Despite significant advancements in immunosuppressive therapies, kidney transplant rejection continues to pose a substantial challenge, impacting the long-term survival of grafts. TCMR is diagnosed through histological examination of kidney biopsy samples, which reveal the infiltration of mononuclear cells into the allograft tissue. Corticosteroids serve as the primary treatment for TCMR, while severe or steroid-resistant cases may require T-cell-depleting agents, like Thymoglobulin. ABMR occurs due to the binding of antibodies to graft endothelial cells. The most common treatment for ABMR is plasmapheresis, although its efficacy is still a subject of debate. Other current therapies, such as intravenous immunoglobulins, anti-CD20 antibodies, complement inhibitors, and proteasome inhibitors, are also utilized to varying degrees, but their efficacy remains questionable. Management decisions for ABMR depend on the timing of the rejection episode and the presence of chronic changes. In managing both TCMR and ABMR, it is crucial to optimize immunosuppression and address adherence.
1. Introduction
Kidney transplantation is considered the best treatment option for individuals with end-stage kidney disease (ESKD)
[1]. Recent trends have shown an increase in the median survival of kidney allografts, and according to the United Network for Organ Sharing (UNOS), the one-year survival rate for kidney transplants is approximately 95%, while the five-year and ten-year survival rates are about 85% and 65%, respectively
[2][3]. Despite advances in surgical techniques, immunosuppressive drugs, and improved patient management, kidney transplant rejection remains a significant issue that affects long-term graft survival
[4]. Therefore, it is crucial to develop effective strategies to prevent and manage rejection in kidney transplant recipients to improve long-term graft survival and patient outcomes. Despite maximizing treatment of rejection episodes, some cases may not be reversed and may impact allograft survival. Even if maximum antirejection treatment is administered, certain kidney allografts may not recover function. Additionally, acute rejection episodes can negatively impact long-term graft survival for those who do recover
[5].
Kidney transplant rejection can manifest as either subclinical or clinical. Subclinical rejection often lacks evident symptoms or laboratory abnormalities and is typically identified through protocol biopsy or biopsy for cause after observing an increase in surveillance biomarkers, such as donor-derived cell-free DNA or the detection of new donor-specific antibodies (DSAs). Surveillance methods for detecting subclinical rejection vary among centers, with some employing routine laboratory tests only, while others utilize a combination of protocol biopsies, DSA assessments, and biomarker monitoring. Consensus on the optimal surveillance approach for subclinical rejection has yet to be reached. Conversely, clinical rejection can manifest with a decline in kidney function, proteinuria, or hematuria, or it can be symptomatic and cause fever, allograft pain, hematuria, or decreased urine output, in addition to the laboratory abnormalities mentioned. The diagnosis of rejection typically requires assessing kidney allograft pathology, with findings being classified according to the Banff classification.
The Banff classification is a standardized system that grades and categorizes kidney transplant rejection based on histological findings. Initially established in 1991, the classification has undergone multiple revisions to enhance its accuracy and clinical relevance
[6]. The Banff classification system categorizes rejection into acute T-cell-mediated rejection (aTCMR), chronic active T-cell-mediated rejection (caTCMR), active antibody-mediated rejection (aABMR), chronic active antibody-mediated rejection (caABMR), and chronic (inactive) antibody-mediated rejection (cABMR). However, it is common for patients to exhibit more than one type of rejection simultaneously. The system also includes specific criteria for borderline changes that do not meet the criteria for aTCMR.
The classification takes into account various acute and chronic histological features to diagnose rejection. The acute rejection changes include
i (interstitial inflammation),
t (tubulitis),
v (arteritis),
g (glomerulitis), PTC (peritubular capillary inflammation), and
c4d (complement 4 d product), while chronic rejection changes include
ct (tubular atrophy),
ci (interstitial fibrosis),
cv (arteriolopathy),
cg (chronic glomerulopathy), and peritubular capillary multilayering. Most changes are typically diagnosed using light microscopy; however,
c4d, a histologic marker of complement activation, is a distinctive histological feature that can be diagnosed using either immunofluorescence or immunohistochemistry. Additionally, peritubular capillary basement membrane multilayering can be visualized using electron microscopy
[6][7]. Each change is graded from 0 to 3, and the severity of rejection is based on the severity of one or a combination of the lesions above.
2. T-Cell-Mediated Rejection (TCMR)
2.1. Diagnosis
The gold standard for diagnosing acute T-cell-mediated rejection is through histological examination of kidney biopsy samples. The biopsy samples typically reveal infiltration of mononuclear cells in the interstitium, tubules, and/or vessels (
t,
i, or
v). caTCMR is characterized by chronic changes that include
ct,
ci, and/or
cv lesions, in addition to acute inflammation changes (
t,
i, or
v)
[7]. The severity of aTCMR is graded into IA, IB, IIA, IIB, and III, which are outlined in
Table 1 according to the Banff 2019 classification
[7]. The majority of biopsy-proven acute rejections (BPARs) occurring within the first year post-transplant are typically TCMR
[8].
Table 1. Summary of TCMR and borderline changes classification and pathological findings.
| Class/Grade of Rejection |
Minimal Pathological Findings Required for Diagnosis |
| aTCMR α |
Grade IA |
t2 + (i2 or i3) |
| Grade IB |
t3 + (i2 or i3) |
| Grade IIA |
v1 with or without interstitial inflammation and/or tubulitis |
| Grade IIB |
v2 with or without interstitial inflammation and/or tubulitis |
| Grade III |
v3 with or without interstitial inflammation and/or tubulitis |
| caTCMR β |
Grade IA |
interstitial inflammation that affects more than 25% of sclerotic cortical parenchyma and more than 25% of the total cortical parenchyma (ti2 or ti3) with t2 |
| Grade IB |
interstitial inflammation that affects more than 25% of sclerotic cortical parenchyma AND more than 25% of the total cortical parenchyma (ti2 or ti3) AND t3 |
| Grade II |
Chronic allograft arteriopathy (arterial intimal fibrosis with mononuclear cell inflammation in fibrosis and formation of neointima) |
| Borderline Changes |
i1, with t1, t2, or t3
or
t1 with i2 or i3 |
2.2. Current Therapies
2.2.1. Corticosteroids
Corticosteroids are the first-line treatment for acute inflammation in TCMR. Typically, a bolus dose of 3–5 mg/kg of intravenous (IV) methylprednisolone is administered over 3–6 consecutive days, followed by an oral prednisone taper. Corticosteroids act by inhibiting cytokine transcription by blocking transcription factors, such as NF-kβ and activator protein-1. This leads to downstream effects of T-cell depletion (by IL-2 inhibition), inhibition of T-Helper 1 differentiation and apoptosis, eosinophil apoptosis, and macrophage dysfunction
[9].
The response to treatment for TCMR can vary based on the severity of the condition. Although the literature and guidelines may not explicitly define the criteria for response to therapy, it is generally understood that response to therapy in the context of TCMR is typically assessed by observing resolutions of histological findings and improvements in kidney function
[10]. A study found that aTCMR Banff grade II had a decreased response to steroid therapy, resulting in poor allograft survival when compared to TCMR Banff grade IA. Steroid therapy alone was able to reverse rejection in 36% of cases. However, 86% of the non-responders were successfully treated with anti-lymphocyte antibody therapy
[11]. In a systematic review of literature aimed at assessing the response to treatment in TCMR, the authors observed that different criteria were employed across various studies for defining response to therapy. Despite the limitations posed by this heterogeneity in the criteria used, the pooled data revealed that the response to therapy was similar in Banff IA, IB rejections (44–73%) and Banff IIA rejections (52–80%), whereas the response was significantly lower in Banff IIB rejections (10%)
[12].
2.2.2. T-Cell-Depleting Agents
KDIGO recommends the use of T-cell-depleting agents in the treatment of steroid-resistant cellular rejection
[13]. Thymoglobulin, which is a polyclonal agent containing antibodies to various antigens, is the most commonly used T-cell-depleting agent. It can interact with several receptors on T cells, as well as some shared receptors on B cells, monocytes, and neutrophils. The primary mechanism of action is lymphocyte depletion through complement-dependent lysis and induction of T-cell activation-induced apoptosis
[14]. There are two preparations available: Thymoglobulin, made by immunizing rabbits with human thymocytes, and anti-thymocyte globulin (rATG), made by immunizing rabbits with lymphocytes from the Jurkat T-cell leukemia line. Both products induce cytokine release due to their non-human immunoglobulins by activation of NK cells and macrocytes/monocytes binding to the Fc receptor, as well as cellular cytotoxicity. Thymoglobulin is generally used at a dose of 1–1.5 mg/kg per dose for a total of 4–6 doses
[15]. Leukopenia and thrombocytopenia are commonly encountered. Peripheral CD3 counts can be used to monitor response. In patients who develop thymoglobulin antibodies, ATGAM (horse anti-thymocyte globulin) can be used at a dose of 1 gm IV for 10 days, with further dosing adjusted based on peripheral CD2-positive T-cell measurement. However, the use of ATGAM is associated with significant side effects, including serum sickness and cytokine release, and it is less well tolerated than thymoglobulin.
2.3. Approach to Treatment
TCMR can be the clinical diagnosis via for-cause biopsy or the subclinical diagnosis through protocol biopsies. Although there are currently no established guidelines on how to treat clinical versus subclinical TCMR, most transplant nephrologists would recommend treatment of both entities, as long as there are no contraindications
[16]. The approach to managing TCMR can vary among transplant centers. For TCMR IA and IB, despite lack of data showing their beneficial impact on kidney transplant rejection outcomes, high-dose corticosteroids are typically used, while thymoglobulin is usually utilized for grade II or III TCMR or if TCMR IA or IB does not respond to treatment. Some centers may perform follow-up biopsies after treatment to assess the response to therapy. While there is no consensus on the exact timing of these biopsies, it is typically performed 2–4 weeks after completing the therapy.
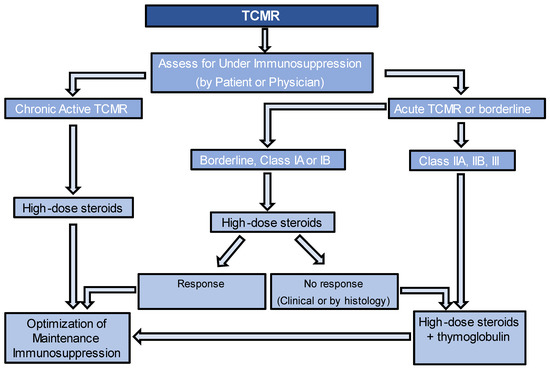
Figure 1 provides an algorithm for managing TCMR. For caTCMR, high-dose steroids alone are usually considered. Also, it is important to check for the presence of DeNovo DSA at the time of diagnosis, even in the absence of histological findings of ABMR. Regardless of the type of TCMR, it is important to optimize maintenance immunosuppression, unless there are contraindications. This may involve targeting higher levels of tacrolimus or cyclosporine, adding prednisone if it is not already being used, and ensuring patients are on the highest tolerated dose of mycophenolate up to 2000 mg per day. If patients are not already taking mycophenolate, it should be added to their regimen or switched from azathioprine, if that is currently being used. Another key point to consider is the evaluation of nonadherence, as it is important to recognize that if nonadherence is identified, increasing the targets of immunosuppression may not be necessary. The guidelines for monitoring the response to therapy in TCMR lack consensus. However, various approaches can be employed, either individually or in combination, to assess the response. These include follow-up kidney function tests and follow-up biopsies.
Figure 1. Algorithm for management of T-Cell-Mediated Rejection.
3. Borderline Changes
Borderline changes in kidney transplant rejection refer to a spectrum of histological findings that lie between normal graft histology and acute cellular rejection (Table 1). Borderline changes are often characterized by mild inflammation, scattered infiltrating immune cells, and subtle signs of tissue damage. Although these changes do not meet the strict criteria for acute cellular rejection, they are considered significant because they indicate an ongoing immunological response against the transplanted organ.
The management of borderline changes typically involves optimizing immunosuppressive therapy if there are no contraindications. Additionally, most centers typically choose to address borderline rejection by administering a course of oral or IV steroids. This is due to the proven negative outcomes of borderline changes. For example, in a prospective study comparing the outcomes of patients with subclinical inflammation (Banff < 1A,
n = 129) to those without inflammation (
i + t = 0,
n = 71) on a 3-month protocol biopsy showed that the group with subclinical inflammation had higher serum creatinine levels (
p = 0.02) and chronicity scores on the follow-up biopsy at 12 months (
ci,
ct,
cv,
cg) (
p = 0.02), and a greater likelihood of developing de novo DSA and experiencing rejection episodes requiring treatment during the two-year follow-up period
[17].