Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sahar Shekoohi | -- | 2027 | 2023-08-08 13:03:09 | | | |
| 2 | Jessie Wu | Meta information modification | 2027 | 2023-08-09 05:10:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vega, A.J.; Smith, C.; Matejowsky, H.G.; Thornhill, K.J.; Borne, G.E.; Mosieri, C.N.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Various Antibiotic Interactions with Warfarin. Encyclopedia. Available online: https://encyclopedia.pub/entry/47788 (accessed on 07 February 2026).
Vega AJ, Smith C, Matejowsky HG, Thornhill KJ, Borne GE, Mosieri CN, et al. Various Antibiotic Interactions with Warfarin. Encyclopedia. Available at: https://encyclopedia.pub/entry/47788. Accessed February 07, 2026.
Vega, Alexis J., Caitlin Smith, Hannah Grace Matejowsky, Katherine J. Thornhill, Grant E. Borne, Chizoba N. Mosieri, Sahar Shekoohi, Elyse M. Cornett, Alan D. Kaye. "Various Antibiotic Interactions with Warfarin" Encyclopedia, https://encyclopedia.pub/entry/47788 (accessed February 07, 2026).
Vega, A.J., Smith, C., Matejowsky, H.G., Thornhill, K.J., Borne, G.E., Mosieri, C.N., Shekoohi, S., Cornett, E.M., & Kaye, A.D. (2023, August 08). Various Antibiotic Interactions with Warfarin. In Encyclopedia. https://encyclopedia.pub/entry/47788
Vega, Alexis J., et al. "Various Antibiotic Interactions with Warfarin." Encyclopedia. Web. 08 August, 2023.
Copy Citation
Warfarin is the most widely used oral anticoagulant in North America and in the world. It has a long-established efficacy for the prevention of thromboembolic events in patients with cardiovascular risk factors such as chronic atrial fibrillation, prosthetic heart valves, venous thromboembolism, and coronary artery disease.
warfarin
antibiotics
penicillin
macrolides
fluoroquinolones
cephalosporins
rifampin
1. Penicillins
There are four major categories of penicillin drugs, all of which have the same mechanism of action. Penicillin V and G are prototype beta-lactam antibiotics and function by acting as D-Ala-D-Ala structural analogs, binding penicillin-binding proteins and blocking the transpeptidase cross-linking of peptidoglycan within the bacterial cell walls [1]. Another group of penicillin drugs is penicillinase-sensitive: Amoxicillin, Ampicillin, and Aminopenicillin. Likewise, there are a group of penicillinase-resistant penicillin drugs whose structure blocks access of the beta-lactamase to the beta-lactam. Lastly, antipseudomonal penicillin drugs are a special group of piperacillin and ticarcillin [1].
Penicillin drugs most commonly treat Gram-positive pathogens due to their thick cell wall filled with peptidoglycan [1]. Some examples include staphylococcal and streptococcal species. Penicillinase-sensitive penicillins have coverage against H. influenzae, H. pylori, E. coli, L. monocytogenes, P. mirabilis, Salmonella, Shigella, and enterococci species related to their extended spectrum. Antipseudomonal penicillins have exceptional coverage against Pseudomonas.
In a case review of a 39-year-old man with a history of deep vein thrombosis and septic arthritis, concurrent cefazolin and nafcillin with warfarin was examined [2]. Initially receiving cefazolin, the patient’s INR remained within the reference range. When he was later given nafcillin as a replacement for cefazolin, he experienced extreme INR decline. The study demonstrated that the co-administration of nafcillin with warfarin may require a 2–4 fold-increased dose of warfarin to reach INR levels within the reference range. Hepatic cytochrome-P450 induction via nafcillin and dicloxacillin decreases the efficacy of warfarin by enhancing warfarin’s metabolism using cytochrome-P450 [3].
Some sources have stated that oral amoxicillin has not been shown to interact with warfarin [3]. However, many cases of the co-administration of amoxicillin or amoxicillin/clavulanate and warfarin are reported in the literature demonstrating INR elevation and/or bleeding anywhere between 7 days post-amoxicillin initiation to 9 days after amoxicillin therapy was discontinued [4][5]. In a 2003 case report by Davydof et al., a 58-year-old Hawaiian/Asian/European woman developed microscopic hematuria and an elevated INR 2.5 weeks after stopping antibiotic therapy with amoxicillin/clavulanate [3]. This case report describes the importance of considering other contributing factors when describing warfarin’s interaction with antibiotics, including warfarin’s 30 h half-life, the rate of clotting factor synthesis, and depletion time for vitamin K liver stores. Thus, the onset of coagulopathy can range from 7 days after initiating antibiotics to 4 weeks after discontinuation.
When administered high doses, 85.7% of patients experienced INR levels greater than four in a study by Mahmoud et al. The study reported high doses of amoxicillin/clavulanate are associated with a higher risk of over-anticoagulation when combined with warfarin than when administered in lower doses [6]. This is likely due to most penicillin drugs, other than nafcillin and dicloxacillin, reducing the gut flora, leading to decreased vitamin K-producing bacteria. This manifests as an enhancement of warfarin’s effects and vitamin K deficiency due to reduced vitamin K concentrations [3]. Lower quantities of vitamin K-producing gut bacteria would likely lead to decreased vitamin K-dependent activated clotting factors, enhancing warfarin’s anticoagulant effects.
2. Fluoroquinolones
Fluoroquinolone antibiotics consist of ciprofloxacin, enoxacin, norfloxacin, ofloxacin, as well as a few respiratory fluoroquinolones, gemifloxacin, levofloxacin, and moxifloxacin. The mechanism of action of fluoroquinolones is via inhibition of the prokaryotic enzymes topoisomerase II and topoisomerase IV [7]. Fluoroquinolones are especially useful in treating urinary tract infections and gastrointestinal and respiratory infections. Fluoroquinolones are commonly used to treat infections with Gram-negative rods. They can also be used for some Gram-positive organisms, Pseudomonas, and otitis externa [7].
Ciprofloxacin and levofloxacin are cytochrome-P450 inhibitors, meaning they displace warfarin from binding sites and prevent warfarin metabolism, thus causing a prolonged bleeding time and increased INR levels [7][8]. These adverse effects are seen commonly in patients receiving chronic warfarin therapy for clotting disorders and are placed on a fluoroquinolone antibiotic to treat an infection. In a case study examining four patients on chronic warfarin therapy with concurrent levofloxacin use, three patients experienced INR increase from within the reference range 2–3 to a 3.5, 8.12, and 11.5 as the study progressed. The fourth patient only experienced mild bleeding throughout the trial [8].
In a statistical review of 30 patients who were hospitalized and concurrently taking warfarin and levofloxacin, statistical analysis revealed a significant increase in the INR with a supporting p-value of 0.0001 [9].
3. Cephalosporins
Cephalosporin antibiotics also have a beta-lactam ring, like penicillin drugs. They inhibit cell wall synthesis and are less susceptible to penicillinases [10]. There are five generations of cephalosporin antibiotics. First-generation cephalosporins, including cefazolin and cephalexin, are commonly used during surgery to prevent wound infections. Susceptible organisms include P. mirabilis, E. coli, and K. pneumoniae. Second-generation cephalosporins are cefaclor, cefoxitin, cefuroxime, and cefotetan. These are also used to treat the previously mentioned organisms: Enterobacter species, H. influenzae, Neisseria species, and Serratia. Third-generation cephalosporins include ceftriaxone, cefpodoxime, and ceftazidime, which are commonly used for severe Gram-negative infections such as N. meningitidis and can cross the blood–brain barrier. Fourth-generation cephalosporins include cefepime which is increasingly used against Pseudomonas. Lastly, fifth-generation cephalosporins include ceftaroline, which treats MRSA, unlike the first to fourth generation cephalosporins [10].
A case review reports ceftaroline prescribed to an 85-year-old woman with a therapeutic INR level who was hospitalized for cellulitis treatment. After a subsequent hospitalization for shoulder pain, her INR level was above therapeutic [11]. It has been found that cephalosporins interact with warfarin by potentiating the risk of hypoprothrombinemia, inhibiting p-glycoprotein, and altering the gastrointestinal flora [11].
A retrospective chart review was conducted by Saum et al. in which INR increases from the baseline were compared in patients taking chronic warfarin therapy with diagnoses of UTI and were treated with ceftriaxone, a first-generation cephalosporin (cefazolin or cephalexin), penicillin (ampicillin/sulbactam, amoxicillin/clavulanate, piperacillin/tazobactam), or ciprofloxacin in a community teaching hospital between June 2011 and September 2012. The ceftriaxone group was found to have a statistically significant higher peak INR value compared to all other antibiotics that were studied (ceftriaxone: 3.56, first-generation cephalosporins: 2.66, penicillins: 2.98, ciprofloxacin: 2.3; p = 0.004), a statistically significant greater extent of change in the INR value (+1.19, +0.66, +0.8, +0.275; p = 0.006), and a statistically significant greater percentage change in the INR value when compared to ciprofloxacin (54.4% vs. 12.7%; p = 0.037) [12]. Ceftriaxone interacts with warfarin to increase a patient’s INR value more than other commonly administered antibiotics for UTI treatment. It was concluded that first-generation cephalosporins, penicillin, or ciprofloxacin should be preferred for UTI treatment in patients on warfarin [12].
4. TMP-SMX
TMP-SMX is theorized to interact with warfarin via two mechanisms. The first is the disruption of the gut flora, reducing vitamin K synthesis, a shared mechanism with many other classes of antibiotics. The second interaction mechanism is via the inhibition of cytochrome P450 isozyme 2C9, which is also involved in warfarin metabolism [13].
In a study by Fischer et al. investigating the interactions between TMP-SMX and warfarin in an elderly population with urinary tract infections, hospitalized cases of upper GI tract hemorrhage in patients on long-term warfarin therapy had an increased risk of having received a prescription for TMP-SMX within 14 days of hospitalization with an odds ratio of 3.84 (95% CI, 2.33–6.33) [14]. Additionally, TMP-SMX prescription showed an increased risk of upper GI tract hemorrhage in patients taking warfarin with an odds ratio of 2.80 (95% CI, 1.48–5.32) compared with amoxicillin or ampicillin, two medications with no observed significant interaction with warfarin [14].
In a study by Vitry et al., patients on chronic warfarin therapy who were administered TMP-SMX were associated with increased bleeding-related hospitalizations with a 5.08 adjusted rate ratio (95% CI, 2.00–12.88) [15].
In another study by Baillargeon et al., TMP-SMX treatment in chronic warfarin users aged 65 years or older had an increased risk of hospitalization for bleeding with an odds ratio of 2.70 (95% CI, 1.46–5.05) [16]. Lane et al. evaluated a population of veterans who were prescribed warfarin for ≥30 days and were found to have an increased risk of serious bleeding events when co-prescribed TMP-SMX with a hazard ratio of 2.09 (95% CI, 1.45–3.02). Additionally, 31% of patients prescribed TMP-SMX were found to have an elevation of INR ≥5 [17]. Schelleman et al. investigated the interaction between TMP-SMX and warfarin, which was observed over time intervals after the administration of TMP-SMX was tracked via hospitalization for GI bleeding [18]. When fully adjusted for confounding variables, 0–5 days had an odds ratio of 1.46 (95% CI, 1.16–1.85); 6–10 days had an odds ratio of 2.54 (95% CI, 2.08–3.10); 11–15 days had an odds ratio of 2.04 (95% CI, 1.64–2.54); and 16–20 days had an odds ratio of 1.18 (95% CI, 0.89–1.57) [13].
5. Rifampin
Interaction between rifampin and warfarin is due to rifampin’s nonspecific induction activity on hepatic enzymes, including cytochrome P450 enzymes, with CYP3A4 being affected preferentially [18]. The current literature and data shows that it can take up to 2 weeks for rifampin’s full effect on these enzymes. After the discontinuation of rifampin, it can take four weeks or longer for warfarin effects to level out again, though this level may not be the same as before rifampin use [18].
Yang et al. studied patients on prior steady-state warfarin who received rifampin. They were investigated for changes in warfarin dosing over time, including after the discontinuation of rifampin. During the onset phase, defined as rifampin initiation in patients who were previously taking warfarin, the study found a median increase in steady-state warfarin dosing on rifampin by 165% (n = 8, IQR 99, 227), which occurred in a median of 30 days (n = 8, IQR 19, 34). Although the overall warfarin dosing increased, the study showed a decrease of 38% (IQR −52, 32) in warfarin dosing in the first week of rifampin initiation. During the offset phase, defined as when rifampin was discontinued and warfarin continued, the study found a median decrease in steady-state warfarin dosing by 67% (IQR −70, −58), which took a median of 6 weeks to achieve (IQR 4, 8). Compared to before rifampin use, the steady-state dosing of warfarin after discontinuation increased by a median of 8% (n = 8, IQE 1, 38) [18].
6. Macrolides
The macrolide family of antibiotics prevents bacterial protein synthesis by binding to the 50 S subunit of the bacterial ribosome (Figure 1) [19]. Macrolides are considered bacteriostatic because they inhibit protein synthesis without directly killing the bacteria. The bacterial ribosome structure is highly conserved across almost all bacterial species, allowing macrolides to be classified as broad spectrum [20]. The most commonly used macrolides are erythromycin, clarithromycin, azithromycin, and fidaxomicin. Macrolides are indicated in the treatment of H. pylori, atypical mycobacterial infections, as well as Gram-positive infections of the skin, respiratory tract, and soft tissue [21].
Extensive research has been conducted regarding the side effects of concomitantly prescribing macrolides alongside warfarin. One study found that macrolide use during warfarin therapy is associated with an increased risk of bleeding with an OR of 1.86, while another study specifically looking at this same interaction in the elderly population found an adjusted risk ratio of 3.07 [15].
Another study looking at azithromycin specifically found it to have a twofold increased risk of a serious bleeding event, compared to low-risk antibiotic usage during warfarin therapy [17].
Other studies also demonstrate an increase in the mean INR from 2.7 to 3.6 when receiving macrolides alongside warfarin treatment in pediatric cardiac patients [22].
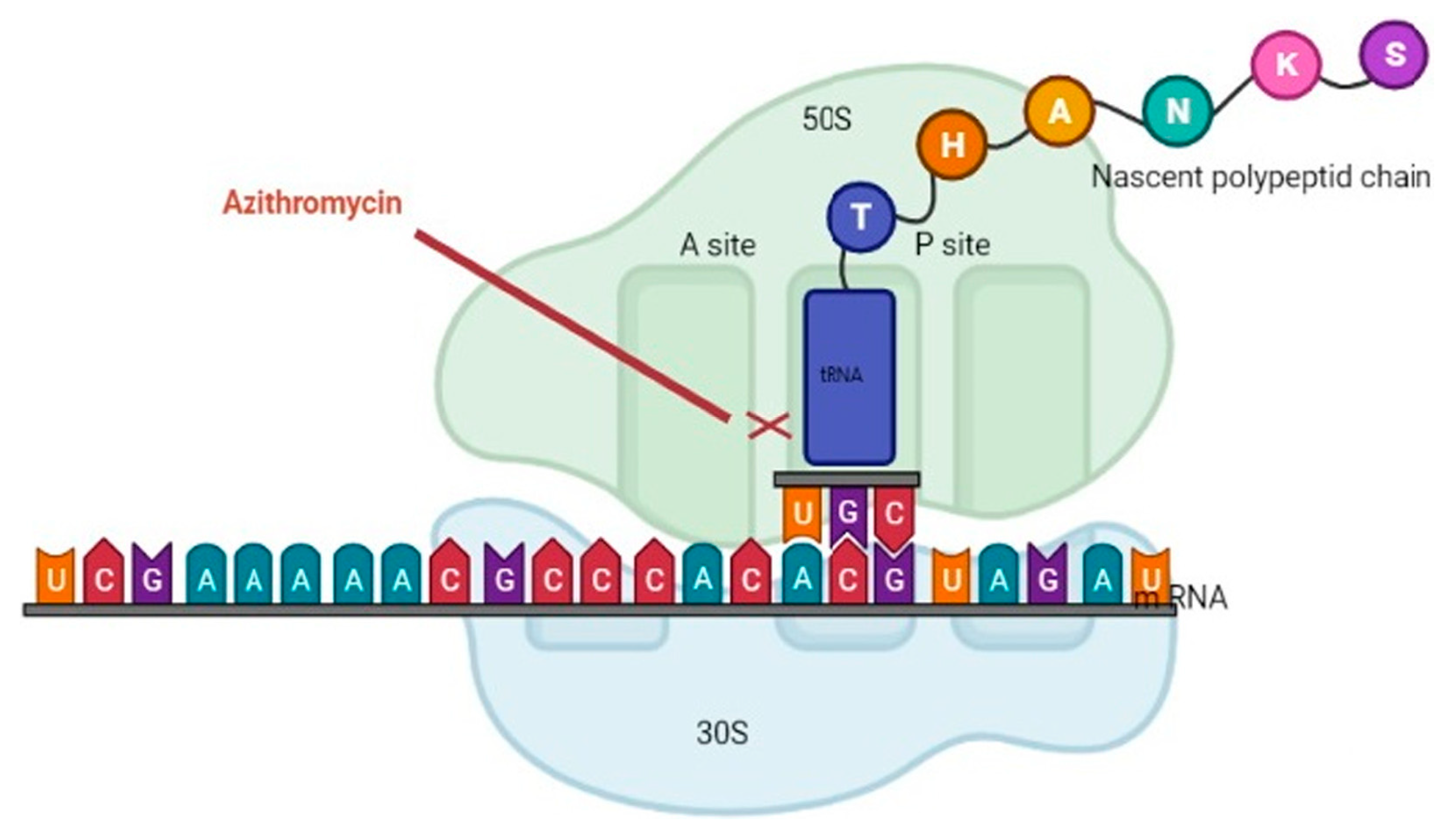
Figure 1. The macrolide family of antibiotics prevents bacterial protein synthesis by binding to the 50 S subunit of the bacterial ribosome. This figure was adapted from Ref. [23] Clinical Laboratory Analysis, Volume: 36, Issue: 6, First published: 21 April 2022.
7. Metronidazole
As the drug of choice for Helicobacter, Bacteroides, Clostridia, Giardia, Trichomonas, and Entamoeba species, metronidazole is a broad-spectrum antibiotic known to inhibit anaerobic Gram-negative rods [24]. A member of the nitroimidazole group of drugs, metronidazole is in a class of its own as an antibiotic due to its ability to also treat a variety of parasitic infections. Metronidazole is thought to assert its effects through its reduced intermediates inhibiting the nucleic acid synthesis in specific organisms [25].
In a study that appraised over 793 citations relating to food and drug interactions with warfarin, metronidazole was named as one of six antibiotics that potentiate warfarin’s anticoagulant effects [26].
The interactions described in the literature with regard to metronidazole and warfarin are usually found to describe the impact this combination of drugs can have on a patient’s INR. One study found that metronidazole, used alongside warfarin, increased a patient’s INR above 6 in 4.9% of patients [17]. Another study, in a similar vein, described metronidazole use with warfarin as having a 23.3% chance of increasing a patient’s INR into supra-therapeutic levels, which meant an INR greater than 3 [27].
References
- Yip, D.W.; Gerriets, V. Penicillin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554560/ (accessed on 27 March 2023).
- Kim, K.Y.; Frey, R.J.; Epplen, K.; Foruhari, F. Interaction between warfarin and nafcillin: Case report and review of the literature. Pharmacotherapy 2007, 27, 1467–1470.
- Davydov, L.; Yermolnik, M.; Cuni, L.J. Warfarin and Amoxicillin/Clavulanate Drug Interaction. Ann. Pharmacother. 2003, 37, 367–370. Available online: https://journals.sagepub.com/doi/10.1345/aph.1C243?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 15 July 2023).
- Bandrowsky, T.; Vorono, A.A.; Borris, T.J.; Marcantoni, H.W. Amoxicillin-related postextraction bleeding in an anticoagulated patient with tranexamic acid rinses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 82, 610–612.
- Wood, G.D.; Deeble, T. Warfarin: Dangers with antibiotics. Dent. Update 1993, 350, 352–353.
- Abdel-Aziz, M.I.; Ali, M.A.S.; Hassan, A.K.M.; Elfaham, T.H. Warfarin-drug interactions: An emphasis on influence of polypharmacy and high doses of amoxicillin/clavulanate. J. Clin. Pharmacol. 2016, 56, 39–46.
- Baggio, D.; Ananda-Rajah, M.R. Fluoroquinolone antibiotics and adverse events. Aust. Prescr. 2021, 44, 161–164.
- Jones, C.B.; Fugate, S.E. Levofloxacin and Warfarin Interaction. Ann. Pharmacother. 2002, 36, 1554–1557.
- Mercadal Orfila, G.; Gracia García, B.; Leiva Badosa, E.; Perayre Badía, M.; Reynaldo Martínez, C.; Jodar Masanés, R. Retrospective assessment of potential interaction between levofloxacin and warfarin. Pharm. World Sci. 2009, 31, 224–229.
- Bui, T.; Preuss, C.V. Cephalosporins. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551517/ (accessed on 27 March 2023).
- Bohm, N.M.; Crosby, B. Hemarthrosis in a Patient on Warfarin Receiving Ceftaroline: A Case Report and Brief Review of Cephalosporin Interactions with Warfarin. Ann. Pharmacother. 2012, 46, e19.
- Saum, L.M.; Balmat, R.P. Ceftriaxone Potentiates Warfarin Activity Greater Than Other Antibiotics in the Treatment of Urinary Tract Infections. J. Pharm. Pract. 2016, 29, 121–124.
- Schelleman, H.; Bilker, W.B.; Brensinger, C.M.; Han, X.; Kimmel, S.E.; Hennessy, S. Warfarin with fluoroquinolones, sulfonamides, or azole antifungals: Interactions and the risk of hospitalization for gastrointestinal bleeding. Clin. Pharmacol. Ther. 2008, 84, 581–588.
- Hemorrhage during Warfarin Therapy Associated with Cotrimoxazole and Other Urinary Tract Anti-Infective Agents: A Population-Based Study. Available online: https://reference.medscape.com/medline/abstract/20386005 (accessed on 12 April 2023).
- Vitry, A.I.; Roughead, E.E.; Ramsay, E.N.; Preiss, A.K.; Ryan, P.; Gilbert, A.L.; Caughey, G.E.; Shakib, S.; Esterman, A.; Zhang, Y.; et al. Major bleeding risk associated with warfarin and co-medications in the elderly population. Pharmacoepidemiol. Drug Saf. 2011, 20, 1057–1063.
- Baillargeon, J.; Holmes, H.M.; Lin, Y.L.; Raji, M.A.; Sharma, G.; Kuo, Y.F. Concurrent Use of Warfarin and Antibiotics and the Risk of Bleeding in Older Adults. Am. J. Med. 2012, 125, 183–189.
- Lane, M.A.; Zeringue, A.; McDonald, J.R. Serious Bleeding Events due to Warfarin and Antibiotic Co-prescription in a Cohort of Veterans. Am. J. Med. 2014, 127, 657–663.e2.
- Yang, C.S.; Boswell, R.; Bungard, T.J. A case series of the rifampin-warfarin drug interaction: Focus on practical warfarin management. Eur. J. Clin. Pharmacol. 2021, 77, 341–348.
- Patel, P.H.; Hashmi, M.F. Macrolides. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551495/ (accessed on 28 March 2023).
- Vázquez-Laslop, N.; Mankin, A.S. How macrolide antibiotics work. Trends Biochem. Sci. 2018, 43, 668–684.
- BPJ 44: The Appropriate Use of Macrolides. Available online: https://bpac.org.nz/BPJ/2012/May/macrolides.aspx (accessed on 28 March 2023).
- Johnson, M.C.; Wood, M.; Vaughn, V.; Cowan, L.; Sharkey, A.M. Interaction of Antibiotics and Warfarin in Pediatric Cardiology Patients. Pediatr. Cardiol. 2005, 26, 589–592.
- Heidary, M.; Samangani, A.E.; Kargari, A.; Nejad, A.K.; Yashmi, I.; Motahar, M.; Taki, E.; Khoshnood, S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J. Clin. Lab. Anal. 2022, 36, e24427.
- Dhand, A.; Snydman, D. Mechanism of Resistance in Metronidazole. In Antimicrobial Drug Resistance; Humana Press: Totowa, NJ, USA, 2009; pp. 223–227.
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082.
- Wells, P.S.; Holbrook, A.M.; Crowther, N.R.; Hirsh, J. Interactions of Warfarin with Drugs and Food. Ann. Intern. Med. 1994, 121, 676–683.
- Martín-Pérez, M.; Gaist, D.; Abajo FJ de Rodríguez, L.A.G. Population Impact of Drug Interactions with Warfarin: A Real-World Data Approach. Thromb. Haemost. 2018, 118, 461–470.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
934
Revisions:
2 times
(View History)
Update Date:
09 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




