
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aleksey Telin | -- | 2235 | 2023-08-08 15:43:06 | | | |
| 2 | Catherine Yang | -1 word(s) | 2234 | 2023-08-09 03:00:24 | | |
Video Upload Options
The use of hydrogels in the development of oil fields by the flooding method has firmly established itself in the oil industry, and this direction is constantly evolving in all oil-producing countries around the world, as evidenced by the growth of publication and patent activity on this topic. Operations for conformance control and flow diversion are impossible to imagine without the use of gel technologies today. Inorganic, organic, and hybrid gels, as well as foam gels, gel-forming and gel-dispersed systems are used. The ability to widely regulate structural-mechanical properties, thermal stability, and shear resistance through the introduction of micro- and nano-sized additives has made hydrogels indispensable tools for petroleum engineers.
1. Gels Based on Acrylamide Polymers
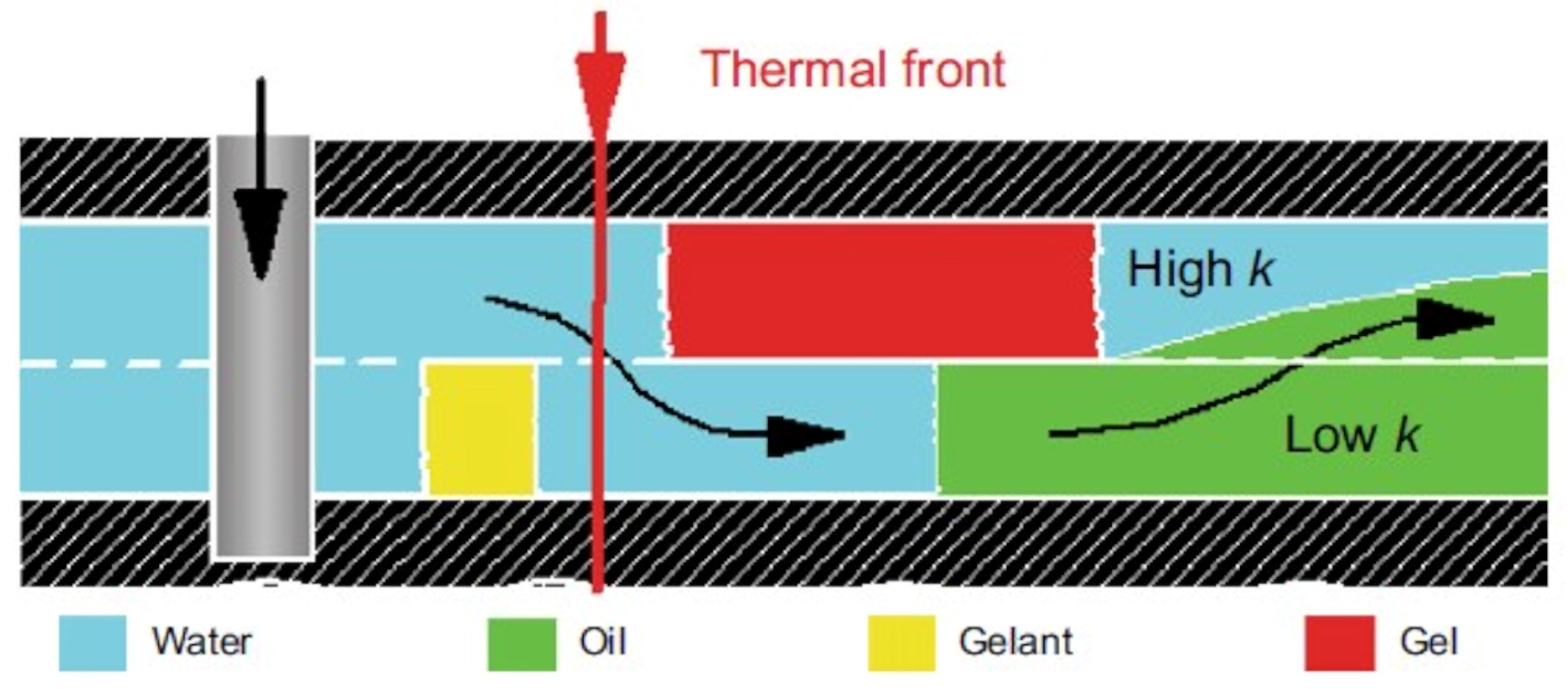
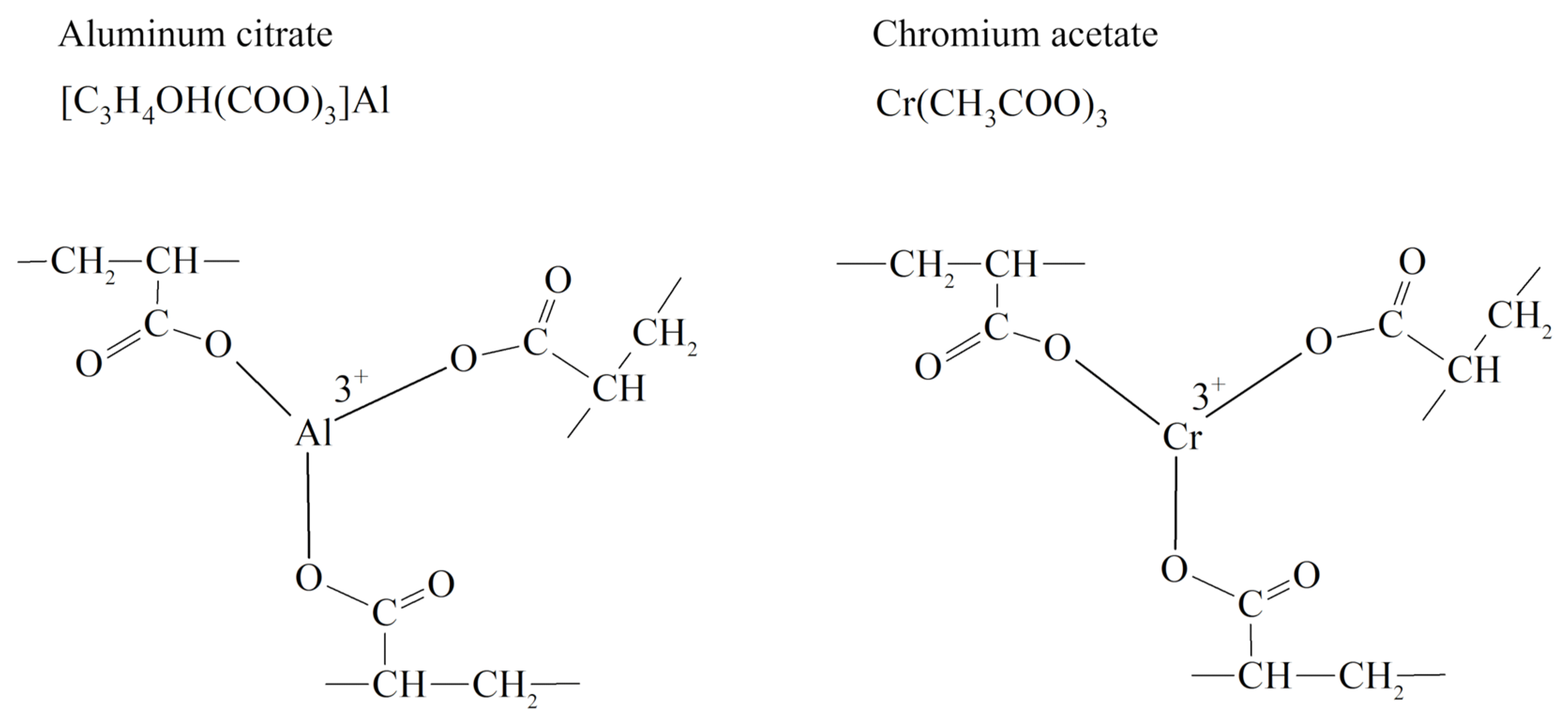
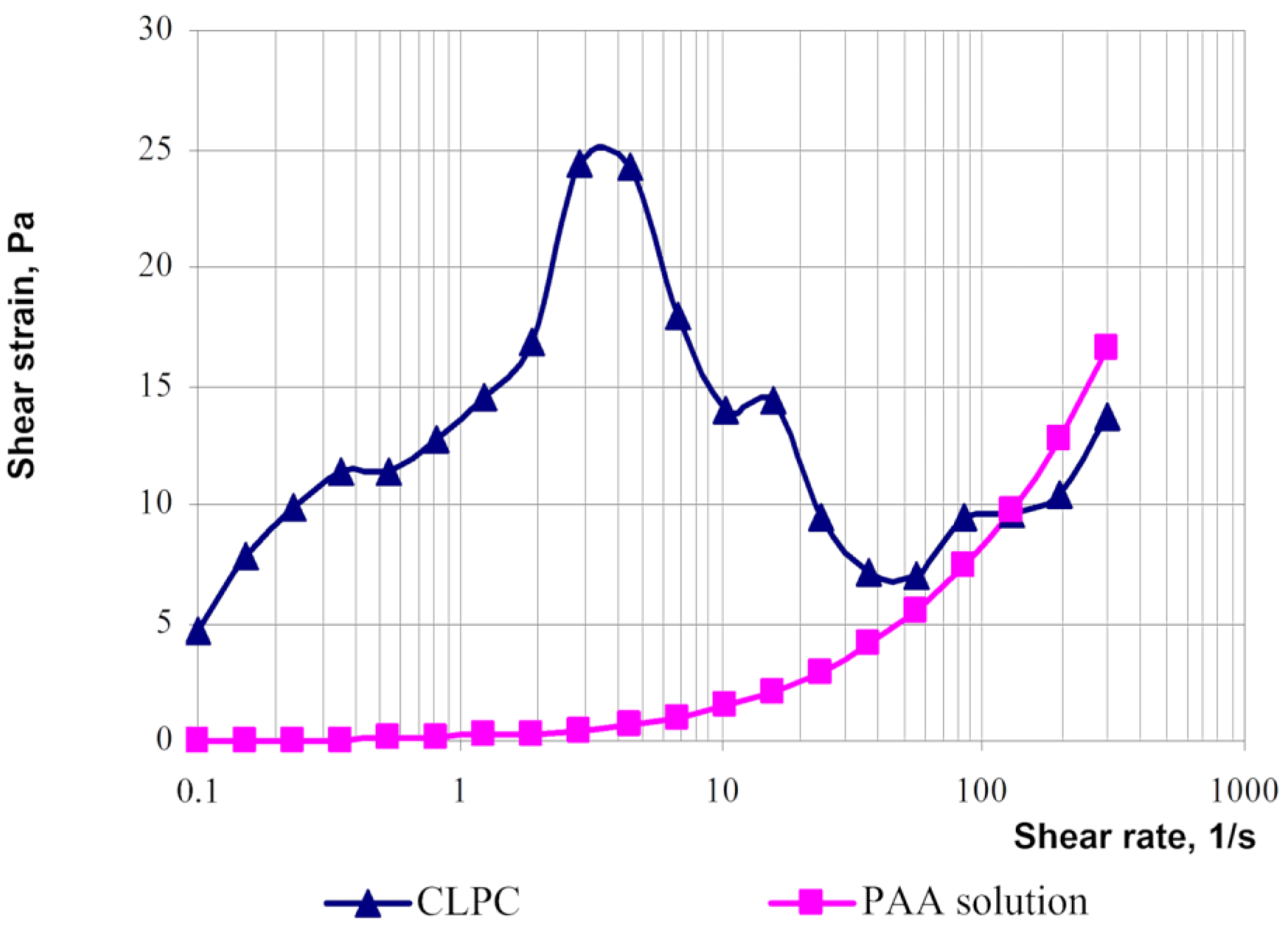
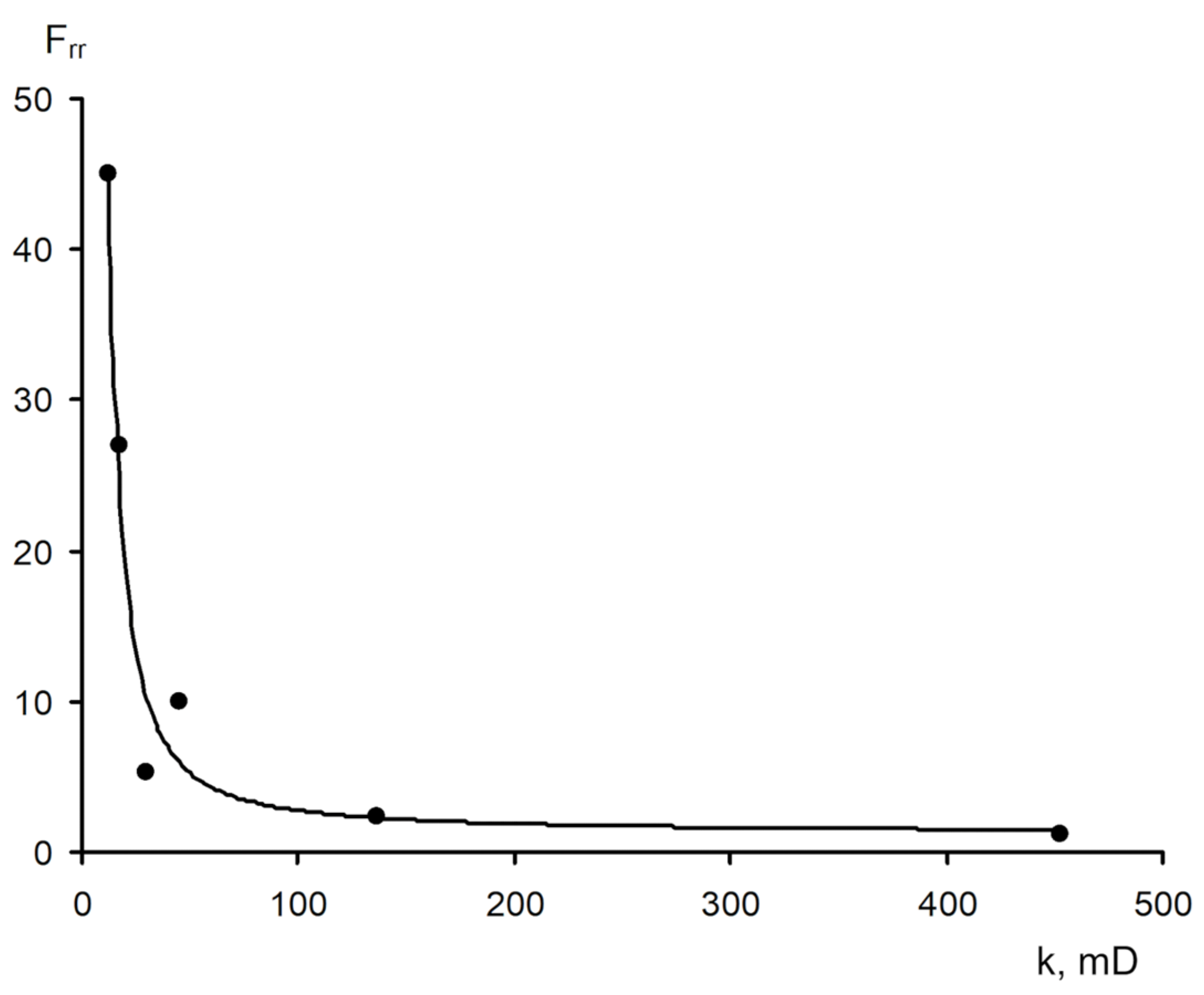
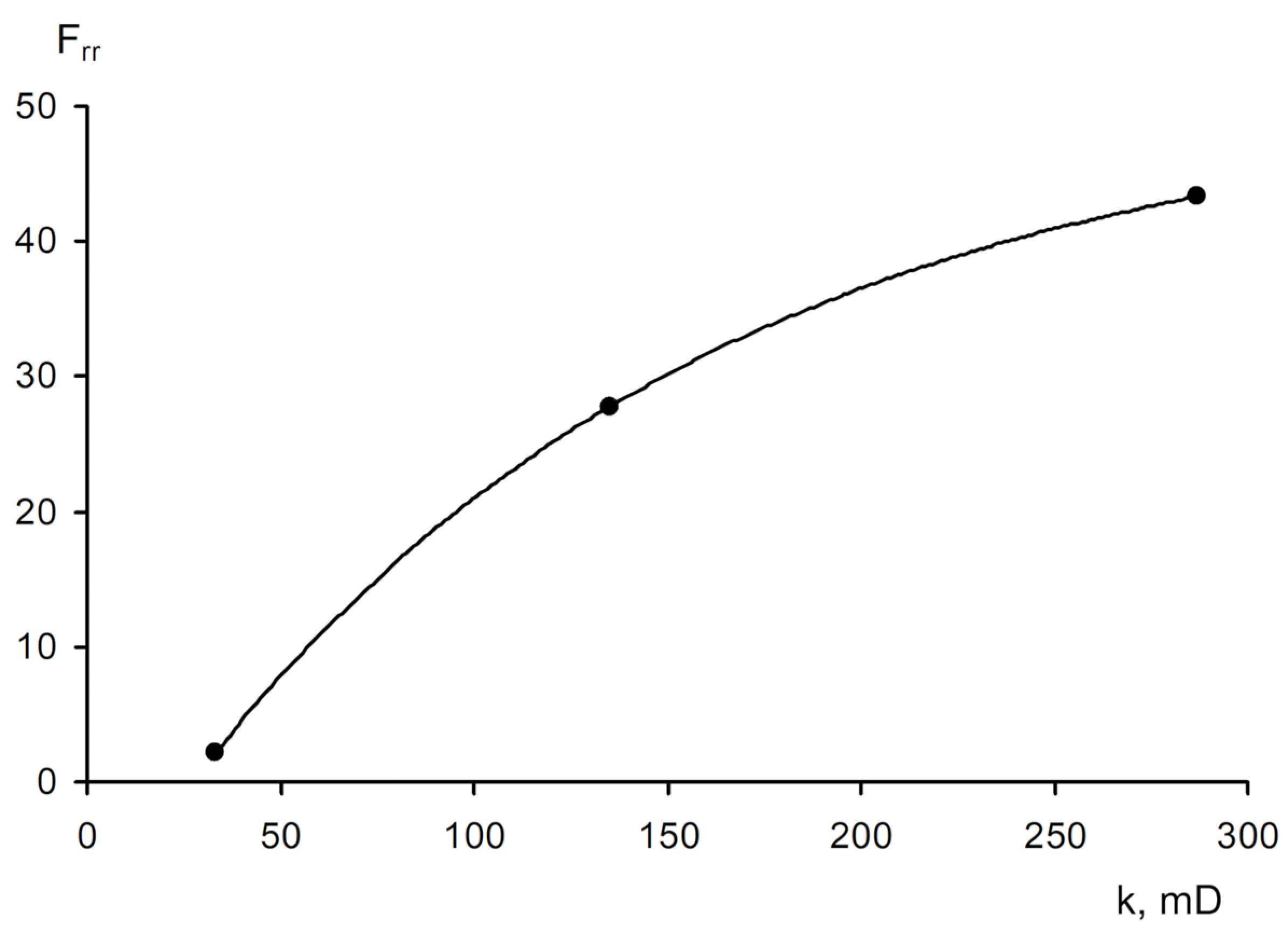
- −
-
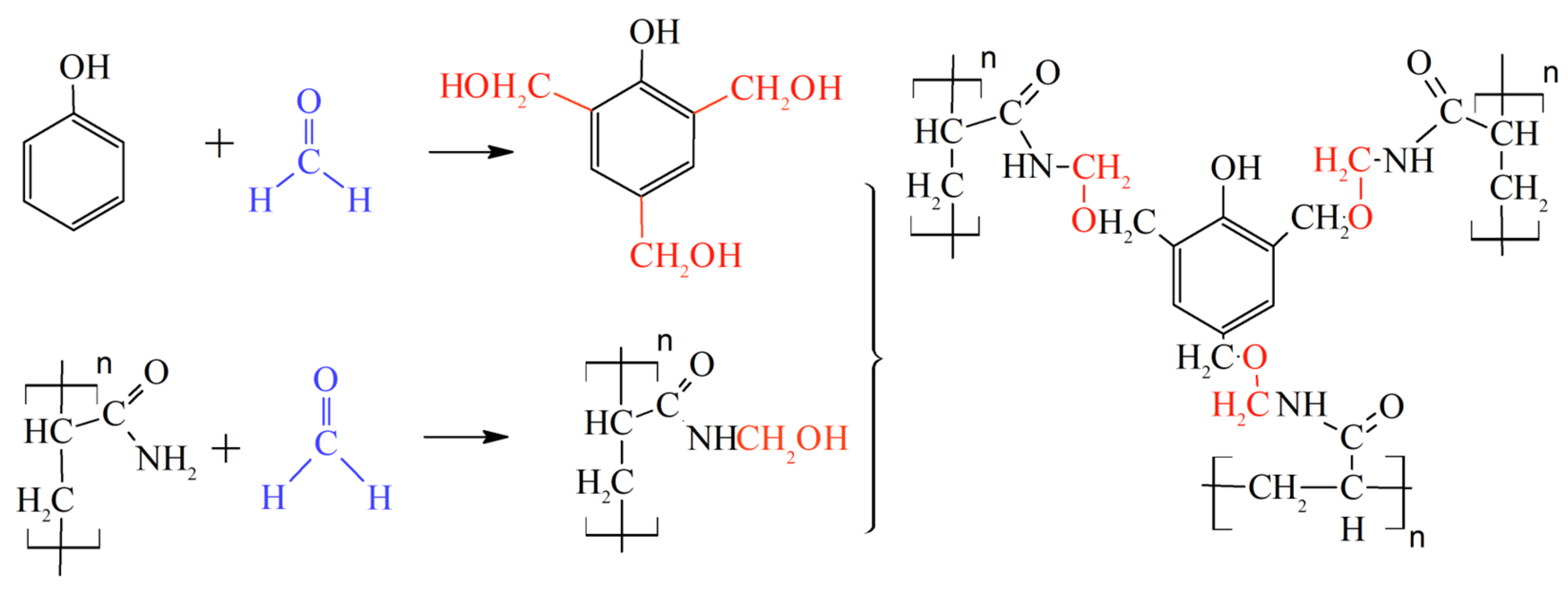
At the polymerization stage, the bifunctional monomer methylene bisacrylamide is introduced into the composition of acrylamide and acrylic acid monomers [13].
- −
-
Heat treatment of polyacrylamide at moderate temperatures when the cross-linking of macromolecules occurs as a result of the imidization reaction [13].
- −
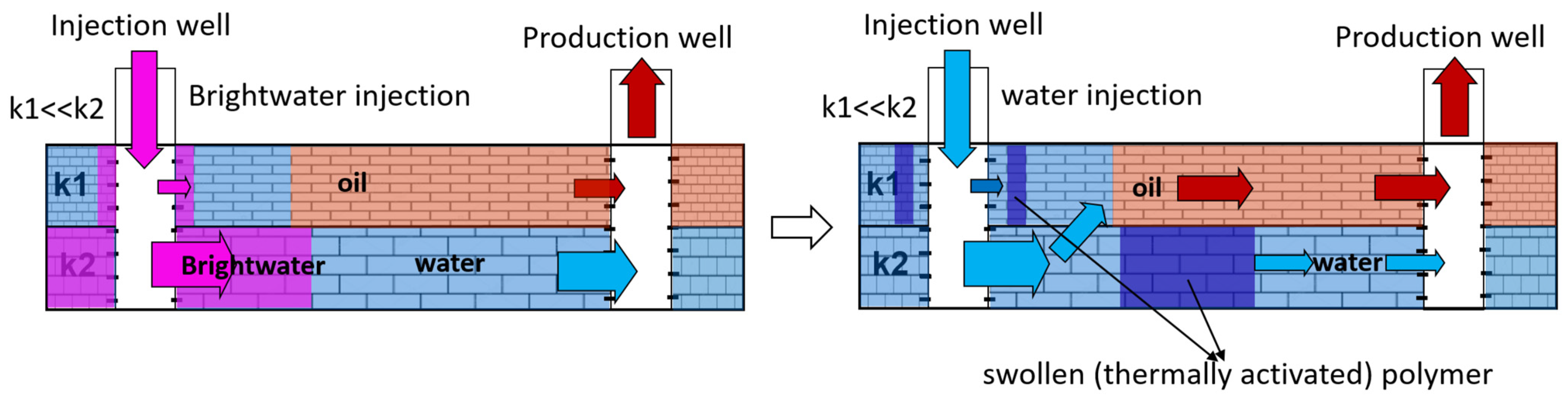
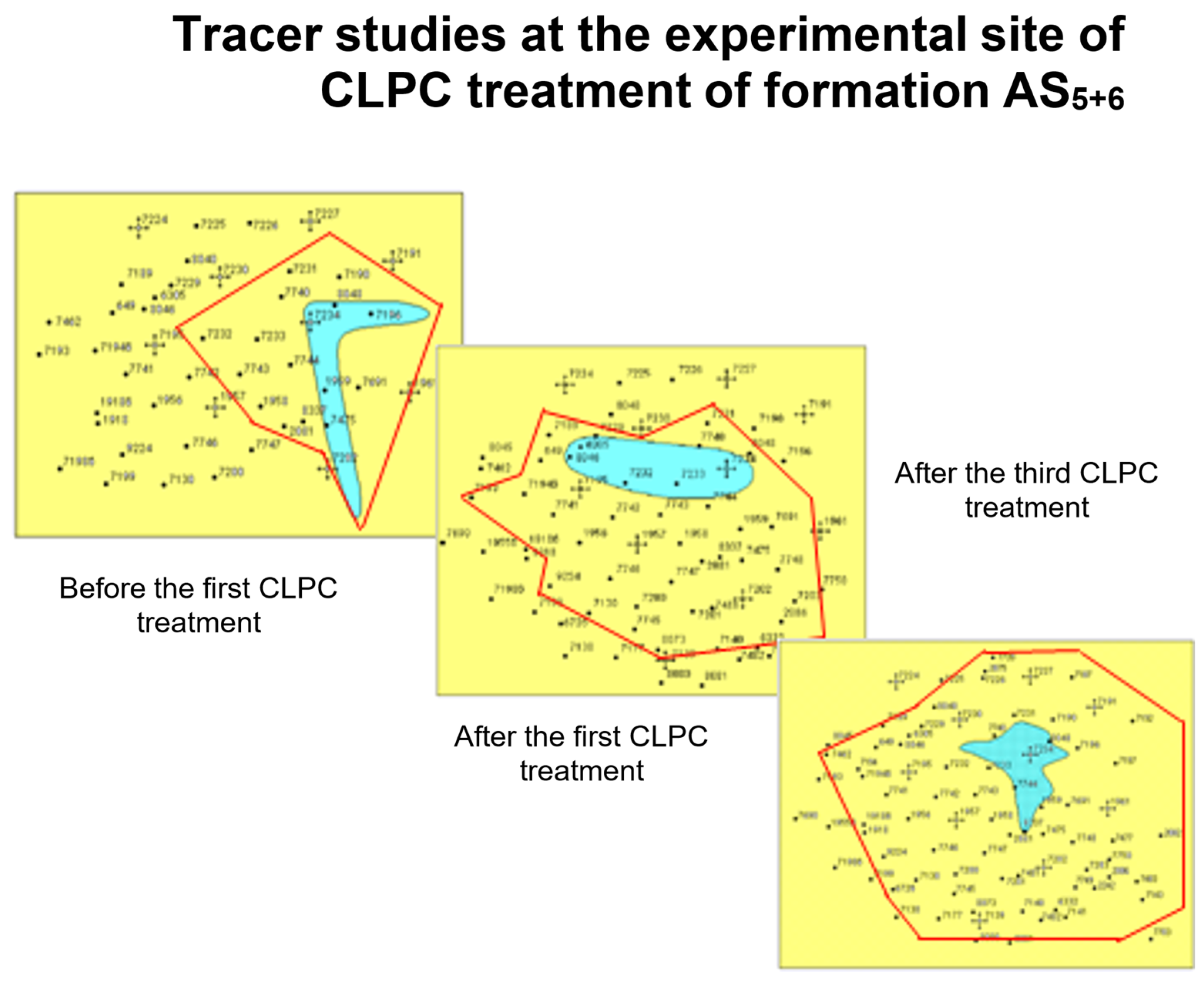
2. Gel-Dispersed and Sedimentary-Gel-Forming Compositions
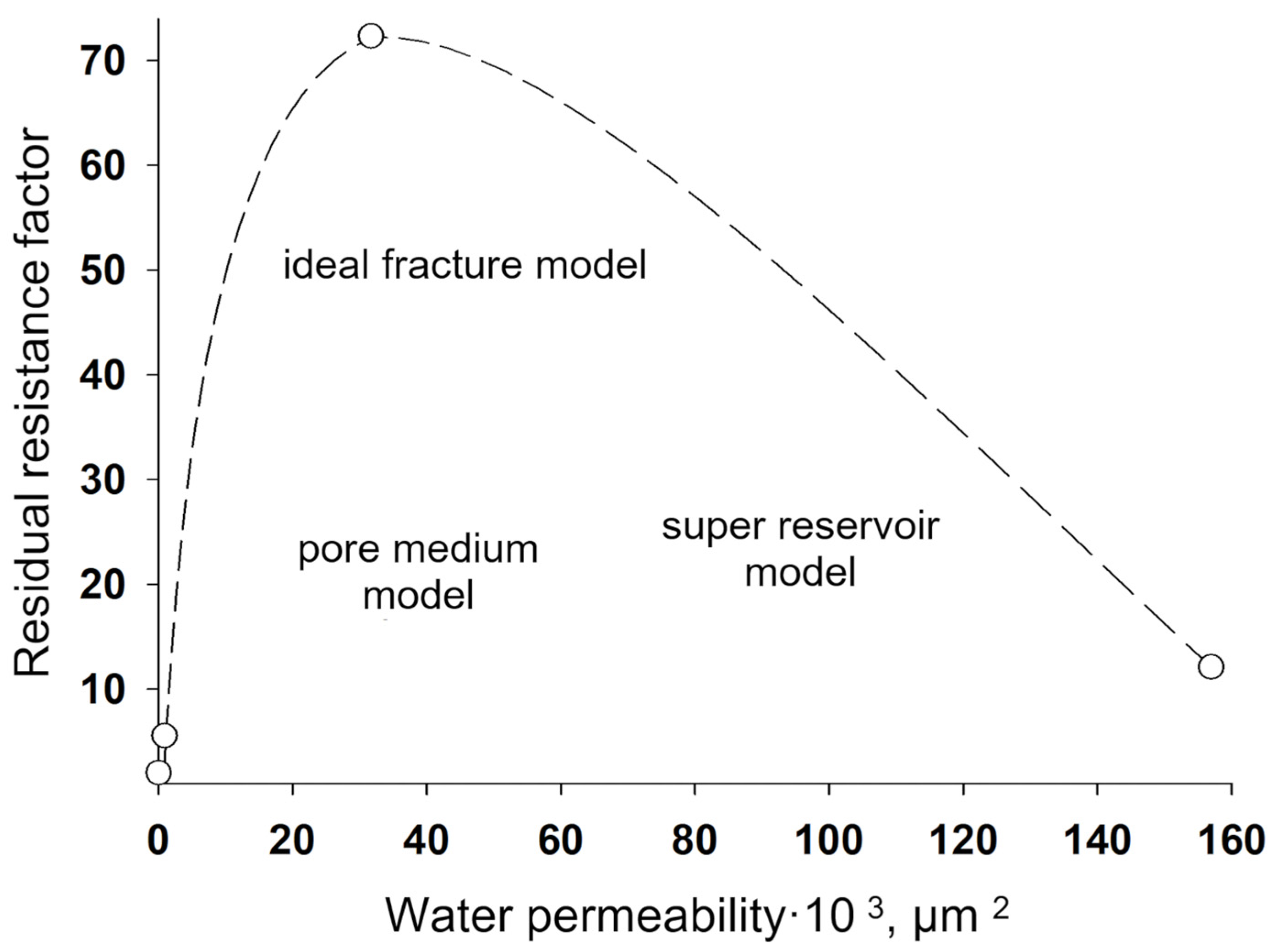
3. Gels Based on Inorganic Compounds
References
- Seright, R.; Brattekas, B. Water shutoff and conformance improvement: An introduction. Pet. Sci. 2021, 18, 450–478.
- Seright, R.S. Improved Methods for Water Shut Off; Annual Technical Progress Report; New Mexico Inst. of Mining and Technology, New Mexico Petroleum Recovery Research Center: Socorro, NM, USA, 1997.
- Gumerova, G.R.; Yarkeeva, N.R. Technology of application of crosslinked polymeric compositions. Oil Gas Bus. 2017, 2, 63–79.
- Zakharov, V.P.; Ismagilov, T.A.; Telin, A.G.; Silin, M.A. Regulation of Filtration Flows by Water-Isolating Technologies in the Development of Oil Fields; Gubkin Russian State University of Oil and Gas Publishing Center: Moscow, Russia, 2011.
- Khasanov, M.M.; Ismagilov, T.A.; Mangazeev, V.P.; Rastrogin, A.E.; Kolchugin, I.S.; Tyan, N.S. Application of cross-linked polymer-gel compositions for enhanced oil recovery. Oil Ind. 2002, 7, 110–112.
- Prada, A.; Civan, F.; Dalrymple, E.D. Evaluation of gelation systems for conformance control. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2000.
- Chen, Z. Polyacrylamide and Its Derivatives for Oil Recovery. Doctoral Dissertation, Missouri University of Science and Technology, Rolla, MO, USA, 2016. Available online: https://scholarsmine.mst.edu/doctoral_dissertations/2532 (accessed on 24 May 2023).
- Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Zhu, X.; Ran, Y.; Han, S. Ultrastable Hydrogel for Enhanced Oil Recovery Based on Double-Groups Cross-Linking. Energy Fuels 2015, 29, 7196–7203.
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087.
- Akhmetshin, I.D.; Popov, V.I.; Osenov, N.L.; Kolchugin, I.S.; Koscheev, I.G.; Revyakin, V.A.; Telin, A.G. Property of Deep Formation Treatment with Viscoelastic Composition. Russia Patent RU2125155C1, 20 January 1999.
- Mikhailov, A.V.; Rizvanov, R.Z.; Ganeeva, Z.M.; Abrosimova, N.N.; Elizarova, T.Y. Technology of increase of formation recovery at the late stage of oil field development with application of cross-linked cellulose ethers (DKM technology). Interval 2006, 12, 48–49.
- Ganiev, I.M.; Yakovlev, K.V.; Belykh, A.M.; Ismagilov, T.A. On specifics of using flow redirection technologies at late stages of fractured carbonate reservoirs development. Pet. Eng. 2020, 18, 51–60.
- Kurochkin, B.M.; Khisamov, R.S. Technology of carrying out insulating works with use of water-swelling polymer. Oil Ind. 2003, 1, 48–53.
- Kaushansky, D.A. Improvement of oil field development indicators by using polymer-gel systems “Temposcreen”. Geol. Geophys. Dev. Oil Gas Fields 2008, 7, 36–46.
- Kaushansky, D.A.; Demjanovsky, V.B.; Batyrbaev, M.D. Creation and industrial introduction of the technology of physical and chemical influence on productive formations of oil fields by the polymer-gel system “Temposcreen”—A technology of new generation. Oilfield Eng. 2006, 8, 28–37.
- Ismagilov, T.A.; Igdavletova, M.Z. Results of complex application of geological-technical actions and methods of oil recovery increase on BC10 horizon of Ust’-Balykskoye deposit. Oil Ind. 2005, 8, 72–75.
- Platov, A.I. Radiochemical technology for the oil industry. Oil Ind. 2007, 2, 70–71.
- Telin, A.G.; Svirsky, D.S.; Khalilov, L.M.; Remnev, G.E. Structural features of radiation crosslinking of sodium acrylamide-acrylate copolymer. Bashkir Chem. J. 2001, 3, 63–67.
- Telin, A.; Khlebnikova, M.; Singizova, V.; Kalimullina, G.; Khakimov, A.; Kolchugin, I.; Ismagilov, T. Regulation of rheological and filtration properties of cross-linked polymer systems in order to improve the efficiency of the impact on the reservoir. Bull. Yukos Eng. Cent. 2002, 4, 41–45.
- Telin, A.G.; Zaynetdinov, T.I.; Khlebnikova, M.E. Study of rheological properties of water swelling polyacrylamide of mark FS 305 for the development of technologies of water shut-off works in oil wells. Proc. Inst. Mech. UNTs RAS 2007, 4, 207–223.
- Ganiev, I.M.; Kalimullina, G.Z.; Mingalishev, F.K.; Valeev, R.F.; Belykh, A.M. Experience in using pre-crosslinked polymer systems for enhanced oil recovery in carbonate reservoirs. Pet. Eng. 2023, 21, 105–113.
- Pritchett, J.; Frampton, H.; Brinkman, J.; Cheung, S.; Morgan, J.; Chang, K.T.; Williams, D.; Goodgame, J. Field Application of a New In-Depth Waterflood Conformance Improvement Tool. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 20–21 October 2003; p. SPE-84897-MS.
- Frampton, H.; Morgan, J.C.; Cheung, S.K.; Munson, L.; Chang, K.T.; Williams, D. Development of a novel waterflood conformance control system. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2004; p. SPE-89391-MS.
- Matveev, S.; Gazizov, A.; Gazizov, A. Polymer Dispersed Systems for Conformance Improvement in Fractured Carbonate Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference; Abu Dhabi, United Arab Emirates, 9–12 November 2020.
- Zemtsov, Y.V.; Mazaev, V.V. The current state of physico-chemical methods of production enhancement. In Literary-Patent Review; Publisher Solutions: Ekaterinburg, Russia, 2021.
- Safarov, F.E.; Karazeev, D.V.; Vezhnin, S.A.; Ratner, A.A.; Koptyaeva, E.I.; Khasanova, G.I.; Telin, A.G. Development of compositions for leveling the injectivity profile of injection wells in conditions of low-permeability high-temperature reservoirs with a complex structure of the pore space. Oil Gas Innov. 2017, 4, 40–45.
- Lenchenkova, L.E. Enhancement of Oil Recovery by Physical and Chemical Methods; Nedra-Business-Center: Moscow, Russia, 1998.
- Ganeeva, Z.M.; Khisametdinov, N.A.; Khisametdinov, M.R.; Rizvanov, R.Z.; Musabirov, R. Kh Development of silica gel based EOR technologies in Tatneft OAO. Oil Ind. 2013, 8, 82–84.
- Ganeeva, Z.M.; Elizarova, T.Y.; Rizvanov, R.Z.; Mikhailov, A.V.; Khisametdinov, M.R. Application of sodium silicate-based dispersant systems to increase sweep efficiency. Oil Ind. 2011, 7, 33–36.
- Fahretdinov, R.N.; Enikeev, P.M.; Mukhametzyanova, R.S.; Rizvanova, Z.I. Prospects of application of gel-forming systems to enhance oil recovery at the late stage of field development. Oilfield Eng. 1994, 5, 12.
- Iler, R. The Chemistry of Silica; Mir: Moscow, Russia, 1982.
- Selimov, F.A.; Kononova, T.G.; Blinov, S.A. Gel compositions based on acidic aluminosilicate solutions. Interval 2003, 5, 8–40.
- Khlebnikov, V.N.; Lenchenkova, L.E. Kinetic regularity of gelation in hydrochloric acid aluminosilicate solutions. Bashkir Chem. J. 1998, 5, 18–21.
- Yakimenko, G.K.; Alvard, A.L.; Yagafarov, Y.N.; Stenichkin, Y.N. Application of gel-forming technology based on acidic aluminosilicate solutions. Oil Ind. 2005, 1, 64–66.
- Strizhnev, V.A.; Presnyakov, A.Y.; Nigmatullin, T.E.; Emaletdinova, L.D.; Elesin, V.A.; Urusov, S.A.; Zhumagaziev, E.T. Gel Composition. Russian Patent RU2472836C1, 20 January 2013.
- Akchurin, H.I.; Nasryev, A.M.; Lenchenkov, N.S.; Lenchenkova, L.E. Experemental tests blocking out technology in highly watered layers by using gelforming composition “CAS”. Oil Gas Bus. 2014, 5, 71–90.
- Rogachev, M.K.; Lenchenkov, N.S.; Lenchenkova, L.E. Laboratory investigation of gelling composition physico-chemical properties on aluminum silicate bas for oil recovery technology. Pet. Eng. 2009, 7, 168–171.
- Badalyants, G.A.; Buchenkov, L.N.; Rogova, T.S.; Starkovsky, A.V. Study of the possibility and efficiency of using silicate-polymer gels for formation isolation. Proc. VNIIneft 1993, 116, 49–58.
- Sermyagin, K.V.; Khlebnikova, M.E.; Singizova, V.H.; Telin, A.G. Mineral and mineral-polymer water-insulating compositions. Interval 2001, 3, 5–10.
- Altunina, L.K.; Kuvshinov, V.A. Physicochemical methods for enhancing oil recovery from oil fields. Russ. Chem. Rev. 2007, 76, 971–987.
- Altunina, L.K.; Kuvshinov, V.A.; Stas’eva, L.A. Enhancement of coverage of high-viscosity oil reservoirs by means of gels. Interval 2001, 1, 18–20.
- Altunina, L.K.; Kuvshinov, V.A.; Selimov, F.A. Gel-technologies for increasing the coverage of high-viscosity oil reservoirs. Interval 2001, 1, 9–11.
- Kuvshinov, V.A.; Altunina, L.K.; Shevlyuk, V.V. Application of the gel-forming composition “Label” for elimination of behind-the-casing water overflow in the gas production well of the Myldzhinskoye gas condensate field. Interval 2003, 2, 72–73.
- Kuvshinov, I.V.; Altunina, L.K. Thermotropic gel-forming compositions for elimination of steam breakthroughs in horizontal steam injection wells. Oil Gas Innov. 2023, 1, 62–66.





