Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bashar Saad | -- | 2850 | 2023-08-08 13:06:29 | | | |
| 2 | Alfred Zheng | Meta information modification | 2850 | 2023-08-09 03:39:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Saad, B. The Molecular Mechanisms of Anti-Obesity Medicinal Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/47789 (accessed on 07 February 2026).
Saad B. The Molecular Mechanisms of Anti-Obesity Medicinal Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/47789. Accessed February 07, 2026.
Saad, Bashar. "The Molecular Mechanisms of Anti-Obesity Medicinal Plants" Encyclopedia, https://encyclopedia.pub/entry/47789 (accessed February 07, 2026).
Saad, B. (2023, August 08). The Molecular Mechanisms of Anti-Obesity Medicinal Plants. In Encyclopedia. https://encyclopedia.pub/entry/47789
Saad, Bashar. "The Molecular Mechanisms of Anti-Obesity Medicinal Plants." Encyclopedia. Web. 08 August, 2023.
Copy Citation
Inflammation is a crucial factor in the development and progression of cardiovascular diseases (CVD). Cardiac remodeling in the presence of persistent inflammation leads to myocardial fibrosis and extracellular matrix changes, which reduce cardiac function, induce arrhythmias, and finally, cause heart failure. Medicinal plants and phytochemicals can cure and prevent obesity and inflammation. In comparison to conventional therapies, the synergistic effects of several phytochemicals boost their bioavailability and impact numerous cellular and molecular targets.
inflammation
obesity
diabetes
CVDs
1. Introduction
Inflammation is a physiological response of the body to injury or infection, representing a universal reaction to various local tissue changes [1][2]. It is characterized by a set of standard vascular changes that result in swelling, followed by the movement of white blood cells to the affected area, forming an inflammatory focus [3]. The primary goal of inflammation is to identify the damaging element, remove it, and then regenerate or repair the affected tissue [4].
Cytokines are important signaling proteins involved in the inflammatory host response to inflammatory stimuli. They are classified as either pro-inflammatory (e.g., tumor necrosis factor-alpha (TNF-α), interleukine (IL)-1 (IL-1), IL-6, IL-15, IL-17, and IL-23), or anti-inflammatory (e.g., transforming growth factor beta(TGFβ), and interferon (IFN), IL-4, IL-10, and IL-13) [5]. Chronic and persistent inflammation, as well as auto-immune response, have been linked to atherosclerosis, myocardial infarction, asthma, diabetes, psoriasis, osteoporosis, angiotensin II-derived hypertension, tumor progression, and cardiovascular disease (CVD) [1][2][6][7]. The latter, the world’s largest cause of morbidity and mortality, includes hypertension, heart diseases, peripheral vascular disease, heart failure, and cardiomyopathies [6][7].
Because the number and kinds of secondary metabolites in a single herb vary [8], the pharmacological effects of herbs are specific to certain plant species or groups of plants. Secondary metabolites do not play a part in normal cell/tissue growth, development, and reproduction; rather, they serve as a defensive factor to protect a plant from potential environmental and interspecies injury. As a result, secondary metabolites are often produced by plants to regulate their metabolism in response to the local presence of herbivores, pollinators, and microorganisms. These metabolites are often derived from primary metabolites or share substrates with them and are produced to meet specific needs. Thousands of these secondary metabolites have been found, and the list is growing. Secondary metabolites are broadly classed as alkaloids, terpenoids, and phenolics [8][9][10] (Figure 1).
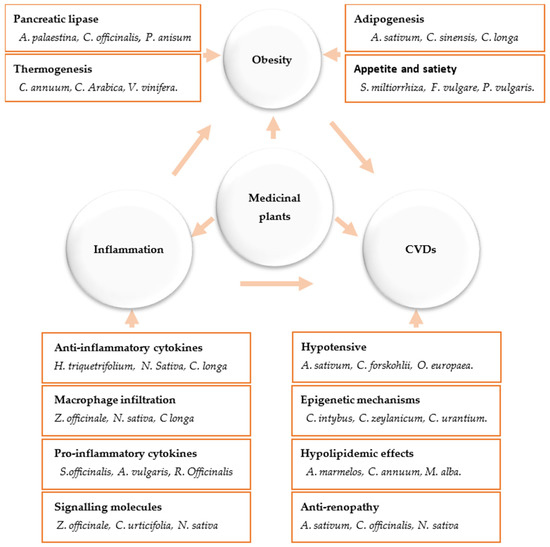
Figure 1. Anti-obesity and anti-inflammatory action mechanisms of medicinal plants and their impact on cardiovascular diseases.
2. Anti-Obesity Medicinal Plants and the Molecular Mechanisms Underlying Their Activities
Currently, medicinal plants, unprocessed extracts, and phytochemicals that aim to regulate weight are becoming more widespread. Significant decrease in body weight was seen by Nigella sativa (black seeds), Curcuma longa (curcumin), Cinnamomum verum (cinnamon), Panax ginseng (ginseng), Foeniculum vulgare, Phaseolus vulgaris, Camellia sinensis (green tea), Allium sativum (garlic), Crocus sativus, and virgin olive oil. These plants have been used in various traditional medical systems for a long time, and their anti-obesity and antidiabetic effects have been confirmed in numerous in vitro animal and clinical testing [11][12][13][14][15].
There has been a lot of progress in understanding how phytochemicals can reduce weight, according to a number of lines of evidence. One of the main categories of plant secondary metabolites is polyphenols, which are abundantly present in medicinal plants as well as in fruits, vegetables, grains, and legumes [16]. Their anti-obesity abilities have been repeatedly demonstrated in cell culture, animal, and clinical research. Examples of substances that have been shown to have weight-reducing properties include genistein and diadzein, cyanidin, grape seed proanthocyanidin extract, xanthohumol, apigenin and luteolin, kaempferol, myricetin and quercetin, and (-)-Epigallo-cathechin gallate (EGCG). Likewise, phytosterols, polyunsaturated fatty acids (PUFA), and organosulfur compounds are other bioactive dietary ingredients having anti-obesity characteristics [17][18][19][20][21][22][23][24][25][26][27][28][29][30][31]. The primary goals of medicinal plants and the products generated from them include inhibition of pancreatic lipase activity, suppression of hunger, stimulation of thermogenesis and lipid metabolism, prevention of lipolysis, and promotion of adipogenesis (Figure 2). A better treatment method for treating obesity is to target adipogenesis in adipose tissue. With weight reduction, mature adipocytes undergo apoptosis and/or lipolysis, which results in a decrease in adipose tissue mass [17][18] (Figure 2).
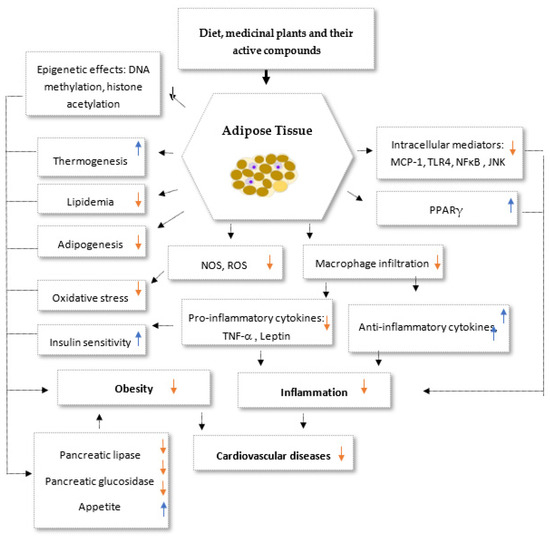
Figure 2. Potential synergistic anti-obesity molecular multi-target of medicinal plants and their secondary metabolites. PPARγ: Peroxisome proliferator-activated receptors gamma, NOS: nitric oxide synthase, ROS: Reactive oxygen species, TNF-α: Tumor necrosis factor alpha, TLR4: Toll-like receptor 4, MCP-1: Monocyte Chemoattractant Protei-1, NF-κB: Nuclear Factor-κB. Up arrows: incresesed effects; down arrows: decresed effects.
2.1. Major Basic Anti-Obesity Mechanisms
Targeting appetite: Some anti-obesity phytochemicals that act through appetite reduction include thymoquinone from Nigella sativa, saponins from ginseng, HCA from Garcinia cambogia, EGCG from green tea, steroidal glycoside from Hoodia gordonii and Hoodia Pilifera, lectins from Phaseolus vulgaris (common bean), and ephedrine from Ephedra species [14][15][18][32]. Appetite and satiety are regulated by a coordinated action of anorexigenic and orexigenic hormones and neuropeptides produced in the hypothalamus as well as in the digestive tract, liver, and adipose tissue [32]. In the short term, neuronal and hormonal transmission from the gastrointestinal tract regulates hunger. The gastrointestinal tract is the biggest endocrine organ in the body and is thought to play a significant role in appetite control through the release of multiple peptide hormones. As a result, these hormones (e.g., ghrelin, melanin-concentrating hormone receptor) might be used to treat obesity. In addition, the inhibition of fatty acid synthase, an adipocyte enzyme, catalyzes the synthesis of fatty acids and represents a potential therapeutic target for appetite suppression. Indeed, many medicinal herbs and their extracts were reported to exert their weight-reducing effect through the inhibition of fatty acid synthase activity and, thus, reduce appetite [17][32].
Targeting pancreatic lipase: Inhibition of fat digestion and absorption is one of the currently used strategies to reduce energy intake through inhibition of the activity of the pancreatic lipase. It is responsible for the hydrolysis of 50–70% of total dietary fats to glycerol and free fatty acids. The latter are then incorporated into bile acid-phospholipid micelles, absorbed in the small intestine, and finally entered the peripheral circulation as chylomicrons [33]. Pancreatic lipase inhibitory effects of medicinal plants and phytochemicals have been extensively investigated. Hitherto, many herbs, as well as isolated phytochemicals, were found to attenuate pancreatic lipase activity. Some phytochemicals that can help reduce weight by inhibiting pancreatic lipase include saponins and catechins from green tea, polyphenols like mangiferin and catechins and condensed tannins from Salacia reticulate, punicalagin, ellagic acid, and anthocyanins from pomegranate, rosmarinic acid and carnosic acid from rosemary, and proteins and isoflavones from soybean [14][15][33][34].
Stimulators of thermogenesis: Thermogenesis is literally defined as heat production. It takes place in the mitochondria of the brown adipose tissue through uncoupling of the mitochondrial electron transport chain by uncoupling protein 1 (UCP1). The “adaptive thermogenesis” that maintains an ideal environment for weight recovery and is active in both lean and obese persons attempting to sustain reduced body weights is what causes the over 80% weight regain to pre-weight loss following a successful weight loss. The adipocyte-derived hormone leptin has a significant role in mediating this resistance to long-term weight loss. Thermogenesis is influenced by phytochemicals and macromolecules in food as well as dietary carbohydrates. Numerous animal and human studies have demonstrated the ability of several phytochemicals to reduce body weight, including oleuropein, capsaicin, resveratrol, epigallocatechin gallate, gingerol, caffeine, and ephedrine [14][15][18][34][35]. Capsaicin, for example, increases catecholamine release from the adrenal medulla to exert its thermogenic action, whereas EGCG promotes thermogenesis by inhibiting catechol methyl-transferase, an enzyme that destroys norepinephrine. Caffeine mediates thermogenic effects by inhibiting the phosphodiesterase-induced degradation of intracellular cAMP, and it decreases energy intake by reducing food intake [14][15][18][34][35].
Increase satiety: Long-term studies have established the favorable weight-loss effects of dietary fiber [36] in obese people, and they are related to a lower BMI. Many entire plant meals include substantial levels of dietary fiber, including pectin, gum, mucilage, cellulose, hemicellulose, lignin, and soluble fibers. Supplementing regular meals with gel-forming fibers, such as guar gum, increases satiation, most likely owing to delayed stomach emptying. Dietary fibers are normally indigestible by humans; however, they may be fermented by gut microorganisms. Soluble or fermentable fibers and insoluble fibers can both be fermented by gut microorganisms to give bulk. Natural hydrogel-forming fibers such as pectin, gum, and mucilage are examples of soluble fibers, whereas structural fibers such as cellulose and lignin are examples of insoluble fibers. Through the hydrogel effect, which slows the absorption of energy-rich macromolecules, insoluble fibers are known to reduce hunger and, thereby, meal intake [14][35][36]. A high-fiber diet decreases both food intake during meals and food intake at the next meal, according to a number of clinical studies. Foods high in pectin cause the stomach to take longer to empty and make you feel fuller [14][35][36]. Recent studies have found a connection between satiation and changes in the hormones orexigenic and anorexigenic. People believe that systematic analyses of the key gut hormone responses to diverse fiber types and formulations will significantly advance our knowledge of this subject. While leaving the HDL fraction alone, hydrogel-forming fibers are particularly effective at lowering high LDL cholesterol. Additionally, they support those with diabetes or impaired glucose tolerance. These effects are most likely connected to the fiber’s gelling characteristic, which slows the absorption process [17].
2.2. Targeting Adipocyte Apoptosis
Antiobesity medicinal plants and phytochemicals can reduce the mass of adipose tissue by apoptosis. At the cellular level, obesity is characterized by an increase in the number or size or both of the adipocytes. The adipocyte life cycle consists of alteration of cell shape and growth arrest, clonal expansion, and a complex sequence of changes in gene expression leading to storage of lipids and finally to apoptosis [14][17][37]. During the terminal phase of adipocyte maturation, mRNA levels for enzymes involved in triacylglycerol metabolism (e.g., glyceraldehyde-3-phosphate dehydrogenase, fatty acid synthase, and glycerol-3-phosphate dehydrogenase) rise significantly. While it was previously thought that the number of fat cells in the body stays the same throughout a person’s life, recent evidence suggests that new fat cells can be created and old ones can be removed through a process called apoptosis [17][37].
Targeting preadipocyte apoptosis: Natural compounds that control the adipocyte life cycle have as their main target preadipocyte maturation [38]. It has been demonstrated that several phytochemicals can increase apoptosis and reduce preadipocyte growth. For instance, it has been discovered that the antioxidant activity of flavonoids is related to the triggering of apoptosis in preadipocytes. It has been demonstrated that a number of flavonoids, including naringenin, rutin, hesperidin, resveratrol, naringin, and genistein, have cytostatic effects on preadipocytes. One of the most current flavonoids, quercetin is present in a variety of fruits and vegetables; the green tea polyphenol EGCG, and capsaicin from paprika have been shown to induce preadipocyte apoptosis. Induction of apoptosis in preadipocytes is intermediated by the activation of caspase-3, Bax, and Bak, followed by fractionalization of PARP and downregulation of Bcl- 2. Likewise, treatment of preadipocytes with phenolic acids similar to o- coumaric acid, m- coumaric acid, and chlorogenic acid redounded in cell cycle arrest in the G1 phase in a time- and cure-dependent manner. CLA has recently been found to induce apoptosis in human preadipocytes. During insulin therapy, EGCG also promotes apoptosis in post-confluent premature preadipocytes, although the molecular pathways involved remain unclear. Induction of apoptosis in post-confluent differentiated cells leads to a decrease in the number of adipocytes, as preadipocytes undergo multiple rounds of replication during the first two days of differentiation [17][38][39][40][41][42][43][44][45][46][47][48][49]. The effect of curcumin on the cell cycle was recently determined. It significantly decreased the viability of preadipocytes at a concentration that caused apoptosis in preadipocytes and induced activation of caspases 8, 9, and 3. A non-cytotoxic dose of curcumin (15 µM) inhibited MCE, attenuated the expression of PPARγ and C/EBPα, prevented differentiation medium-induced β-catenin downregulation, and decreased the lipid accumulation in 3T3-L1 adipocytes. These data show that curcumin can induce preadipocyte apoptosis and attenuate adipocyte differentiation, leading to inhibition of adipogenesis [17][38].
Targeting adipocyte apoptosis: Animal studies have shown that EGCG, genistein, capsaicin, soy isoflavones, and CLA reduce body fat. However, the mechanism by which they exert their apoptosis-inducing effects on adipocytes has only recently been investigated. EGCG-induced apoptosis is thought to be mediated by protein 1, nuclear factor kappa B (NF-kB), p53, and caspase-3 activity. Although the effects of conjugated linoleic acid (CLA) on body fat are not fully understood, the marked increase in TNF-α mRNA observed after treatment of adipocytes with uncoupling protein 2 (UCP2) suggests that CLA-induced suspected to be responsible for apoptosis [17][45][46][47][48][49]. Genistein, ajoene, EGCG, and capsaicin exert apoptotic effects by stimulating the release of reactive oxygen species (ROS), thereby activating AMP-activated protein kinase (AMPK), an important target for anti-obesity therapy. Ajoene also induces apoptosis in leukemia cells through the formation of ROS, and more recently, it was shown that ajoene also induces ROS-mediated apoptosis in adipocytes [17][46][47][48][49][50][51][52][53].
Synergistic apoptosis-inducing effects of phytochemicals: The apoptosis regulator Bcl-2 and its homologs, which control the outer mitochondrial membrane, are members of the Bcl-2 family. They are either anti-apoptotic (Bcl-2, Bcl-xL, Bcl-w, etc.) or pro-apoptotic (particularly Bax, Bak, Bok, etc.). The Bcl-2 family of proteins induces the release of cytochrome c and translocates from the cytosol to the mitochondria, where it displays pro-apoptotic action. In 3T3-L1 adipocytes, CLA promotes apoptosis brought on by ajoene. Individually, neither CLA nor ajoene had an impact on cytochrome c, whereas ajoene enhanced and CLA had no impact on the expression of the Bax protein [15][54][55]. However, the expression of cytochrome c and Bax was enhanced when ajoene and CLA were combined. Similarly to this, vitamin D3 increases genistein’s ability to induce apoptosis and prevent adipogenesis in mature 3T3-L1 preadipocytes. Vitamin D3 alone boosted VDR protein levels in 3T3-L1 adipocytes by only 40%, while genistein alone did not enhance these levels at the dosages studied. In contrast, the combination of genistein and vitamin D3 elevated VDR protein levels by more than 100%. With combination therapy, this impact on VDR was associated with a roughly 200% increase in apoptosis. The results of such synergy point to the possibility of reducing the harmful effects of two or more chemicals while still producing desired effects on adipocytes. Despite the fact that outcomes from in vitro and animal experiments cannot be directly extrapolated to clinical efficacy, such studies indicate that selected phytochemicals, alone or in combination, are widely used to treat obesity through adipocyte apoptosis contribute to our understanding of important molecular and cellular pathways. It may be effective in inhibiting adipogenesis [17][54][55][56].
2.3. Targeting of Adipogenesis
Adipogenesis is reduced by medicinal herbs and phytochemicals such as resveratrol, guggulsterone, CLA, capsaicin, baicalein, EGCG, genistein, esculetin, DHA, berberine, and procyanidins. Capsaicin, genistein, berberine, and EGCG inhibited the synthesis of CCAAT/enhancer-binding protein (C/EBP) and peroxisome proliferator-activated receptor (PPAR). These transcription factors have been implicated in the suppression of preadipocyte proliferation, which is essential for adipocyte differentiation. Resveratrol, for example, has been found to decrease adipogenesis by enhancing the expression of the Sirt1 gene, which activates fat mobilization by suppressing PPARγ. Capsaicin, genistein, and EGCG have been shown to inhibit adipocyte differentiation through activation of AMPK [17][37][57]. At the same time, PUFA has been shown to suppress adipogenesis by lowering the expression of sterol-regulatory element-binding proteins and slowing the late phases of adipocyte differentiation. Many cell lines have recently been proven in vitro to undergo lipogenic differentiation into adipocytes. The 3T3-L1 preadipocyte is a well-studied biological model for understanding the adipogenesis process [58][59][60]. To assess the effect of test plant extracts on 3T3-L1 cell adipogenesis, 3T3-L1 preadipocytes are differentiated into mature adipocytes in the presence of different concentrations of the test plants (0–2 days), and the treatment is maintained for a total of 10 days [15][61][62]. Grape seed, green tea, Allium sativum, Camellia sinensis, Capsicum annuum, Curcuma longa, Ginkgo biloba, Olea europea, Mentha longifolia, Alchemilla vulgaris, Vitis vinifera, and Salvia officinalis extracts [15][61][62] have been reported to reduce adipogenesis in the 3T3-L1 cell line. Salvia officinalis leaves act through synergistic mechanisms to reduce the weight of overweight and obese patients [13]. Further research is needed to determine if combining these extracts could create a more effective way to suppress the formation of fat cells. Adipogenesis, the process of creating new fat cells, is controlled by adipogenic signaling, which activates transcriptional activators, mainly from the PPAR and C/EBP families, to regulate fat cell differentiation and fat storage within cells [58][59]. During the final stages of preadipocyte development, PPAR and C/EBP coordinate the activation of various adipogenic gene products, including ADRP, aP2, CD36, and perilipin. These gene products work together to produce the adipocyte phenotype [58][59].
Lipolysis: Lipolysis in adipocytes and the release of free fatty acids play a major role in regulating energy homeostasis. Hormone-sensitive lipase is the key enzyme that catalyzes lipolysis in adipocytes. Several medicinal plants and phytochemicals can also affect lipolysis [63][64]. Flavonoids genistein, diadzein, coumestrol, and zearalenone stimulate lipolysis in a dose-dependent manner. Quercetin, luteolin, and fisetin increase lipolysis in rat adipocytes in a dosage and time-dependent manner that was synergistic with epinephrine [65][66]. It has also been observed that these flavonoids inhibit phosphodiesterase. Proanthocyanidins produced from grape seeds promote long-term lipolysis in 3T3-L1 adipocytes through elevating cAMP and protein kinase A. In human adipocytes and 3T3-L1 adipocytes, conjugated linoleic acid was observed to increase basal lipolysis. When docosahexaenoic acid (an omega-3 fatty acid) is introduced to mature adipocytes, it also induces lipolysis [17][67][68][69][70].
References
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596.
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–nature’s way to efficiently respond to all types of challenges: Implications for understanding and managing “the epidemic” of chronic diseases. Front. Med. 2018, 5, 316.
- Granger, D.N.; Senchenkova, E. Inflammation and the Microcirculation; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010.
- Plytycz, B.; Seljelid, R. From inflammation to sickness: Historical perspective. Arch. Immunol. Et Ther. Exp. 2003, 51, 105–109.
- Cucu, I. Signaling Pathways in Inflammation and Cardiovascular Diseases: An Update of Therapeutic Strategies. Immuno 2022, 2, 630–650.
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S.
- Rana, M.N.; Neeland, I.J. Adipose Tissue Inflammation and Cardiovascular Disease: An Update. Curr. Diabetes Rep. 2022, 22, 27–37.
- Fakhri, S.; Moradi, S.Z.; Ash-Rafzadeh, A.; Bishayee, A. Targeting cellular senescence in cancer by plant secondary metabolites: A systematic review. Pharmacol. Res. 2021, 177, 105961.
- Kashyap, A.K.; Dubey, S.K.; Shah, S.; Kumar, A. A Short Review on Genes Regulating Biosynthesis of Major Secondary. Metabolites 2022, 1, 501–519.
- Ain, Q.-U.; Khan, H.; Mubarak, M.S.; Pervaiz, A. Plant alkaloids as antiplatelet agent: Drugs of the future in the light of recent developments. Front. Pharmacol. 2016, 7, 292.
- Saad, B. Prevention and Treatment of Obesity-Related Inflammatory Diseases by Edible and Medicinal Plants and Their Active Compounds. Immuno 2022, 2, 609–629.
- Hasani-Ranjbar, S.; Jouyandeh, Z.; Abdollahi, M. A systematic review of anti-obesity medicinal plants-An update. J. Diabetes Metab. Disord. 2013, 12, 28.
- Said, O.; Saad, B.; Fulder, S.; Khalil, K.; Kassis, E. Weight Loss in Animals and Humans Treated with “Weighlevel”, a Combination of Four Medicinal Plants Used in Traditional Arabic and Islamic Medicine. Evid.-Based Complement. Altern. Med. 2011, 2011, 874538.
- Saad, B.; Zaid, H.; Shanak, S.; Kadan, S. Anti-Diabetes and Anti-Obesity Medicinal Plants and Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2017.
- Saad, B.; Ghareeb, B.; Kmail, A. Metabolic and Epigenetics Action Mechanisms of Antiobesity Medicinal Plants and Phytochemicals. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–19.
- Nisar, A. Medicinal plants and phenolic compounds. In Phenolic Compounds: Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2022; p. 131.
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157.
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465.
- Han, L.K.; Sumiyoshi, M.; Zheng, Y.N.; Okuda, H.; Kimura, Y. Anti-obesity action of Salix matsudana leaves (Part 2). Isolation of anti-obesity effectors from polyphenol fractions of Salix matsudana. Phytother. Res. 2003, 17, 1195–1198.
- Yu, S.F.; Shun, C.T.; Chen, T.M.; Chen, Y.H. 3-O-beta-D-glucosyl-(1–N6)-beta-D-glucosyl-kaempferol isolated from Sauropus androgenus reduces body weight gain in Wistar rats. Biol. Pharm. Bull 2006, 29, 2510–2513.
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377.
- Kim, H.K.; Nelson-Dooley, C.; Della-Fera, M.A.; Yang, J.Y.; Zhang, W.; Duan, J.; Hartzell, D.L.; Hamrick, M.W.; Baile, C.A. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J. Nutr. 2006, 136, 409–414.
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The Soy Isoflavone Genistein Decreases Adipose Deposition in Mice. Endocrinology 2003, 144, 3315–3320.
- Dang, Z.; Lowik, C.W. The balance between concurrent activation of ERs and PPARs determines diadzein-induced osteogenesis and adipogenesis. J. Bone Miner Res. 2004, 19, 853–861.
- Tsuda, T.; Ueno, Y.; Kojo, H.; Yoshikawa, T.; Osawa, T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2005, 1733, 137–147.
- Preuss, H.G.; Wallerstedt, D.; Talpur, N.; Tutuncuoglu, S.O.; Echard, B.; Myers, A.; Bui, M.; Bagchi, D. Effects of niacin-bound chromium and grape seed proanthocyanidin extract on the lipid profile of hypercholesterolemic subjects: A pilot study. J. Med. 2000, 31, 227–246.
- Nakagawa, Y.; Iinuma, M.; Matsuura, N.; Yi, K.; Naoi, M.; Nakayama, T.; Nozawa, Y.; Akao, Y. A potent apoptosis-inducing activity of a sesquiterpene lactone, arucanolide, in HL60 cells: A crucial role of apoptosis-inducing factor. J. Pharmacol. Sci. 2005, 97, 242–252.
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006, 136, 2512–2518.
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397.
- Yang, J.-Y.; Della-Fera, M.A.; Hartzell, D.L.; Nelson-Dooley, C.; Hausman, D.B.; Baile, C.A. Esculetin Induces Apoptosis and Inhibits Adipogenesis in 3T3-L1 Cells. Obesity 2006, 14, 1691–1699.
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776.
- Valjevac, A. Neuropeptides and Adipokines in The Control of Food Intake. Meta-Inflamm. Obes. 2020, 29, 44–62.
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681.
- Ahmad, B.; Friar, E.P.; Vohra, M.S.; Garrett, M.D.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Mechanisms of action for the anti-obesogenic activities of phytochemicals. Phytochemistry 2020, 180, 112513.
- Li, H.; Qi, J.; Li, L. Phytochemicals as potential candidates to combat obesity via adipose non-shivering thermogenesis. Pharmacol. Res. 2019, 147, 104393.
- Stuby, J.; Gravestock, I.; Wolfram, E.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Appetite-Suppressing and Satiety-Increasing Bioactive Phytochemicals: A Systematic Review. Nutrients 2019, 11, 2238.
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83.
- Wu, L.-Y.; Chen, C.-W.; Chen, L.-K.; Chou, H.-Y.; Chang, C.-L.; Juan, C.-C. Curcumin Attenuates Adipogenesis by Inducing Preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients 2019, 11, 2307.
- Hsu, C.L.; Yen, G.C. Induction of cell apoptosis in 3T3-L1 preadipocytes by flavonoids is associated with their antioxidant activity. Mol. Nutr. Food Res. 2006, 50, 1072–1079.
- Hsu, C.-L.; Huang, S.-L.; Yen, G.-C. Inhibitory Effect of Phenolic Acids on the Proliferation of 3T3-L1 Preadipocytes in Relation to Their Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 4191–4197.
- Harmon, A.; Harp, J. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 2001, 280, C807–C813.
- Kim, H.K.; Della Fera, M.A.; Lin, J.; Baile, C.A. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J. Nutr. 2006, 136, 2965–2969.
- Roncari, D.A.; Lau, D.C.; Kindler, S. Exaggerated replication in culture of adipocyte precursors from massively obese persons. Metabolism 1981, 30, 425–427.
- Scott, R.E.; Florine, D.L.; Wille, J.J., Jr.; Yun, K. Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle:GD. Proc. Natl. Acad. Sci. USA 1982, 79, 845–849.
- Li, T.; Zhang, L.; Jin, C.; Xiong, Y.; Cheng, Y.Y.; Chen, K. Pomegranate flower extract bidirectionally regulates the proliferation, differentiation and apoptosis of 3T3-L1 cells through regulation of PPARγ expression mediated by PI3K-AKT signaling pathway. Biomed. Pharmacother. 2020, 131, 110769.
- Evans, M.; Geigerman, C.; Cook, J.; Curtis, L.; Kuebler, B.; McIntosh, M. Conjugated linoleic acid suppresses triglyceride accumulation and induces apoptosis in 3T3-L1 preadipocytes. Lipids 2000, 35, 899–910.
- Lin, J.; Della-Fera, M.A.; Baile, C.A. Green Tea Polyphenol Epigallocatechin Gallate Inhibits Adipogenesis and Induces Apoptosis in 3T3-L1 Adipocytes. Obes. Res. 2005, 13, 982–990.
- Kao, Y.H.; Hiipakka, R.A.; Liao, S. Modulation of obesity by a green tea catechin. Am. J. Clin. Nutr. 2000, 72, 1232–1234.
- Hsu, C.L.; Yen, G.C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736.
- Iliakis, G.; Wang, Y.; Guan, J.; Wang, H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 2003, 22, 5834–5847.
- Fischer-Posovszky, P.; Kukulus, V.; Zulet, M.A.; Debatin, K.M.; Wabitsch, M. Conjugated Linoleic Acids Promote Human Fat Cell Apoptosis. Horm. Metab. Res. 2007, 39, 186–191.
- West, D.B.; DeLany, J.; Camet, P.M.; Blohm, F.; Truett, A.A.; Scimeca, J. Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R667–R672.
- Tsuboyama-Kasaoka, N.; Takahashi, M.; Tanemura, K.; Kim, H.J.; Tange, T.; Okuyama, H.; Kasai, M.; Ikemoto, S.; Ezaki, O. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes 2000, 49, 1534–1542.
- Li, Z.; Wang, S.; He, Y.; Li, Q.; Gao, G.; Tong, G. Regulation of Apelin-13 on Bcl-2 and Caspase-3 and Its Effects on Adipocyte Apoptosis. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–8.
- Yang, J.-Y.; Della-Fera, M.A.; Hausman, D.B.; Baile, C.A. Enhancement of ajoene-induced apoptosis by conjugated linoleic acid in 3T3-L1 adipocytes. Apoptosis 2007, 12, 1117–1128.
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326.
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258.
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643.
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734.
- Fajas, L. Adipogenesis: A cross-talk between cell proliferation and cell differentiation. Ann. Med. 2003, 35, 79–85.
- Saad, B.; AbouAtta, B.S.; Basha, W.; Hmade, A.; Kmail, A.; Khasib, S.; Said, O. Hypericum triquetrifolium—Derived Factors Downregulate the Production Levels of LPS-Induced Nitric Oxide and Tumor Necrosis Factor-α in THP-1 Cells. Evid.-Based Complement. Altern. Med. 2011, 2011, 586470.
- Saad, B.; Embaslat, W.H.; Abu-Farich, B.; Mahajna, S.; Azab, M. Hypericum triquetrifolium extracts modulate IL-6, IL-10 and TNF-α protein and mRNA expression in LPS-activated human peripheral blood mononuclear cells and THP-1-derived macrophages. Med. Aromat. Plants S 2016, 3, 2167-0412.
- Zechner, R.; Bogner-Strauss, J.; Haemmerle, G.; Lass, A.; Zimmermann, R. Lipolysis: Pathway under construction. Curr. Opin. Infect. Dis. 2005, 16, 333–340.
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020, 477, 985–1008.
- Kim, J.H.; Lee, S.; Cho, E.J. Flavonoids from Acer okamotoanum Inhibit Adipocyte Differentiation and Promote Lipolysis in the 3T3-L1 Cells. Molecules 2020, 25, 1920.
- Oliveira, A.K.D.S.; Silva, A.M.D.O.E.; Pereira, R.O.; Santos, A.S.; Junior, E.V.B.; Bezerra, M.T.; Barreto, R.S.S.; Quintans-Junior, L.J.; Quintans, J.S.S. Anti-obesity properties and mechanism of action of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 3, 1–22.
- Kuryłowicz, A.; Cąkała-Jakimowicz, M.; Puzianowska-Kuźnicka, M. Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients 2020, 12, 582.
- Szkudelska, K.; Szkudelski, T.; Nogowski, L. Daidzein, coumestrol and zearalenone affect lipogenesis and lipolysis in rat adipocytes. Phytomedicine 2002, 9, 338–345.
- Kuppusamy, U.; Das, N. Effects of flavonoids on cyclic AMP phosphodiesterase and lipid mobilization in rat adipocytes. Biochem. Pharmacol. 1992, 44, 1307–1315.
- Pinent, M.; Bladé, M.C.; Salvadó, M.J.; Arola, L.; Ardévol, A. Intracellular Mediators of Procyanidin-Induced Lipolysis in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2005, 53, 262–266.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
617
Revisions:
2 times
(View History)
Update Date:
09 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




