
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Feiqing Ding | -- | 1066 | 2023-08-08 10:54:49 | | | |
| 2 | Dean Liu | -7 word(s) | 1059 | 2023-08-09 02:03:55 | | |
Video Upload Options
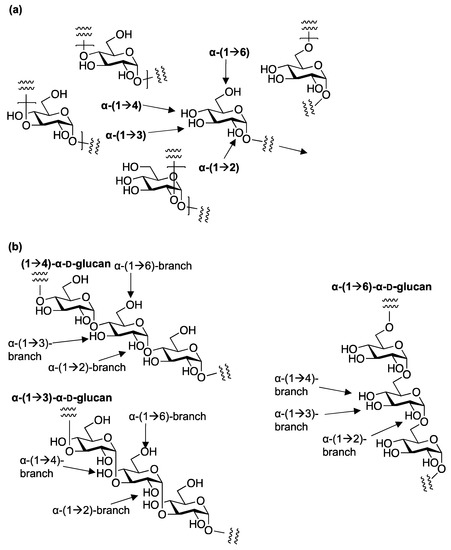
Controlling the stereoselectivity of 1,2-cis glycosylation is one of the most challenging tasks in the chemical synthesis of glycans. There are various 1,2-cis glycosides in nature, such as α-glucoside and β-mannoside in glycoproteins, glycolipids, proteoglycans, microbial polysaccharides, and bioactive natural products. In the structure of polysaccharides such as α-glucan, 1,2-cis α-glucosides were found to be the major linkage between the glucopyranosides. Various regioisomeric linkages, 1→3, 1→4, and 1→6 for the backbone structure, and 1→2/3/4/6 for branching in the polysaccharide as well as in the oligosaccharides were identified. To achieve highly stereoselective 1,2-cis glycosylation, including α-glucosylation, a number of strategies using inter- and intra-molecular methodologies have been explored.
1. Introduction
α-D-glucans
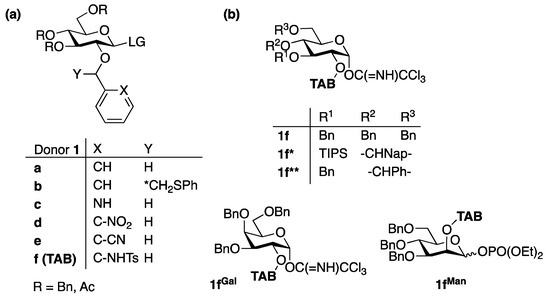
2. 1,2-cis glycosylation
References
- Andreana, P.R.; Crich, D. Guidelines for O-Glycoside Formation from First Principles. ACS Cent. Sci. 2021, 7, 1454–1462.
- Gangoiti, J.; Corwin, S.F.; Lamothe, L.M.; Vafiadi, C.; Hamaker, B.R.; Dijkhuizen, L. Synthesis of novel α-glucans with potential health benefits through controlled glucose release in the human gastrointestinal tract. Crit. Rev. Food Sci. Nutr. 2020, 60, 123–146.
- Shivatare, S.S.; Wong, C.-H. Synthetic Carbohydrate Chemistry and Translational Medicine. J. Org. Chem. 2020, 85, 15780–15800.
- Loh, C.C.J. Exploiting non-covalent interactions in selective carbohydrate synthesis. Nat. Rev. Chem. 2021, 5, 792–815.
- Wang, L.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Reagent Controlled Stereoselective Synthesis of α-Glucans. J. Am. Chem. Soc. 2018, 140, 4632–4638.
- Wang, L.; Zhang, Y.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Reagent Controlled Glycosylations for the Assembly of Well-Defined Pel Oligosaccharides. J. Org. Chem. 2020, 85, 15872–15884.
- Inuki, S.; Tabuchi, H.; Matsuzaki, C.; Yonejima, Y.; Hisa, K.; Kimura, I.; Yamamoto, K.; Ohno, H. Chemical Synthesis and Evaluation of Exopolysaccharide Fragments Produced by Leuconostoc mesenteroides Strain NTM048. Chem. Pharm. Bull. 2022, 70, 155–161.
- Shetty, P.R.; Batchu, U.R.; Buddana, S.K.; Sambasiva Rao, K.; Penna, S. A comprehensive review on α-D-Glucans: Structural and functional diversity, derivatization and bioapplications. Carbohydr. Res. 2021, 503, 108297.
- Wang, G.-L.; Li, J.-Y.; Wang, Y.; Chen, Y.; Wen, Q.-L. Extraction, Structure and Bioactivity of Polysaccharides from Tricholoma matsutake (S. Ito et Imai) Singer (Review). Appl. Biochem. Microbiol. 2022, 58, 375–381.
- Stephens, Z.; Wilson, L.F.L.; Zimmer, J. Diverse mechanisms of polysaccharide biosynthesis, assembly and secretion across kingdoms. Curr. Opin. Struct. Biol. 2023, 79, 102564.
- Thitipraphunkul, K.; Uttapap, D.; Piyachomkwan, K.; Takeda, Y. A comparative study of edible canna (Canna edulis) starch from different cultivars. Part II. Molecular structure of amylose and amylopectin. Carbohydr. Polym. 2003, 54, 489–498.
- Sarko, A.; Wu, H.-C.H. The Crystal Structures of A-, B- and C-Polymorphs of Amylose and Starch. Starch 1978, 30, 73–78.
- Helbert, W.; Chanzy, H. Single crystals of V amylose complexed with n-butanol or n-pentanol: Structural features and properties. Int. J. Biol. Macromol. 1994, 16, 207–213.
- Bail, P.L.; Rondeau, C.; Buleon, A. Structural investigation of amylose complexes with small ligands: Helical conformation, crystalline structure and thermostability. Int. J. Biol. Macromol. 2005, 35, 1–7.
- Rappenecker, G.; Zugenmaier, P. Detailed refinement of the crystal structure of Vh-amylose. Carbonhydr. Res. 1981, 89, 11–19.
- Zhang, Q.; Lu, Z.; Hu, H.; Yang, W.; Marszalek, P.E. Direct detection of the formation of V-amylose helix by single molecule force spectroscopy. J. Am. Chem. Soc. 2006, 128, 9387–9393.
- Sivak, M.N.; Preiss, J. (Eds.) Starch: Basic Science to Biotechnology. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 1998; Volume 41.
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112.
- Wang, T.L.; Bogracheva, T.Y.; Hedley, C.L. Starch: As simple as A, B, C? J. Exp. Bot. 1998, 49, 481–502.
- James, M.G.; Robertson, D.S.; Myers, A.M. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 1995, 7, 417–429.
- Hehre, E.J.; Hamilton, D.M.; Carlson, A.S. Synthesis of a polsaccharide of the starch glycogen class from sucrose by a cell-free, bacterial enzyme system (amylosucrase). J. Biol. Chem. 1949, 177, 267–279.
- Potocki de Montalk, G.; Remaud-Simeon, M.; Willemot, R.-M.; Sarçabal, P.; Planchot, V.; Monsan, P. Amylosucrase from Neisseria polysaccharea: Novel catalytic properties. FEBS Lett. 2000, 471, 219–223.
- Kim, B.-S.; Kim, H.-S.; Hong, J.-S.; Huber, K.C.; Shim, J.-H.; Yoo, S.-H. Effects of amylosucrase treatment on molecular structure and digestion resistance of pre-gelatinised rice and barley starches. Food Chem. 2013, 138, 966–975.
- Jung, Y.-S.; Hong, M.-G.; Park, S.-H.; Lee, B.-H.; Yoo, S.-H. Biocatalytic Fabrication of α-Glucan-Coated Porous Starch Granules by Amylolytic and Glucan-Synthesizing Enzymes as a Target-Specific Delivery Carrier. Biomacromolecules 2019, 20, 4143–4149.
- Li, Y.; Ren, J.; Liu, J.; Sun, L.; Wang, Y.; Liu, B.; Li, C.; Li, Z. Modification by α-D-glucan branching enzyme lowers the in vitro digestibility of starch from different sources. Int. J. Biol. Macromol. 2018, 107, 1758–1764.
- Park, I.; Park, M.; Yoon, N.; Cha, J. Comparison of the Structural Properties and Nutritional Fraction of Corn Starch Treated with Thermophilic GH13 and GH57 α-Glucan Branching Enzymes. Foods 2019, 8, 452.
- Ban, X.; Dhoble, A.S.; Li, C.; Gu, Z.; Hong, Y.; Cheng, L.; Holler, T.P.; Kaustubh, B.; Li, Z. Bacterial 1,4-α-glucan branching enzymes: Characteristics, preparation and commercial applications. Crit. Rev. Biotechnol. 2020, 40, 380–396.
- Yu, L.; Kong, H.; Gu, Z.; Li, C.; Ban, X.; Cheng, L.; Hong, Y.; Li, Z. Two 1,4-α-glucan branching enzymes successively rearrange glycosidic bonds: A novel synergistic approach for reducing starch digestibility. Carbohydr. Polym. 2021, 262, 117968.
- Xu, T.; Li, Z.; Gu, Z.; Li, C.; Cheng, L.; Hong, Y.; Ban, X. The N-terminus of 1,4-α-glucan branching enzyme plays an important role in its non-classical secretion in Bacillus subtilis. Food Biosci. 2023, 52, 102491.
- Lambré, C.; Baviera, J.M.B.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; Mortensen, A.; et al. Safety evaluation of the food enzyme 1,4-α-glucan branching enzyme from the non-genetically modified Geobacillus thermodenitrificans strain TRBE14. EFSA J. 2023, 21, e07834.
- Carbonero, E.R.; Montai, A.V.; Woranovicz-Barreira, S.; Gorin, P.A.J.; Lacomini, M. Polysaccharides of lichenized fungi of three Cladina spp.: Significance as chemotypes. Phytochemistry 2002, 61, 681–686.
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17–31.
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Leuconostoc dextransucrase and dextran: Production, properties and applications. J. Chem. Technol. Biotechnol. 2005, 80, 845–860.
- Zhong, X.; Wang, G.; Fang, S.; Zhou, S.; Ishiwata, A.; Cai, H.; Ding, F. Immunomodulatory Effect and Biological Significance of β-Glucans. Pharmaceutics 2023, 15, 1615.
- Okuyama, M.; Saburi, W.; Mori, H.; Kimura, A. α-Glucosidases and α-1,4-Glucan Lyases: Structures, Functions, and Physiological Actions. Cell. Mol. Life Sci. 2016, 73, 2727–2751.
- Synytsya, A.; Novák, M. Structural Diversity of Fungal Glucans. Carbohydr. Polym. 2013, 92, 792–809.
- Luo, X.; Xu, X.; Yu, M.; Yang, Z.; Zheng, L. Characterisation and immunostimulatory activity of an α-(1→6)-D-glucan from the cultured Armillariella tabescens mycelia. Food Chem. 2008, 111, 357–363.
- Han, X.Q.; Wu, X.M.; Chai, X.Y.; Chen, D.; Dai, H.; Dong, H.L.; Ma, Z.Z.; Gao, X.M.; Tu, P.F. Isolation, characterization and immunological activity of a polysaccharide from the fruit bodies of an edible mushroom, Sarcodon aspratus (Berk.) S. Ito. Food. Res. Int. 2011, 44, 489–493.
- Painter, T.J. Details of the fine structure of nigeran revealed by the kinetics of its oxidation by periodate. Carbohydr. Res. 1990, 200, 403–408.
- Pasteur, L. On the viscous fermentation and the butyrous fermentation. Bull. Soc. Chim. Paris 1861, 11, 30–31.
- Leemhuis, H.; Pijning, T.; Dobruchowska, J.M.; van Leeuwen, S.S.; Kralj, S.; Dijkstra, B.W.; Dijkhuizen, L. Glucansucrases: Three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. J. Biotechnol. 2013, 163, 250–272.
- van Hijum, S.A.F.T.; Kralj, S.; Ozimek, L.K.; Dijkhuizen, L.; van Geel-Schutten, I.G.H. Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 157–176.
- Simpson, C.L.; Cheetham, N.W.H.; Jacques, N.A. Four glucosyltransferases, gtfJ, gtfK, gtfL and gtfM, from Streptococcus salivarius ATCC 25975. Microbiology 1995, 141, 1451–1460.
- Kang, H.-K.; Oh, J.-S.; Kim, D. Molecular characterization and expression analysis of the glucansucrase DSRWC from Weissella cibaria synthesizing a α(1→6) glucan. FEMS Microbiol. Lett. 2009, 292, 33–41.
- Mondal, S.; Chakraborty, I.; Pramanik, M.; Rout, D.; Islam, S.S. Structural studies of water-soluble polysaccharides of an edible mushroom, Termitomyces eurhizus. A reinvestigation. Carbohydr. Res. 2004, 339, 1135–1140.
- Purama, R.K.; Goswami, P.; Khan, A.T.; Goyal, A. Structural analysis and properties of dextran produced by Leuconostoc mesenteroides NRRL B-640. Carbohydr. Polym. 2009, 76, 30–35.
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353.
- He, Q.; Kobayashi, K.; Kusumi, R.; Kimura, S.; Enomoto, Y.; Yoshida, M.; Kim, U.-J.; Wada, M. In vitro Synthesis of Branchless Linear (1→6)-α-D-Glucan by Glucosyltransferase K: Mechanical and Swelling Properties of Its Hydrogels Crosslinked with Diglycidyl Ethers. ACS Omega 2020, 5, 31272–31280.
- Rosenfeld, E.L.; Lukomskaya, I.S. The splitting of dextran and isomaltose by animal tissues. Clin. Chim. Acta 1957, 2, 105–114.
- Wang, R.; Dijkstra, P.J.; Karperien, M. Dextran. Biomaterials from Nature for Advanced Devices and Therapies; Wiley: Hoboken, NJ, USA, 2016; pp. 307–319.
- Hong, M.-G.; Yoo, S.-H.; Lee, B.-H. Effect of highly branched α-glucans synthesized by dual glycosyltransferases on the glucose release rate. Carbohydr. Polymer 2022, 278, 119016.
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301.
- Lamothe, L.M.; Francey, C.; Lerea-Antes, J.S.; Rytz, A.; D’Urzo, C.; Delodder, F.; Piccardi, N.; Curti, D.; Murciano Martinez, P.; Darimont, C.; et al. Effects of α-D-glucans with alternating 1,3/1,6 α-D-glucopyranosyl linkages on postprandial glycemic response in healthy subjects. Carbohydr. Polym. Technol. Appl. 2022, 4, 100256.
- Zonneveld, B.J.M. The Significance of α-1,3-glucan of the cell wall and α-1,3-glucanase for cleistothecium development. Biochim. Biophys. Acta. 1972, 273, 174–187.
- Johnston, I.R. The composition of the cell wall of Aspergillus niger. Biochem. J. 1965, 96, 651–658.
- Zonneveld, B.J.M. Biochemical analysis of the cell wall of Aspergillus nidulans. Biochim. Biophys. Acta. 1971, 249, 506–514.
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and Biosynthesis of Cell Wall α-1,3-Glucan in Fungi. J. Fungi 2017, 3, 63.
- Van der Kaaij, R.M.; Janecek, S.; van der Maarel, M.J.E.C.; Dijkhuizen, L. Phylogenetic and biochemical characterization of a novel cluster of intracellular fungal α-amylase enzymes. Microbiology 2007, 153, 4003–4015.
- Marion, C.L.; Rappleye, C.A.; Engle, J.T.; Goldman, W.E. An α-(1,4)-amylase is essential for α-(1,3)-glucan production and virulence in Histoplasma capsulatum. Mol. Microbiol. 2006, 62, 970–983.
- Camacho, E.; Sepulveda, V.E.; Goldman, W.E.; San-Blas, G.; Niño-Vega, G.A. Expression of Paracoccidioides brasiliensis AMY1 in a Histoplasma capsulatum amy1 mutant, relates an α-(1,4)-amylase to cell wall α-(1,3)-glucan synthesis. PLoS ONE 2012, 7, e50201.
- Koizumi, A.; Miyazawa, K.; Ogata, M.; Takahashi, Y.; Yano, S.; Yoshimi, A.; Sano, M.; Hidaka, M.; Nihira, T.; Nakai, H.; et al. Cleavage of α-1,4-glycosidic linkages by the glycosylphosphatidylinositol-anchored α-amylase AgtA decreases the molecular weight of cell wall α-1,3-glucan in Aspergillus oryzae. Front. Fungal Biol. 2023, 3, 1061841.
- Jelsma, J.; Kreger, D.R. Polymorphism in crystalline (1→3)-α-D-glucan from fungal cell-walls. Carbohydr. Res. 1979, 71, 51–64.
- Złotko, K.; Wiater, A.; Waśko, A.; Pleszczyńska, M.; Paduch, R.; Jaroszuk-Ściseł, J.; Bieganowski, A. A Report on Fungal (1→3)-α-D-glucans: Properties, Functions and Application. Molecules 2019, 24, 3972.
- Moreno-Mendieta, S.; Guillén, D.; Hernández-Pando, R.; Sánchez, S.; Rodríguez-Sanoja, R. Potential of glucans as vaccine adjuvants: A review of the α-glucans case. Carbohydr. Polym. 2017, 165, 103–114.
- Patra, S.; Maity, P.; Chakraborty, I.; Sen, I.K.; Ghosh, D.; Rout, D.; Bhanja, S.K. Structural studies of immunomodulatory (1→3)-, (1→4)-α glucan from an edible mushroom Polyporus grammocephalus. Int. J. Biol. Macromol. 2021, 168, 649–655.
- Chen, R.; Xu, J.; Wu, W.; Wen, Y.; Lu, S.; El-Seedi, H.R.; Zhao, C. Structure–immunomodulatory activity relationships of dietary polysaccharides. Curr. Res. Food Sci. 2022, 5, 1330–1341.
- Zhang, Y.; Kong, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioact. Carbohydr. Diet. Fibre 2013, 1, 53–71.
- Olennikov, D.N.; Agafonova, S.V.; Rokhin, A.V.; Penzina, T.A.; Borovskii, G.B. Branched glucan from the fruiting bodies of Piptoporus betulinus (Bull.:Fr) Karst. Appl. Biochem. Microbiol. 2012, 48, 65–70.
- Pozsgay, V.; Nánási, P.; Neszmélyi, A. Utilisation of the d-glucopyranosyl group as a non-participating group in stereoselective glycosylation: Synthesis of O-α-D-glucopyranosyl-(1→2)-O-α-D-glucopyranosyl-(1→6)-D-glucose. Carbohydr. Res. 1979, 75, 310–313.
- Rychener, M.; Bigler, P.; Pfander, H. Synthese und 1H-NMR-Studie der vier unverzweigten peracetylierten β-D-Glucopyranosyl-β-gentiobiosen. Helv. Chim. Acta 1984, 67, 378–385.
- Gómez de Segura, A.; Alcalde, M.; Bernabé, M.; Ballesteros, A.; Plou, F.J. Synthesis of methyl α-D-glucooligosaccharides by entrapped dextransucrase from Leuconostoc mesenteroides B-1299. J. Biotechnol. 2006, 124, 439–445.
- Brissonnet, Y.; Ladevèze, S.; Tezé, D.; Fabre, E.; Deniaud, D.; Daligault, F.; Tellier, C.; Šesták, S.; Remaud-Simeon, M.; Potocki-Veronese, G.; et al. Polymeric Iminosugars Improve the Activity of Carbohydrate-Processing Enzymes. Bioconjugate Chem. 2015, 26, 766–772.
- Ahrazem, O.; Rubio-Moraga, A.; Jimeno, M.; Gómez-Gómez, L. Structural characterization of highly glucosylated crocins and regulation of their biosynthesis during flower development in Crocus. Front. Plant Sci. 2015, 6, 971–985.
- Hoshi, H.; Yagi, Y.; Iijima, H.; Matsunaga, K.; Ishihara, Y.; Yasunara, T. Isolation and Characterization of a Novel Immunomodulatory α-Glucan-Protein Complex from the Mycelium of Tricholoma matsutake in Basidiomycetes. J. Agric. Food Chem. 2005, 53, 8948–8956.
- Kroon-Batenburg, L.M.; Kroon, J. The crystal and molecular structures of cellulose I and II. Glycoconj. J. 1997, 14, 677–690.
- Chawla, P.R.; Bajaj, I.B.; Survase, S.A.; Singhal, R.S. Microbial Cellulose: Fermentative Production and Applications. Food Technol. Biotechnol. 2009, 47, 107–124.
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for β-glucans. Nature 2001, 413, 36–37.
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 2003, 197, 1119–1124.
- Zipfel, C.; Robatzek, S. Pathogen-Associated Molecular Pattern-Triggered Immunity: Veni, Vidi…? Plant Physiol. 2010, 154, 551–554.
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766.
- Adachi, Y. Role of the 1,3-β-D-Glucan Receptor Dectin-1 in Fungal Infection and Activation of Innate and Anti-Tumor Immunity. Trends Glycosci. Glycotechnol. 2007, 19, 195–207.
- Fesel, P.H.; Zuccaro, A. β-Glucan: Crucial Component of the Fungal Cell Wall and Elusive MAMP in Plants. Fungal Genet. Biol. 2016, 90, 53–60.
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251–1268.
- Miyagawa, A. Chemical Synthesis of β-(1,3)-Glucan Oligosaccharide and Its Application. Trends Glycosci. Glycotechnol. 2018, 30, E117–E127.
- Morelli, L.; Compostella, F.; Panza, L.; Imperio, D. Unusual Promoters and Leaving Groups in Glycosylation Reactions: The Evolution of Carbohydrate Synthesis. Carbohydr. Res. 2022, 519, 108625.
- Singh, Y.; Geringer, S.A.; Demchenko, A.V. Synthesis and Glycosidation of Anomeric Halides: Evolution from Early Studies to Modern Methods of the 21st Century. Chem. Rev. 2022, 122, 11701–11758.
- Ishiwata, A.; Tanaka, K.; Ao, J.; Ding, F.; Ito, Y. Recent advances in stereoselective 1,2-cis-O-glycosylations. Front. Chem. 2022, 10, 972429.
- Takahashi, D.; Toshima, K. 1,2-cis O-glycosylation methods. In Comprehensive Glycoscience; Barchi, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2021; Volume 2, pp. 365–412.
- Lv, Z.; Liu, H.; Hao, H.; Rahman, F.-U.; Zhang, Y. Chemical synthesis of oligosaccharides and their application in new drug research. Eur. J. Med. Chem. 2023, 249, 115164.
- Shadrick, M.; Singh, Y.; Demchenko, A.V. Stereocontrolled α-galactosylation under Cooperative Catalysis. J. Org. Chem. 2020, 85, 15936–15944.
- Ishiwata, A.; Lee, Y.J.; Ito, Y. Recent advances in stereoselective glycosylation through intramolecular aglycon delivery. Org. Biomol. Chem. 2010, 8, 3596–3608.
- Ishiwata, A.; Ito, Y. Intramolecular Aglycon Delivery. In Selective Glycosylations—Synthetic Methods and Catalysts; Bennett, C.S., Ed.; Wiley: Weinheim, Germany, 2017; Chapter II-4; pp. 81–96.
- Ishiwata, A. Synthetic Study on Glycoconjugates Containing 1,2-cis Glycoside and Their Application. Trends Glycosci. Glycotech. 2019, 31, SE53–SE54.
- Nigudkar, S.S.; Demchenko, A.V. Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chem. Sci. 2015, 6, 2687–2704.
- Leng, W.-L.; Yao, H.; He, J.-X.; Liu, X.-W. Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity. Acc. Chem. Res. 2018, 51, 628–639.
- van der Vorm, S.; Hansen, T.; van Hengst, J.M.A.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Acceptor reactivity in glycosylation reactions. Chem. Soc. Rev. 2019, 48, 4688–4706.
- Njeri, D.K.; Valenzuela, E.A.; Ragains, J.R. Leveraging Trifluoromethylated Benzyl Groups toward the Highly 1,2-cis-Selective Glucosylation of Reactive Alcohols. Org. Lett. 2021, 23, 8214–8218.
- Kobayashi, Y.; Takemoto, Y. Regio- and stereoselective glycosylation of 1,2-O-unprotected sugars using organoboron catalysts. Tetrahedron 2020, 76, 131328.
- Feng, Y.; Guo, T.; Yang, H.; Liu, G.; Zhang, Q.; Zhang, S.; Chai, Y. Ni(II)-Catalyzed Regio- and Stereoselective O-Alkylation for the Construction of 1,2-cis-Glycosidic Linkages. Org. Lett. 2022, 24, 6282–6287.
- Ma, Z.; Hu, Y.; Li, X.; Liu, R.; Xia, E.; Xu, P.; Yang, Y. Stereoselective synthesis of α-glucosides with glucosyl (Z)-Ynenoates as donors. Carbohydr. Res. 2023, 523, 108710.
- Szarek, W.A.; Horton, D. Anomeric Effect; American Chemical Society: Washington, DC, USA, 1979.
- Deslongchamps, P. Stereoelectronic Effect in Organic Chemistry; Pergamon: Oxford, UK, 1983.
- Juaristi, E.; Cuevas, G. The Anomeric Effect; CRC: Boca Raton, FL, USA, 1995.
- Kirby, A.J. Stereoelectronic Effect; Oxford University Press: New York, NY, USA, 1996.
- Perrin, C.L. Reverse anomeric effect: Fact or fiction? Tetrahedron 1995, 51, 11901–11935.
- Randell, K.D.; Johnston, B.D.; Green, D.F.; Pinto, B.M. Is there a generalized reverse anomeric effect? Substituent and solvent effects on the configurational equilibria of neutral and protonated N-Arylglucopyranosylamines and N-Aryl-5-thioglucopyranosylamines. J. Org. Chem. 2000, 65, 220–226.
- Vaino, A.R.; Szarek, W.A. An examination of the purported reverse anomeric effect beyond acetylated N-xylosyl-and N-glucosylimidazoles. J. Org. Chem. 2001, 66, 1097–1102.
- Perrin, C.L.; Kuperman, J. Anomeric effects versus steric hindrance to ionic solvation in protonated glucosylanilines and cyclohexylanilines. J. Am. Chem. Soc. 2003, 125, 8846–8851.
- Reichardt, C. (Ed.) Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003.
- Lemieux, R.U.; Pavia, A.A.; Martin, J.C.; Watanabe, K.A. Solvation effects on conformational equilibria. Studies related to the conformational properties of 2-methoxytetrahydropyran and related methyl glycopyranosides. Can. J. Chem. 1969, 47, 4427–4439.
- Eby, R.; Schuerch, C. The Use of 1-O-Tosyl-D-glucopyranose Derivatives in α-D-Glucoside Synthesis. Carbohydr. Res. 1974, 34, 79–90.
- Schmidt, R.R.; Rücker, E. Stereoselective glycosidations of uronic acids. Tetrahedron Lett. 1980, 21, 421–1424.
- Lemieux, R.U.; Ratcliffe, R.M. The azidonitration of tri-O-acetyl-D-galactal. Can. J. Chem. 1979, 57, 1244–1251.
- Ishiwata, A.; Ito, Y. High throughput screening of O-glycosylation conditions. Tetrahedron Lett. 2005, 46, 3521–3524.
- Ishiwata, A.; Munemura, Y.; Ito, Y. Synergistic solvent effect in 1,2-cis-glycoside formation. Tetrahedron 2008, 64, 92–102.
- Chao, C.-S.; Li, C.-W.; Chen, M.-C.; Chang, S.-S.; Mong, K.-K.T. Low-Concentration 1,2-trans β-Selective Glycosylation Strategy and Its Applications in Oligosaccharide Synthesis. Chem. Eur. J. 2009, 15, 10972–10982.
- Chao, C.-S.; Lin, C.-Y.; Mulani, S.; Hung, W.-C.; Mong, K.-K.T. Neighboring-group participation by C-2 ether functions in glycosylations directed by nitrile solvents. Chem. Eur. J. 2011, 17, 12193–12202.
- Demchenko, A.; Stauch, T.; Boons, G.J. Solvent and other effects on the stereoselectivity of thioglycoside glycosidations. Synlett 1997, 1997, 818–820.
- Takatani, M.; Nakano, J.; Arai, M.A.; Ishiwata, A.; Ohta, H.; Ito, Y. Accelerated glycosylation under frozen conditions. Tetrahedron Lett. 2004, 45, 3929–3932.
- Ishiwata, A.; Sakurai, A.; Dürr, K.; Ito, Y. Effects of frozen conditions on stereoselectivity and velocity of O-glycosylation reactions. Bioorg. Med. Chem. 2010, 18, 3687–3695.
- Csávás, M.; Herczeg, M.; Bajza, I.; Borbás, A. Protecting Group Manipulations in Carbohydrate Synthesis, Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Jr., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 464–524.
- Ghosh, B.; Kulkarni, S.S. Advances in Protecting Groups for Oligosaccharide Synthesis. Chem. Asian J. 2020, 15, 450–462.
- Meyer, A.G.; Bissember, A.C.; Hyland, C.J.T.; Williams, C.C.; Szabo, M.; Pearsall, M.A.; Hyland, I.K.; Olivier, W.J. Seven-Membered Rings. In Progress in Heterocyclic Chemistry; Gribble, G.W., Joule, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 579–633.
- Ma, X.; Zheng, Z.; Fu, Y.; Zhu, X.; Liu, P.; Zhang, L. A “Traceless” Directing Group Enables Catalytic SN2 Glycosylation toward 1,2-cis-Glycopyranosides. J. Am. Chem. Soc. 2021, 143, 11908–11913.
- Ma, X.; Zhang, Y.; Zhu, X.; Wei, Y.; Zhang, L. Directed SN2 Glycosylation Employing an Amide-Functionalized 1-Naphthoate Platform Featuring a Selectivity-Safeguarding Mechanism. J. Am. Chem. Soc. 2023, 145, 11921–11926.
- Ding, F.; Ishiwata, A.; Ito, Y. Bimodal Glycosyl Donors Protected by 2-O-(ortho-Tosylamido)benzyl Group. Org. Lett. 2018, 20, 4384–4388.
- Ding, F.; Ishiwata, A.; Ito, Y. Recent advances of the stereoselective bimodal glycosylations for the synthesis of various glucans. Stud. Nat. Prod. Chem. 2022, 74, 1–40.
- Ding, F.; Ishiwata, A.; Zhou, S.; Zhong, X.; Ito, Y. Unified Strategy toward Stereocontrolled Assembly of Various Glucans Based on Bimodal Glycosyl Donors. J. Org. Chem. 2020, 85, 5536–5558.
- Zhou, S.; Zhong, X.; Guo, A.; Xiao, Q.; Ao, J.; Zhu, W.; Cai, H.; Ishiwata, A.; Ito, Y.; Liu, X.-W.; et al. ZnI2-Directed Stereocontrolled α-glucosylation. Org. Lett. 2021, 23, 6841–6845.




