aschner, M.; Cheang, W.S. Therapeutic potential of marine peptides in cervical and ovarian cancers. Mol. Cell. Biochem. 2022, 477, 605–619.
- Zhu, J.; Zhang, S.; Zechel, D.L.; Paululat, T.; Bechthold, A. Rational Design of Hybrid Natural Products by Utilizing the Promiscuity of an Amide Synthetase. ACS Chem. Biol. 2019, 14, 1793–1801.
- Byeon, H.E.; Park, B.K.; Yim, J.H.; Lee, H.K.; Moon, E.Y.; Rhee, D.K.; Pyo, S. Stereocalpin A inhibits the expression of adhesion molecules in activated vascular smooth muscle cells. Int. Immunopharmacol. 2012, 12, 315–325.
- Giltrap, A.M.; Haeckl, F.P.J.; Kurita, K.L.; Linington, R.G.; Payne, R.J. Synthetic Studies Toward the Skyllamycins: Total Synthesis and Generation of Simplified Analogues. J. Org. Chem. 2018, 83, 7250–7270.
- Cimmino, A.; Nimis, P.L.; Masi, M.; De Gara, L.; van Otterlo, W.A.L.; Kiss, R.; Evidente, A.; Lefranc, F. Have lichenized fungi delivered promising anticancer small molecules? Phytochem. Rev. 2019, 18, 1–36.
- Murai, J.; Thomas, A.; Miettinen, M.; Pommier, Y. Schlafen 11 (SLFN11), a restriction factor for replicative stress induced by DNA-targeting anti-cancer therapies. Pharmacol. Ther. 2019, 201, 94–102.
- Mohan, C.D.; Rangappa, S.; Nayak, S.C.; Jadimurthy, R.; Wang, L.; Sethi, G.; Garg, M.; Rangappa, K.S. Bacteria as a treasure house of secondary metabolites with anticancer potential. Semin. Cancer Biol. 2021, 86, 998–1013.
- Watters, D.J. Ascidian toxins with potential for drug development. Mar. Drugs 2018, 16, 162.
- Hegge, A.; Lønborg, R.; Nielsen, D.M.; Sørensen, J.L. Factors influencing production of fusaristatin A in Fusarium graminearum. Metabolites 2015, 5, 184–191.
- Puig, B.; Brenna, S.; Magnus, T. Molecular communication of a dying neuron in stroke. Int. J. Mol. Sci. 2018, 19, 2834.
- Weerasinghe, P.; Hallock, S.; Brown, R.; Buja, L.M. Oncosis. In Apoptosis and Beyond; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 567–582.
- Tsai, C.M. AqF026 may act as a cancer therapeutic agent via inducing cancer cell oncosis. Med. Hypotheses 2020, 140, 109685.
- Dayanidhi, D.L.; Thomas, B.C.; Osterberg, J.S.; Vuong, M.; Vargas, G.; Kwartler, S.K.; Schmaltz, E.; Dunphy-Daly, M.M.; Schultz, T.F.; Rittschof, D.; et al. Exploring the Diversity of the Marine Environment for New Anti-cancer Compounds. Front. Mar. Sci. 2021, 7, 614766.
- Kaneda, M.; Sueyoshi, K.; Teruya, T.; Ohno, H.; Fujii, N.; Oishi, S. Total synthesis of odoamide, a novel cyclic depsipeptide, from an Okinawan marine cyanobacterium. Org. Biomol. Chem. 2016, 14, 9093–9104.
- Nguyen, M.M.; Ong, N.; Suggs, L. A general solid phase method for the synthesis of depsipeptides. Org. Biomol. Chem. 2013, 11, 1167–1170.
- Campbell, T.D.; Febrian, R.; Kleinschmidt, H.E.; Smith, K.A.; Bracher, P.J. Quantitative Analysis of Glycine Oligomerization by Ion-Pair Chromatography. ACS Omega 2019, 4, 12745–12752.
- Phyo, Y.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine natural peptides: Determination of absolute configuration using liquid chromatography methods and evaluation of bioactivities. Molecules 2018, 23, 306.
- Greco, C.; Pfannenstiel, B.T.; Liu, J.C.; Keller, N.P. Depsipeptide Aspergillicins Revealed by Chromatin Reader Protein Deletion. ACS Chem. Biol. 2019, 14, 1121–1128.
- Arumugam, V.; Venkatesan, M.; Ramachandran, S.; Sundaresan, U. Bioactive Peptides from Marine Ascidians and Future Drug Development–A Review. Int. J. Pept. Res. Ther. 2018, 24, 13–18.
- Matsuo, Y.; Kanoh, K.; Imagawa, H.; Adachi, K.; Nishizawa, M.; Shizuri, Y. Urukthapelstatin A, a novel cytotoxic substance from marine-derived Mechercharimyces asporophorigenens YM11-542: II. Physico-chemical properties and structural elucidation. J. Antibiot. 2007, 60, 256–260.
- Phyo, M.Y.; Katermeran, N.P.; Goh, J.X.; Tan, L.T. Trikoveramides A-C, cyclic depsipeptides from the marine cyanobacterium Symploca hydnoides. Phytochemistry 2021, 190, 112879.
- Sable, G.A.; Park, J.; Kim, H.; Lim, S.J.; Jang, S.; Lim, D. Solid-Phase Total Synthesis of the Proposed Structure of Coibamide A and Its Derivative: Highly Methylated Cyclic Depsipeptides. Eur. J. Org. Chem. 2015, 2015, 7043–7052.
- Kaur, H.; Harris, P.W.R.; Little, P.J.; Brimble, M.A. Total synthesis of the cyclic depsipeptide YM-280193, a platelet aggregation inhibitor. Org. Lett. 2015, 17, 492–495.
- Pieszka, M.; Sobota, A.M.; Gačanin, J.; Weil, T.; Ng, D.Y.W. Orthogonally Stimulated Assembly/Disassembly of Depsipeptides by Rational Chemical Design. ChemBioChem 2019, 20, 1376–1381.
- Lobo-Ruiz, A.; Tulla-Puche, J. General Fmoc-Based Solid-Phase Synthesis of Complex Depsipeptides Circumventing Problematic Fmoc Removal. Eur. J. Org. Chem. 2020, 2020, 183–192.
- Jin, K.; Sam, I.H.; Po, K.H.L.; Lin, D.; Ghazvini Zadeh, E.H.; Chen, S.; Yuan, Y.; Li, X. Total synthesis of teixobactin. Nat. Commun. 2016, 7, 12394.
- Merlino, F.; Tomassi, S.; Yousif, A.M.; Messere, A.; Marinelli, L.; Grieco, P.; Novellino, E.; Cosconati, S.; Di Maro, S. Boosting Fmoc Solid-Phase Peptide Synthesis by Ultrasonication. Org. Lett. 2019, 21, 6378–6382.
- Farhid, H.; Rostami, M.M.; Shaabani, A.; Notash, B. Synthesis of Depsipeptides via Isocyanide-Based Consecutive Bargellini-Passerini Multicomponent Reactions. SynOpen 2021, 5, 167–172.
- Batiste, S.M.; Johnston, J.N. Evidence for Ion-Templation during Macrocyclooligomerization of Depsipeptides. J. Am. Chem. Soc. 2018, 140, 4560–4568.
- Jing, X.; Jin, K. A gold mine for drug discovery: Strategies to develop cyclic peptides into therapies. Med. Res. Rev. 2020, 40, 753–810.
- Conte, M.; Fontana, E.; Nebbioso, A.; Altucci, L. Marine-derived secondary metabolites as promising epigenetic bio-compounds for anticancer therapy. Mar. Drugs 2021, 19, 15.
- Eckes, K.M.; Baek, K.; Suggs, L.J. Design and Evaluation of Short Self-Assembling Depsipeptides as Bioactive and Biodegradable Hydrogels. ACS Omega 2018, 3, 1635–1644.





