Controlling the stereoselectivity of 1,2-cis glycosylation is one of the most challenging tasks in the chemical synthesis of glycans. There are various 1,2-cis glycosides in nature, such as α-glucoside and β-mannoside in glycoproteins, glycolipids, proteoglycans, microbial polysaccharides, and bioactive natural products. In the structure of polysaccharides such as α-glucan, 1,2-cis α-glucosides were found to be the major linkage between the glucopyranosides. Various regioisomeric linkages, 1→3, 1→4, and 1→6 for the backbone structure, and 1→2/3/4/6 for branching in the polysaccharide as well as in the oligosaccharides were identified. To achieve highly stereoselective 1,2-cis glycosylation, including α-glucosylation, a number of strategies using inter- and intra-molecular methodologies have been explored.
1. Introduction
Stereoselective synthesis of 1,2-
cis glycosides is one of the most challenging issues in the chemical synthesis of glycans [
1,
2,
3,
4,
5,
6,
7]. Various 1,2-
cis glycosides in nature have been found as α-glucoside and β-mannoside in glycoproteins, glycolipids, proteoglycans, microbial polysaccharides, and bioactive natural products. In the structure of polysaccharides such as α-glucan, 1,2-
cis α-glucosides were found to be the major linkage between the glucopyranosides. Various regioisomeric linkages, 1→3, 1→4, and 1→6 for backbone structure, and 1→2/3/4/6 for branching in the polysaccharide as well as in the oligosaccharides were identified.
α-D-glucans
α-D-glucan is a homopolysaccharide and a simple polymer of α-D-glucopyranoside (α-D-Glc
p) [
8,
9]. D-Glucose, the component of the D-glucans, is photosynthesized in plants and widespread in nature and exists in its D-glucopyranose form in α-D-glucans [
10]. The most common and linear example of α-D-glucan is (1→4)-α-D-glucan (amylose), which plays an essential role as an energy source for metabolism [
11]. The chain length of amylose is known to be in the order of 500–6000 glucose units, depending on its botanical origin. Three crystalline forms of amylose, A-, B-, and C- (a mixture of A and B) granules [
12], containing random and short helical segments, have been reported. Crystallized structures were found in the V form [
13,
14,
15], and each segment composed of six glucose residues formed a left-handed, single-stranded helical structure [
16]. Branched (1→4)-α-D-glucans are called amylopectin and glycogen, the analogues of starch for energy storage in plants and animals, fungi, and bacteria, respectively. The structures of amylopectin and glycogen are well known to be more compact than that of linear amylose. (1→4)-α-D-glucan is biologically synthesized by glucosyltransferase [
17,
18,
19,
20] and amylosucrase (sucrose-1,4-α-glucan glucosyltransferase [
21,
22,
23,
24] and (1→4)-α-D-glucan branching enzymes [
25,
26,
27,
28,
29,
30]).
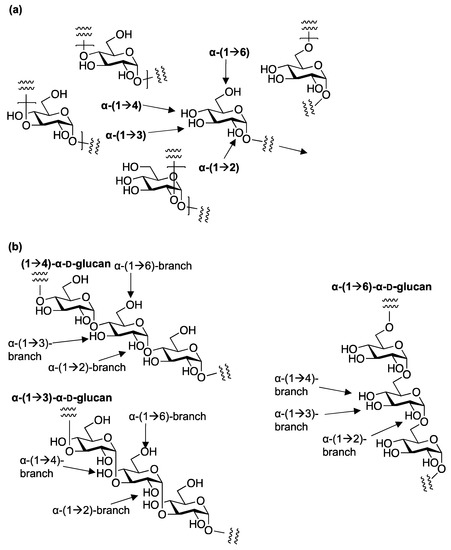
α-D-glucans also have extremely complex structural diversity according to various regioisomers, making non-branched and branched α-D-glucans with (1→6)-, (1→4)-, (1→3)-, and (1→2)-glycosidic linkages and molecular masses according to the degree of polymerization (
Figure 1). The α-D-glucans have been obtained from various species, listed in
Table 1 [
31,
32,
33,
34,
35,
36].
Figure 1. The linkages of α-D-glucans. (a) The linkages in the linear α-D-glucans; (b) the linkages of the branching in the various α-D-glucans.
Regioisomeric linear (1→6)-α-D-glucans (isomaltosides) were isolated from
Amillariella tabescens and
Sarcodon aspratus [
37,
38,
39]. A dextran [
40] obtained from lactic acid bacteria, such as
Lactobacillus,
Leuconostoc,
Weissella, and
Streptococcus, has a (1→6)-α-D-glucan backbone with up to 50% branching as α-(1→3), α-(1→4), or α-(1→2) linkages. Several glucosyl transferase (Gtf) enzymes synthesize dextrans with [
41,
42] and without branching [
43,
44,
45,
46,
47,
48]. The complex branched structures make the dextrans effective energy storage molecules that release D-glucose slowly via enzymatic hydrolysis [
49,
50,
51,
52,
53].
Linear (1→3)-α-D-glucan (pseudonigan) was identified from
Aspergillus niger [
48] as a storage polysaccharide [
54]. To the best of our knowledge, a linear (1→2)-α-D-glucan has not yet been identified. The (1→3)-α-D-glucans are major components of the cell wall of filamentous fungi [
55,
56,
57] and dimorphic yeasts [
58,
59,
60,
61] and are synthesized via the primer for (1→3)-α-D-glucans by intracellular amylases. The structural analysis of (1→3)-α-D-glucan was reported and it was mentioned that three crystalline forms I–III of (1→3)-α-D-glucan were detected and interconverted via dehydration and hydration reactions [
32,
62]. Various biological functions of (1→3)-α-D-glucan were investigated such as immunological activity via Toll-like receptor 4 (TLR4) [
63,
64,
65], which has been shown in the case of (1→4)-α-D-glucans as well as β-D-glucans [
66].
More complex branching structures have been discovered in various linear glucans [
33,
67,
68]. From dextran, NRRL B1397, an α-D-Glc-(1→2)-α-D-Glc-(1→6)-D-Glc structure [
69,
70,
71,
72,
73] was identified and the D-Glc-(1→2)-branching moiety was found to be an α-glucoside to tricholomal (1→4)-α-D-glucan [
74].
The most common and linear example of a stereoisomeric β-D-glucans is cellulose, composed of β-D-Glc
p, which plays a fundamental role as a structural component of the cell wall [
75,
76]. As physiologically active biological response modifiers (BRMs), the structure of glucans and the biological activity relationship of β-D-glucans have been reported to be adjuvants in bacterial, viral, or protozoan infections, and potent antitumor drugs, depending on the molecular weight, degree of branching, conformation, and intermolecular associations of glucans [
76,
77,
78,
79,
80,
81]. In the case of the synthesis of β-D-glucans, a common methodology such as stereoselective β-D-glucopyranosylation via the effect of neighboring group participation from the 2-
O-acyl group can be effectively used [
82,
83,
84].
2. 1,2-cis glycosylation
Stereoselective
O-glycosylation is a key step in the assembly of biologically relevant oligosaccharides. The target oligosaccharide contains 1,2-
cis- or 1,2-
trans-configurated
O-glycosidic linkages to the C-2–O bond of the non-reducing side residue of the glycoside. The 1,2-
cis linkages, such as α-glucopyranoside, α-galactopyranoside, β-mannopyranoside, β-rhamnopyranoside, and other glycosides, are found in natural glycans, including glycoconjugate such as glycoproteins, glycolipids, proteoglycans, microbial polysaccharides, and glycosylated natural products. Controlling the stereoselectivity in the formation of 1,2-
cis glycosides is extremely challenging in synthetic chemistry, as in the case of α-gluco (2-equatorial)- and β-manno (2-axial)-type glycoside formations, although the method for the 1,2-
trans isomers was developed by using the effect of neighboring group participation from theC-2 acyl group as the first choice of the chemist. Various methods using inter- [
150,
151,
152,
153,
154,
155] and intra- [
156,
157,
158] molecular procedures have been developed for the stereoselective synthesis of 1,2-
cis glycosides [
153,
159], depending on the acceptor molecules [
160,
161], and further developments have been reported in recent years [
162,
163,
164,
165].
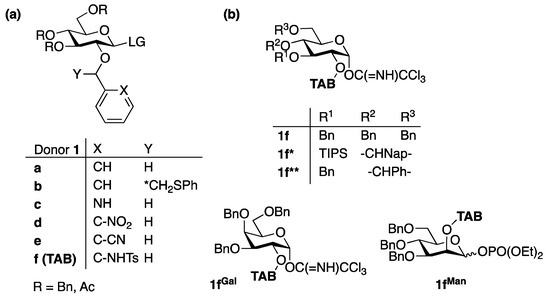
The 2-
O-ether-protected glycosyl donors predominantly afford the axial glycosides via stereoelectronic effects [
166,
167,
168,
169,
170,
171,
172,
173] (
Figure 2). Using this methodology, 1,2-
cis gluco-type pyranosides were selectively obtained. However, the selectivity is not predictable, mainly because of the many controversial results reported from a variety of examinations using many types of donors suitably optimized to the demand of their targets. Based on basic observations, the solvent effect [
174,
175,
176,
177,
178,
179,
180], the concentration effect [
181,
182,
183,
184,
185], and other factors [
186,
187,
188], including a very recent approach using an S
N2-predicting, leaving group enhanced by a coordinating acceptor [
189,
190], were also accepted as factors for the stereoselectivity of glycosylation. This review focuses on two effective and stereoselective methods for glucan synthesis: the use of C2-
o-tosylamide (TsNH)-benzyl (TAB) ether for bimodal glycosylation [
191,
192,
193] and ZnI
2-mediated 1,2-
cis glycosylation [
194].
Figure 2. 2-O-ether-protected glycosyl donors. (a) The 2-O-ether-protected glycosyl donors for stereoselective glycosylation; (b) TAB-protected donors for bimodal glycosylations.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28155644