Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | G.G. Flores-Rojas | -- | 7682 | 2023-08-08 01:10:19 | | | |
| 2 | Camila Xu | Meta information modification | 7682 | 2023-08-08 04:31:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun Scaffolds for Tissue Engineering. Encyclopedia. Available online: https://encyclopedia.pub/entry/47761 (accessed on 08 February 2026).
Flores-Rojas GG, Gómez-Lazaro B, López-Saucedo F, Vera-Graziano R, Bucio E, Mendizábal E. Electrospun Scaffolds for Tissue Engineering. Encyclopedia. Available at: https://encyclopedia.pub/entry/47761. Accessed February 08, 2026.
Flores-Rojas, Guadalupe Gabriel, Bélen Gómez-Lazaro, Felipe López-Saucedo, Ricardo Vera-Graziano, Emilio Bucio, Eduardo Mendizábal. "Electrospun Scaffolds for Tissue Engineering" Encyclopedia, https://encyclopedia.pub/entry/47761 (accessed February 08, 2026).
Flores-Rojas, G.G., Gómez-Lazaro, B., López-Saucedo, F., Vera-Graziano, R., Bucio, E., & Mendizábal, E. (2023, August 08). Electrospun Scaffolds for Tissue Engineering. In Encyclopedia. https://encyclopedia.pub/entry/47761
Flores-Rojas, Guadalupe Gabriel, et al. "Electrospun Scaffolds for Tissue Engineering." Encyclopedia. Web. 08 August, 2023.
Copy Citation
Electrospun fiber scaffolds offer distinct characteristics, including a high surface area-to-volume ratio, excellent porosity, fiber uniformity, compositional diversity, flexibility, and the ease of functionalization with bioactive molecules. These scaffolds can effectively control the release rate of bioactive molecules, making them promising candidates for delivering antibiotics, proteins, and growth factors.
electrospinning
scaffolds
tissue engineering
medical applications
tendon
1. Introduction
Over the past few decades, tissue engineering and regenerative medicine have emerged as promising approaches aimed at improving clinical outcomes in the treatment of tissue lesions and degenerations that can lead to significant health deterioration. This is particularly crucial because human tissues possess limited regenerative capabilities [1]. Regenerative engineering has emerged as a prominent field with the aim of enhancing and regenerating damaged tissues while restoring their functionalities. In recent years, this field has experienced remarkable progress driven by advancements in materials science, tissue engineering, and regenerative medicine, thereby revolutionizing the manufacturing of artificial matrices. These matrices are designed to facilitate biosorption, promote cell adhesion, and utilize electrochemical signals [2][3][4].
Tissue engineering plays a crucial role in the advancement of nano- or micro-structured materials known as scaffolds, which are essential for regulating cell behavior and promoting the regeneration of damaged tissues [5]. Among these materials, those composed of electrospun fibers with diameters ranging from nanometers to micrometers are particularly advantageous due to their ability to mimic the natural extracellular matrix (ECM) [6]. Electrospun fiber scaffolds offer distinct characteristics, including a high surface area-to-volume ratio, excellent porosity, fiber uniformity, compositional diversity, flexibility, and the ease of functionalization with bioactive molecules. These scaffolds can effectively control the release rate of bioactive molecules, making them promising candidates for delivering antibiotics, proteins, and growth factors [6]. The incorporation of these bioactive molecules into the electrospinning process can be achieved through various methods such as mixing, emulsion, coaxial electrospinning, or even surface functionalization in a post-electrospinning process [7].
Therefore, electrospinning has gained recognition within the scientific community as a valuable technique in additive manufacturing. It allows for precise control over fiber deposition, facilitating the development of nano- or micro-structured materials known as scaffolds, which play a fundamental role in regulating cell behavior and promoting tissue regeneration [7]. Moreover, electrospun nanofibers offer notable advantages, allowing for the control of various properties such as hydrophilicity, stimulus-response capacity, and nano- or micro-structuring, including designs like microwires [8], nanotubes [9], nanobelts [10], nanofibers [11], core–sheath [12], multilayer [13], or two- or three-dimensional assembly from one-dimensional structures [14], thus enabling the adoption of complex geometries and structures.
The two- or three-dimensional structures are achieved through a combination of direct electrospinning and predefined translational movement of the collector, resulting in 2D or 3D constructions with high porosity, defined patterns, and controlled features [15]. These variables allow the fabrication of scaffolds that mimic the architecture of the ECM, where the highly porous structure facilitates the diffusion and transport of nutrients and oxygen to the cells. The pore size of the scaffold is essential for supporting and enabling cell–cell and cell–matrix interactions, with the optimal pore size depending on the cell type [16]. Additionally, mechanical properties, such as the stiffness of the ECM, can influence cellular activities, including adhesion, migration, proliferation, and differentiation [16]. The design of biomimetic structures considers various critical stages they need to undergo, encompassing viability, in vitro cell function, implantation, integration, and in vivo remodeling. Hence, the scaffolds must be cell-friendly, supporting cell viability while retaining their shape and mechanical properties over time, facilitating tissue remodeling until the regenerated tissue fully takes over [17].
The 3D electrospun materials hold promise in creating specifically shaped scaffolds for tissue repair; however, they currently face limitations in replicating the complex and organized structures found in native tissues. The scaffolds can only be tailored to the specific functions of a single cell type, lacking the versatility of native tissue structures [18]. Nevertheless, advancements have been made in fabricating ECM-like three-dimensional electrospun structures using diverse methodologies involving one or more materials and phases [19]. Therefore, the ongoing development of structured materials with flexibility, biocompatibility, solubility, and programmability holds great potential for revolutionizing ECM development. This includes exploring a wide variety of biocompatible and biodegradable polymeric materials capable of replacing or augmenting the functions of living tissues [20].
While electrospun materials have shown moderate success in designing and fabricating soft tissue replacements or implants, such as vascular grafts, skin grafts, hernia patches, and artificial ligaments (Figure 1), there are challenges in creating more complex and efficient biomimetic structures. Currently, the spatial organization and structural components of these scaffolds limit their application in areas such as posts, bone grafts, bone plates, joint replacements, spinal rods, intervertebral discs, and spinal cages. Although existing scaffolds are made from synthetic biomaterials and have demonstrated considerable success, further advancements are necessary to bridge the gap between these materials and the complex nature of human body tissues.
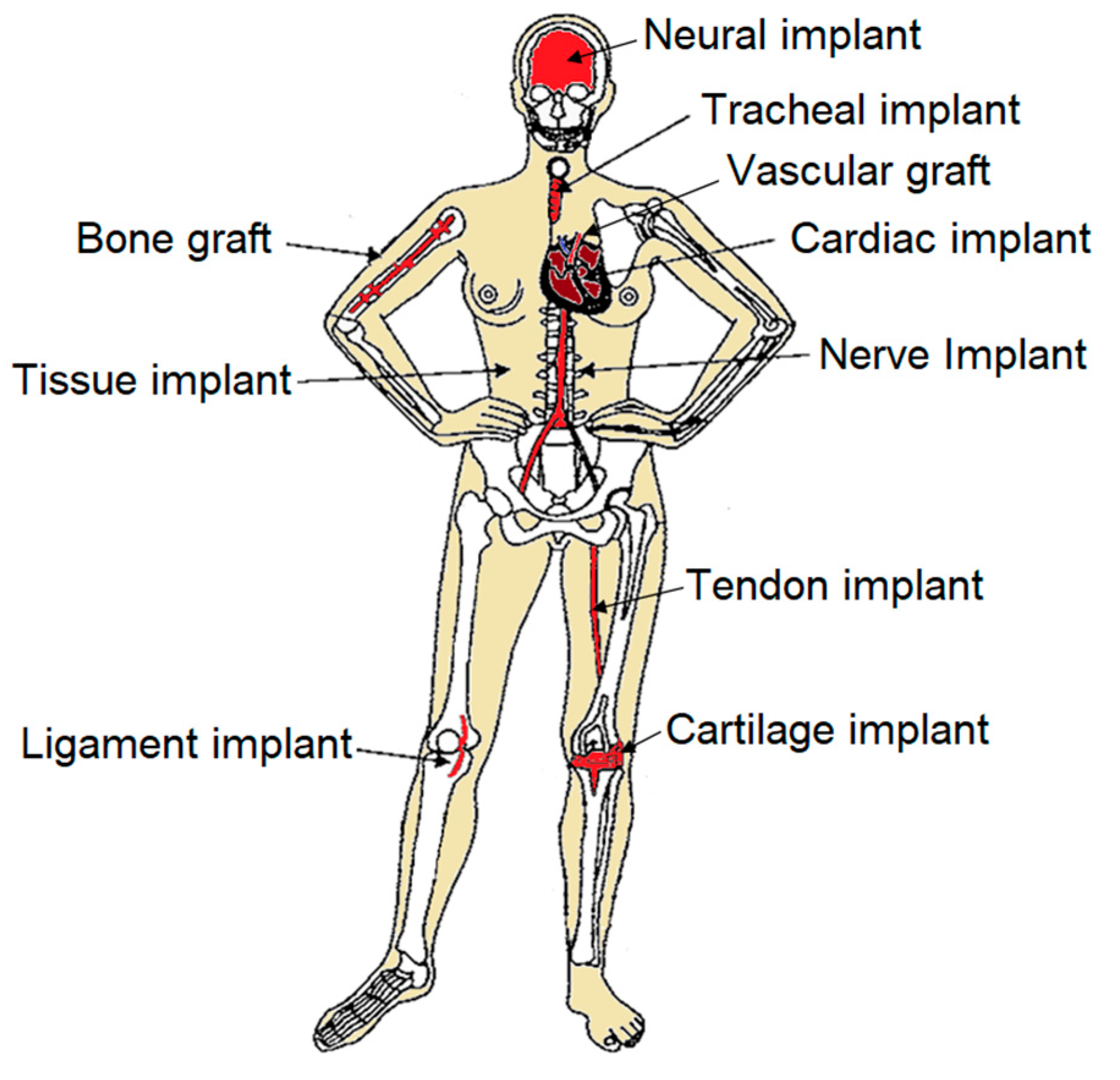
Figure 1. Schematic illustration of electrospun implants.
2. Ideal Scaffold System
Cellular scaffolds must possess specific characteristics to be considered suitable scaffold systems, with requirements varying based on the intended application and tissue type. However, all scaffolds must meet certain minimum criteria to enable their viability as an ECM. These requirements include:
-
The scaffold should feature an interconnected porous structure with controlled pore geometry and size. This structure should maintain mechanical stability over a period of time, facilitating adequate tissue regeneration within the scaffold. The interconnected porous structure is crucial for cell clearance, nutrient transport, and removal of cellular waste, all of which are vital for cell formation and tissue growth. The optimal size and morphology of the pores can vary depending on the cell type, but they should be large enough to support three-dimensional tissue formation through multilayered cell growth. Additionally, a highly porous scaffold not only maximizes tissue growth but also minimizes material usage [24][25][26].
3. Scaffold Materials
Scaffold materials can be broadly classified into three groups: natural, synthetic, and hybrid materials [29][30]. Natural materials are derived from plant sources, microorganisms, animals, or human tissues [31]. They often come with high costs due to the challenges of isolation and processing, and their properties can vary significantly between batches. Synthetic materials, on the other hand, offer a solution to availability issues, as they can be produced synthetically. However, synthetic materials can also exhibit batch-to-batch variations and varying production costs as well as a wide range of properties through chemical functionalization [32].
Synthetic materials can be further categorized as either biodegradable or non-degradable. Biodegradable synthetic materials, such as polyesters [33], polyanhydrides [34], polyphosphoesters [35], and polyorthoesters [36], provide control over scaffold structure and surface properties to accommodate biological requirements for cell adhesion, growth, and functions. Scaffolds can be processed with controlled macro- and microstructures, including size and porosity, and they can be tailored to different degradation times, ranging from days to years, depending on the scaffold’s structure and manufacturing material [37]. Therefore, biodegradable synthetic polymers find widespread use in scaffold manufacturing. For example, hybrid meshes made of dextrin and poly(ethylene oxide) (PEO) can exhibit mechanical properties similar to human skin by combining the mechanical strength of dextrin with the flexibility of PEO. These structures can be adjusted by altering the polymer ratio. On the other hand, non-degradable materials, such as polyethylene (PE), poly(ethylene terephthalate) (PET), poly(tetrafluoroethylene) (PTFE), silicone rubber (SR) [38], and others, offer excellent mechanical properties for various scaffold applications. However, there is a risk of permanent tissue reactions due to wear and tear during implantation.
Among the materials used in tissue engineering, biodegradable polyesters are widely employed compared to other biodegradable polymers (Table 1) [39][40]. Polyesters, such as poly(α-hydroxy acid) (PAHA), have demonstrated considerable success in various applications, including implants [41][42]. However, the mechanical properties and degradation profiles of these polyesters may be insufficient for specific applications, and certain derivatives can be toxic during degradation. Therefore, as tissue engineering continues to advance, there is a need to develop biodegradable polymers or alternative materials of natural, mineral, or composite origin [43][44][45] that meet specific structural and biodegradability requirements, allowing for cell adhesion, growth, and tissue regeneration. The challenge lies in cultivating cells on the scaffold to produce appropriate tissue matrices [46]. Scaffold engineering plays a vital role in regenerative medicine, providing an alternative for cell survival, proliferation, and differentiation for tissue formation under in vitro or in vivo conditions. Additionally, scaffolds can be designed to deliver therapeutic and bioactive agents simultaneously to tissue lesions [47][48][49][50].
Table 1. Polymers used in electrospun scaffolds for tissue engineering.
| Category | Polymers |
|---|---|
| Natural polymers | Cellulose [51] |
| Xanthan gum [52] | |
| Poly(hydroxyalkanoates) [53] | |
| Starch [54] | |
| Chitosan [55] | |
| Chitin [56] | |
| Pullulan [57] | |
| Alginate [58] | |
| Wheat gluten [59] | |
| Gelatin [60] | |
| Collagen [61] | |
| Dextrin [62] | |
| Fibrin [63] | |
| Zein [64] | |
| Poly(hydroxybutyrate) (PHB) [65] | |
| Synthetic polymers | Polyamide-6 [66] |
| Polycaprolactone (PCL) [67] | |
| Polylactic acid (PLA) [68] | |
| Poly(lactic-co-glycolic acid) (PLGA) [69] | |
| Polyvinyl alcohol (PVA) [70] | |
| Poly(glycolic acid) (PGA) [71] | |
| Poly(butylene succinate) [72] | |
| Poly(anhydride-ester) [73] | |
| Polyorthoesters [74] | |
| Polycarbonate [75] | |
| Polyanhydride [76] | |
| Polyfumarate [77] | |
| Polyphosphoester [78] | |
| Polyphosphazenes [79] | |
| Poly(urethane ester)urea [80] | |
| Poly(L-lactide-co-caprolactone) (PLCL) [81] | |
| Polyurethane (PU) [81] | |
| Poly(vinylidene fluoride) (PVDF) [82] |
4. Electrospinning
Electrospinning is a straightforward, cost-effective, and reproducible process that involves the formation of fibers from a solution or fusion of polymers sourced from natural and synthetic materials. This process utilizes an electrical charge to manufacture nano- and micro-structured materials, which exhibit lightweight and soft properties, along with a significant surface area-to-volume ratio and porosity at both the structural and fiber levels [83]. These unique characteristics render these materials highly suitable for a wide range of biomedical applications, including tissue engineering. They have the ability to mimic the ECM, promoting cell migration and proliferation. Additionally, electrospun fibers can effectively incorporate drugs or other biomolecules into their structure or onto the fiber surfaces. Beyond the biomedical field, electrospun fibers find diverse applications in areas such as filtration membranes, multifunctional membranes for photo/chemo/electrocatalysis [84][85], nanodevice electronics [86], biosensors [87], self-powered electronics in textiles [88], electromagnetic interference shielding [89], stealth materials [90], and energy storage and conversion [91].
The most commonly employed electrospinning technique involves the assembly of fibers formed from polymer solutions, which is known as solution electrospinning. This technique is typically conducted at room temperature under atmospheric conditions [11]. The electrospinning system comprises a high-voltage power supply, a syringe pump, a spinneret (usually a blunt-tipped hypodermic needle), and a grounded conductive collector, such as a metal screen, plate, or rotating mandrel. The power source can be either direct current (DC) or alternating current (AC), which imparts a charge of specific polarity to a polymer solution or molten polymer. The charged polymer is then accelerated toward a collector of opposite polarity (Figure 2) [83]. The electrospinning process can be broadly divided into four consecutive steps: (i) charging the liquid drop and forming a Taylor cone or cone-shaped jet; (ii) extending the charged jet along a straight line; (iii) thinning of the jet in the presence of an electric field and increased electrical bending instability, also known as whipping instability; and (iv) solidification and collection of the jet as solid fiber(s) on a grounded collector [84][85][86]. This technique can be implemented in various electrospinning configurations, as depicted below (Figure 3).
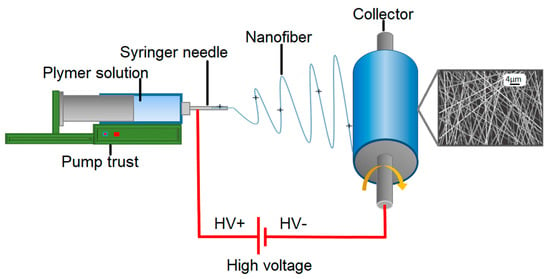
Figure 2. Illustration of electrospinning process.
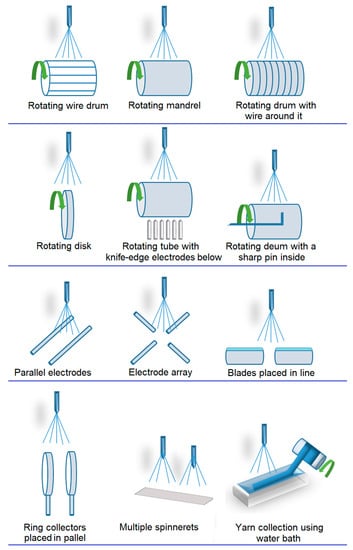
Figure 3. Schematic illustration of electrospinning configurations.
Currently, extensive efforts are being made to develop methods for manufacturing 3D structures, including microembossing [87], fiber felts [88], 3D printing [46], and electrospinning [89]. Among these techniques, electrospinning is considered a simple and versatile approach that enables the production of uniform fibers in a continuous and scalable process. However, the assembly of 3D fibrous structures still poses a significant challenge, as most electrospun materials are limited to 2D structures [90][91]. In general, four strategies are employed for fabricating 3D electrospun structures, which face the issue of hypoxia resulting from limitations in oxygen and nutrient diffusion within 3D scaffolds. Nevertheless, 3D nanofiber scaffolds created by incorporating fibers of different diameters (nano/micro) and forming micro/millimeter-sized pores have the potential to mitigate these diffusion limitations. Consequently, significant efforts have been dedicated to developing 3D nanofiber scaffolds with customized shapes and adjustable pore sizes, facilitating notable advancements in tissue regeneration through various approaches (Figure 4):
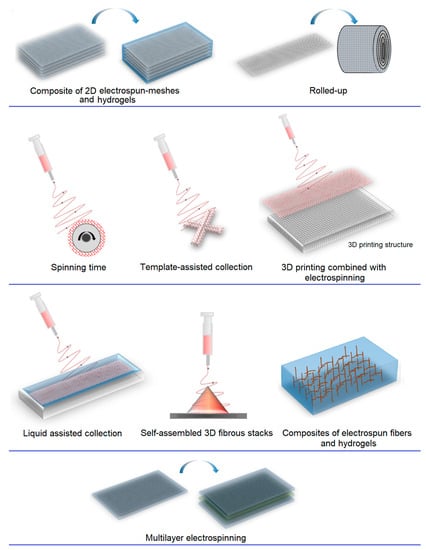
Figure 4. Schematic illustration of 3D electrospun methods.
- (a)
-
Spinning time; electrospinning is particularly suitable for fabricating 2D structures, such as membranes with random or aligned orientations [92]. However, conventional electrospinning leads to changes in membrane thickness over time, resulting in 3D structures with thicknesses ranging from tens to hundreds of microns. Moreover, multilayered 3D macrostructures comprising different materials can be achieved through sequential electrospinning [15][93] or co-electrospinning [94], involving the exchange and electrospinning of polymer solutions under different conditions [95].
- (b)
- (c)
-
Direct assembly using auxiliary factors, such as 3D templates [99], liquid collectors, or porogenic agents. Templates are commonly in the form of mechanical collectors with desired shapes (e.g., rotating collectors or static collectors) or other fibrous structures (e.g., microfibers), which serve as matrix templates [100][101].
- -
-
Rotating collectors enable the fabrication of single or interconnected micro- and macro-tubes with multiple micropatterns [102].
- -
- -
-
Liquid collectors are effective for manufacturing 3D fibrous structures by utilizing liquid deposition or vortex formation to solidify the fibers, resulting in a 3D fibrous structure [106].
- -
-
The addition of porogenic agents, such as ice crystals [107][108], salt particles [109] or even certain polymers (e.g., PEO), is ideal for fabricating highly porous 3D fibrous structures [110][111]. These materials, acting as porogens, are typically mixed with the precursor during the electrospinning process and later washed away after reaching the desired thickness [109].
- (d)
-
Self-assembly is a strategy for creating nest-like fibrous structures through the utilization of electrostatic forces between already collected fibers, where flying fibers are directed to settle on nearby conductive regions to dissipate their charges [112].
5. Applications
Electrospun 3D structures and membranes closely resemble the morphology and mechanical properties of native tissue extracellular matrices [113]. These electrospun scaffolds serve as analogs to the ECM, providing a supportive environment for cells and promoting stem cell regeneration and differentiation into functional tissues. The unique characteristics of electrospun materials, such as their large surface-to-volume ratio, pore size, and fiber diameter, facilitate efficient cell infiltration and proliferation [114][115]. Furthermore, these scaffolds offer the potential to incorporate bioactive agents, enhancing their biocompatibility and making them promising candidates for a wide range of biomedical applications. Examples include nerve regeneration [116], vascular grafts [117], bone grafts [118], and more. Additionally, electrospun structures find utility in controlled drug delivery [119], biosensors, and cancer diagnosis [120].
5.1. Nerve and Neural Regeneration
The human nervous system has a limited capacity for regeneration, particularly in the case of large lesions [121], which poses a significant clinical challenge for repair. The nervous system consists of the central nervous system (CNS) and the peripheral nervous system (PNS) [122]. Within both the CNS and PNS, neurons are surrounded by various regulatory cells collectively known as glia. The interaction between neuronal cells and glial regulatory cells is crucial for maintaining a balanced ECM and facilitating proper electrophysiological functions. One notable distinction between the CNS and PNS is the relatively reduced protection of the PNS, which lacks bone tissue and the blood–brain barrier. Consequently, the PNS is more susceptible to physical trauma, chemical substances, and biological agents, often resulting in injuries or diseases that lead to impaired muscle movement and permanent sensory loss [123]. Autologous nerve grafting is currently the standard treatment for nerve damage, involving the use of donor nerves, which are typically harvested from the patient’s sural nerve [124]. However, this procedure may result in functional loss and permanent morbidity at the donor nerve site [125] as well as potential Schwann cell necrosis in the grafted nerve due to limited perfusion from surrounding blood vessels [126].
Considering the potential complications linked to autologous nerve grafting, electrospun structures offer an appealing alternative for the repair and regeneration of injured nerves. These structured materials provide versatile methods for non-traditional clinical treatments [127]. They utilize synthetic [128][129], semi-synthetic [130] and natural [131][132] polymeric materials to manufacture structures with well-aligned fibers at the nano- or micro-scale, exhibiting minimal defects [133]. These structures serve as supportive scaffolds to connect injured nerves and guide nerve regeneration, promoting attachment, propagation, neurite outgrowth, and axonal regeneration along the fiber direction [134]. Three-dimensional scaffolds composed of uniaxially and radially aligned fibers have demonstrated particular efficacy in regenerating neuronal damage [135], while two-dimensional scaffolds are primarily used for in vitro neuronal regeneration studies [136]. Uniaxially oriented fiber scaffolds effectively differentiate bone marrow-derived mesenchymal stem cells (MSCs) and Schwann cells, whereas radially aligned fibers show enhanced repair capacity in spinal cord injuries by promoting cell proliferation surrounding the injury site [137][138]. Mechanisms such as the migration of neural stem cells from the periphery to the center and along the scaffold contribute to these outcomes [139]. Despite significant advances, precisely replicating all the structural and biochemical features of nervous tissue remains a challenge.
Among the electrospun 3D scaffolds, multitubular structures, often designed with microchannels or intraluminal coatings, are frequently employed as nerve guides (NGCs) in in vivo studies. These multitubular scaffolds aim to provide additional topographic signals, including topographic and biological orientation, simultaneously to facilitate nervous tissue regeneration. Methods to achieve additional topographic orientation include using sacrificial templates such as sucrose fibers [140]. Additionally, piezoelectric polymers and conductive polymers have the capacity to deliver electrical stimulation to cells. For instance, the use of polypyrrole, a conductive polymer, enables Schwann cell proliferation and axonal regeneration in vivo through the application of topographic and electrical signals [141]. The efficiency of electrospun conduits can be further enhanced by incorporating cells [127], biochemical signals, peptides (e.g., IKVAV) [142], bioactive molecules such as fibronectin and laminin [143], neurotrophic growth factors [144][145][146] nerve growth factors [147], antibodies targeting inhibitors present in harsh extracellular environments, small molecules (e.g., paclitaxel) [148], and electrical stimulation [149][150]. These enhancements play crucial roles in facilitating the process of nerve regeneration (Figure 5).
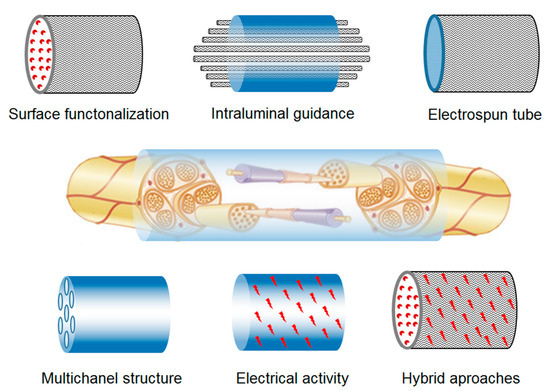
Figure 5. Electrospun scaffold approaches for nerve regeneration.
For instance, chitosan-based oriented fiber conduits have demonstrated remarkable efficacy in aligning Schwann cells and promoting the regeneration of peripheral nerves. These conduits enhance cell adhesion to the aligned chitosan fibers, creating a unique structure that extends between the cells and adjacent fibers. Histology studies have confirmed the formation of nervous tissue with Schwann cell myelinated axons, closely resembling isografts within the conduit. These findings suggest that chitosan-oriented fiber conduits hold promise as a potential alternative to autologous nerve grafting [151][152][153].
Similarly, nanofibrous PLGA scaffolds were engineered with longitudinal 2D and random 3D orientations, featuring mean fiber diameters of 187 ± 121 nm and 197 ± 72 nm, respectively. Characterization studies revealed that the 2D scaffolds exhibited a pore size of 3.5 ± 1.1 μm, which is significantly smaller than the random scaffolds, which had a pore size of 8.0 ± 2.0 μm. The pore size of the scaffold influenced various properties, including degradation rate, with the random fiber scaffolds degrading at a faster pace. Additionally, the random scaffolds demonstrated greater tensile strength and Young’s modulus compared to the aligned fiber scaffolds. In vitro cultures of Schwann cells on the scaffolds revealed that the aligned fibers acted as guides, promoting cell proliferation. In summary, the results demonstrated that aligned fiber scaffolds exhibited superior deformability, slower degradation, comparable porosity, and provided topographic orientation cues compared to random fiber scaffolds. Thus, longitudinally aligned fibers hold significant potential as scaffolds for nerve regeneration [154].
Furthermore, biofunctionalized PCL fibrous scaffolds incorporating glial cell-derived neurotrophic factor (iGDNF) displayed controlled release of the factor, exerting a positive influence on primary cortical neural stem cells in in vitro studies and enhancing cell transplantation within the brain parenchyma. The biofunctionalized scaffolds exhibited notable improvements in the cell viability and proliferation of neural stem/progenitor cells, surpassing conventional two-dimensional culture materials. Moreover, upon implantation, the scaffolds continued to enhance the survival, proliferation, migration, and neurite outgrowth of cortical cells while suppressing reactive inflammatory astroglia [155].
5.2. Skin Regeneration
The field of skin tissue engineering faces significant challenges in developing scaffolds that can effectively mimic the complex ECM of the skin. The skin’s ECM is composed of collagen fibrils arranged in a mesh-like pattern, resembling a basket-shaped tissue structure [156]. It comprises two distinct layers: the epidermis and the dermis, with the epidermis having a lower capacity for regeneration compared to the dermis [157]. Therefore, tissue engineering aims to not only facilitate wound closure but also promote the regeneration of both layers in a manner that closely replicates their natural composition and organization (Figure 6).
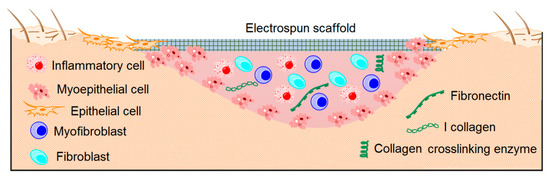
Figure 6. Electrospun scaffolds to promote skin regeneration.
Electrospun materials hold significant promise in tissue engineering due to their structural similarity to the mesh-like ECM [158]. Moreover, they offer biomechanical properties that may surpass those of normal human skin (4–20 MPa) by utilizing naturally derived or synthetic polymers in scaffold fabrication [159]. Studies examining the effect of fiber diameter have revealed that fibers within the range of 350 to 1100 nm facilitate better fibroblast proliferation, leading to the formation of well-distributed cell layers [160]. Additionally, nanofibers with crossed patterns have demonstrated improved migration rates of keratinocytes and fibroblasts compared to randomly or unidirectionally aligned nanofibers [161]. These structural factors have the potential to enhance wound healing [57] and decrease the risk of hypertrophic scarring by preventing abnormal fibroblast proliferation and excessive collagen deposition [162]. Several cell-signaling molecules have been investigated, including basic fibroblast growth factor (bFGF) [163], transforming growth factor-β1 (TGF-β1) [164], and ginsenoside-Rg3 [162]. For instance, the incorporation of ginsenoside-Rg3 and bFGF into PLGA nanofibers using a combination of electrospinning and surface immobilization has shown promising results in promoting normal fibroblast function [162][165]. Similarly, TGF-β1 inhibitors tested on composite nanofibers of PCL/gelatin have exhibited potential in inhibiting hypertrophic healing [166]. Therefore, understanding the impact of nanofiber characteristics, such as diameter and alignment, on cell signaling mechanisms and biochemical pathways is crucial for scar prevention.
Xylan, chitosan, gelatin, collagen, silk fibroin, poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), poly(lactic acid-co-glycolic acid) (PLAGA), PLGA, poly(L-lactic acid)-co-poly(3-caprolactone) (PLLCL), and PCL are among the most commonly employed polymers in skin tissue engineering [150][151][152][153][154][155][156][157]. Notably, electrospun PLAGA 3D scaffolds with varying fiber diameters have exhibited tensile moduli within the range of normal human skin, ranging from 39.23 ± 8.15 to 79.21 ± 13.71 MPa. Studies on the impact of fiber diameter have shown that human skin fibroblasts seeded on matrices with fiber diameters ranging from 350 to 1100 nm display significantly higher proliferation rates compared to matrices with fiber diameters below or above this range. This favorable response results in the formation of well-distributed cell layers on the scaffolds after 28 days in vitro [151].
Moreover, blending PLCL with PU to create electrospun scaffolds with a 50% PLCL content and a suitable degree of fiber crosslinking has demonstrated improved mechanical properties and appropriate porosity. These scaffolds have shown significant biological advantages in terms of human skin fibroblast growth, with cells adopting a sprawling morphology compared to PU membranes, indicating the excellent cytocompatibility of the composite scaffolds. These findings highlight the substantial potential of electrospun PU/PLCL scaffolds in skin regeneration applications [81].
5.3. Bone Regeneration
Bone tissue engineering is a rapidly expanding field that offers a promising approach to repairing and regenerating bone lesions resulting from various conditions, including tumors, trauma, or disease [167]. Typically, bone tissue engineering involves the use of scaffold materials in combination with tissue cells and biological signals. The scaffold provides the necessary support for cells to adhere, grow, and differentiate [168]. Tissue engineering is an excellent alternative to reconstructive techniques such as autologous and allogeneic bone grafts, which require multiple surgeries and carry the risk of complications and patient trauma [169][170].
Although electrospinning offers a suitable solution for soft tissue regeneration, it encounters a significant challenge when designing 3D scaffolds with the desired pore size for bone regeneration. The inherent nature of electrospinning produces very small pores, typically around one micron, which may not be optimal for bone scaffold fabrication that requires larger pores (200–400 µm) [171][172]. Nevertheless, 3D electrospun scaffolds hold great potential as bone substitutes in tissue reconstruction due to their ECM-like morphology [173]. Achieving suitable mechanical properties for the scaffold is crucial as it should mimic native bone, providing mechanical support during bone regeneration while guiding osteogenesis to accelerate bone healing [174][175].
In this context, a wide range of materials and methods (Figure 7) have been investigated for their application in the fabrication of bone structures. These materials can be broadly classified into four categories based on their composition: bioactive inorganics [176][177][178][179][180], degradable polymers [181], non-degradable polymers [182][183], and their composites (hybrids) [184][185]. However, many polymers used in 3D electrospinning structures have a lower Young’s modulus compared to cortical bone, which is approximately 3.1–34.3 GPa, and a strength of 65–238 MPa [18][186][187], thanks to its main structure of type I collagen fibrils and hydroxyapatite nanoparticles. To overcome this limitation, researchers have incorporated bioactive inorganic materials into the electrospinning process, resulting in scaffolds with suitable properties for bone regeneration. The incorporation of bioactive inorganic materials in these scaffolds has been shown to improve their mechanical properties [188][189] and the cytocompatibility of polymeric nanofibers [190] as well as adhesion and cell growth. These improvements facilitate osteogenic differentiation and calcification of the bone matrix, which are crucial in the bone formation process [191].
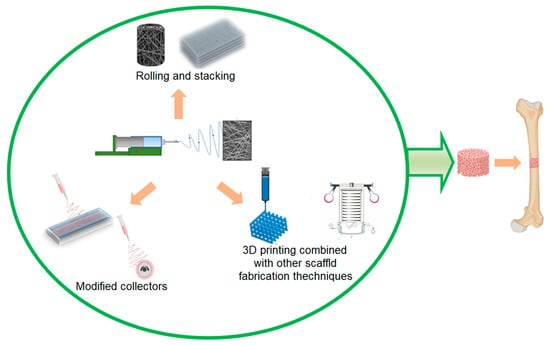
Figure 7. Electrospun scaffold approaches for bone regeneration.
The fragility of certain inorganic materials limits their application as cell supports, particularly in areas where they interface with soft tissue: for example, in subchondral areas involving bone and articular cartilage lesions [192]. Therefore, the introduction of a polymer phase can provide mechanical flexibility while eliminating the need for subsequent treatments. The polymer phase acts as a binding matrix and can also be utilized for drug delivery systems [192].
In practice, the intrinsic properties of polymeric and inorganic materials have been successfully studied and applied in electrospinning nanofiber systems. Special consideration is required for the preparation of polymeric solutions when incorporating inorganic compounds due to their suspension stability [193]. Various methods have been developed to produce hybrid nanofibers through electrospinning with inorganic compounds, including hydroxyapatite [194], polyphosphate [195], bioactive glass [196], ceramic [197], and dicalcium silicate [198], among others. These materials have been proven to be excellent inorganic phases in the production of high-strength scaffolds for hard tissue regeneration.
Some examples of hybrid scaffolds include gelatin nanofibers electrospun with varying amounts of hydroxyapatite using organic solvents and water [199]. The hybrid nanofibers exhibited a uniform distribution, which is attributed to the interactions between hydroxyapatite and gelatin amino acids, which also prevented the precipitation of the inorganic phase. In both cases, the incorporation of the inorganic phase significantly enhanced the chemical stability of gelatin, resulting in a hybrid network and promoting notable osteoblast differentiation [200]. The same approach has been applied to create collagen–hydroxyapatite scaffolds [201] as well as chitosan–hydroxyapatite scaffolds [202], among others. These hybrid scaffolds aim to generate a nanofibrous matrix that closely mimics the bone ECM.
In addition to natural polymers, synthetic degradable polymers have also been utilized in the electrospinning of composite fibers containing bioactive inorganic materials. However, combining these polymers with inorganic phases poses a significant challenge due to their hydrophobic nature. This hydrophobicity hinders the homogenization and organization of the inorganic phase, unlike natural polymers that tend to be more hydrophilic. Some studies have reported the incorporation of CaCO3 nanoparticles into electrospun membranes made of PCL [203]. These CaCO3 nanoparticles exhibit good water affinity, desirable tensile mechanical properties, and promote the favorable adhesion and growth of osteoblasts. To address the challenges associated with homogenizing and organizing the inorganic phase, some methodologies have employed surfactants. These surfactants act as mediators, leveraging their amphiphilic nature to stabilize the interface between the inorganic and organic components as well as the solvent interface. This approach effectively prevents the agglomeration of inorganic nanoparticles and the formation of beads during the electrospinning process [204].
Other reported examples have utilized a 12-hydroxystearic acid surfactant in the manufacturing of PLA composite fibers containing hydroxyapatite nanocrystallites [205]. The electrospun fibers were obtained without beads, with fiber sizes in the range of a few microns, and they exhibited a uniform distribution of hydroxyapatite nanocrystals within the PLA matrix. Cellular studies demonstrated that these composite fibers promoted osteoblast growth and phenotypic expression at a significantly higher level compared to pure PLA polymeric fibers. In general, the focus of electrospinning composite fibers has been primarily on achieving the uniform incorporation of bioactive inorganic nanoparticles within a polymer matrix while maintaining the fibrous morphology and control over homogenization [206].
The incorporation of bioactive inorganic phases in conjunction with degradable polymers of natural and synthetic origin holds promise in fabricating ECMs suitable for bone tissue regeneration, including its interface with cartilage [207]. Therefore, further studies are anticipated to focus on developing composite nanofibers with new compositions that offer both desirable mechanical properties and biological functionalities for bone regeneration. Despite existing challenges, such as achieving morphological and compositional control, reducing fiber size, ensuring homogenization, and guaranteeing mechanical stability, composite nanofibers show potential for surpassing the capabilities of individual polymeric components. This can be achieved through the incorporation of metal nanoparticles [208][209], which exhibit a relatively high modulus, or by developing polymer-free ceramic scaffolds for hard tissue regeneration [210].
In recent years, several research groups have explored another application of 3D fibrous scaffold manufacturing techniques in bone tissue engineering [211]. This involves fabricating PLA microfibrous 3D scaffolds using the electrospinning technique, which is followed by a mechanical expansion process. The resulting scaffolds were evaluated and demonstrated favorable results in terms of cell infiltration and bone formation after 2 to 4 weeks, using the rabbit calvarial model [98].
Cai et al. introduced a novel electrospinning-based fiber assembly technique for fabricating a macroporous 3D scaffold using PLLA/PCL nanofibers. The scaffold had a thickness of 3 mm and a diameter of 15 mm, demonstrating favorable mechanical resistance with an elastic modulus of 71.68 ± 5.61 MPa. Additionally, it exhibited interconnected micropores, resulting in a porosity of 77.61 ± 6.35%. To assess its potential for bone formation, the efficacy of the 3D scaffold was evaluated using a human embryonic stem cell-derived mesenchymal stem cell differentiation model and a rabbit tibial bone defect model. In vitro studies demonstrated robust cell proliferation and growth on the 3D scaffold. Furthermore, when applied to the bone defect, the scaffold promoted the formation of bone tissue. By week 3, there was observable bone tissue formation both around and within the scaffold. By week 6, most regions of the scaffold displayed thicker and more mature bone tissue with newly formed cortical bone exhibiting interconnectedness and serving as a structural and functional unit [212].
5.4. Cartilage Regeneration
Cartilage is a structural component found in various parts of the body, including the rib cage, ear, nose, and joints. There are three types of cartilage, which are distinguished by their ECM composition: elastic cartilage (with elastic fibers in the ECM), fibrous cartilage (with a rich collagen fiber ECM), and hyaline cartilage (predominantly composed of glycosaminoglycans (GAGs) in the ECM). The latter, known as articular cartilage or diarthrosis-like cartilage, is a resilient connective tissue covering the surfaces of bones within joints. It consists of approximately 30% chondrocytes by volume, along with an integrated ECM comprised of type II collagen and proteoglycans, which are secreted by chondrocytes [213][214][215].
Articular cartilage is a tissue that is structurally and functionally complex. Its ECM exhibits spatial variations in organization and composition from the superficial to the deep zones. In the superficial zone, the concentration of type II collagen decreases, while the amount of proteoglycans increases. This zone is acellular and consists of collagen fibrils aligned parallel to the articular surface. On the other hand, the middle and deep zones are composed of densely packed collagen fibrils oriented perpendicularly to the articular surface. A zone of calcified cartilage separates the deep zone, which is non-calcified, from the subchondral bone, which is rich in type X collagen [216][217].
The articular cartilage serves as a deformable, low-friction surface that facilitates the movement of synovial joints. It supports high dynamic compression loads due to its gradient structure and mechanical properties, including a resistance ranging from 9 to 40 MPa, toughness (fracture energy of 1000 to 15,000 J m−2), elasticity (fracture deformation of 60 to 120%), and a coefficient of friction of 0.010 [218][219]. Despite its crucial functions and mechanical properties, cartilage lacks blood vessels and nerves, and it has limited capacity for repair [217][220]. Various approaches have been used for cartilage repair, such as autologous chondrocyte implantation and the microfracture technique [221]. Tissue engineering has sought alternative solutions for cartilage repair, including the use of hydrogels [222]. However, hydrogels have certain disadvantages due to their poor mechanical properties and limited ability to mimic the ECM of cartilage [223][224][225]. Consequently, electrospun materials have emerged as a promising alternative to hydrogels, gaining increasing interest due to their biomimetic properties similar to the ECM as well as superior mechanical properties. Electrospun hydrogels have proven to be an excellent alternative in this regard.
Continuous advancements in tissue engineering have facilitated the production of highly organized fiber scaffolds capable of emulating cartilage and serving as temporary replacements for damaged articular cartilage with 3D scaffolds [226]. Hydrogels show promise as materials for cartilage regeneration, especially when combined with electrospun fibers to reinforce their structure [227]. These scaffolds are able to mimic the natural ECM due to their high porosity and water content. Additionally, the presence of fibers allows for improved mechanical properties as they can reorient themselves under deformation, thereby strengthening and enhancing the system [228]. In this sense, efforts have been made to optimize the mechanical properties of hydrogels by incorporating various polymer fibers with specific diameters, alignment, porosity, and the number of scaffold layers to facilitate cell infiltration [229]. Studies have suggested that nanofibrous scaffolds can promote the expression of chondrogenic markers such as type II collagen and aggrecan. This may be attributed to the fact that the size and alignment of the fibers can influence the morphology of chondrocytes, ultimately affecting gene expression [230][231].
Various natural and synthetic polymers have been utilized to fabricate electrospun scaffolds for applications in cartilage regeneration. These polymers include polycaprolactone (PCL) [232], poly(lactic-co-glycolic acid) (PLGA) [233], poly(L-lactic acid) (PLLA) [234], polyvinyl alcohol (PVA) [179], hyaluronic acid (HA) [235], silk fibroin [236], and chitosan [237], among others. In this regard, techniques have been developed to reinforce hydrogel scaffolds by incorporating nanofibers of varying sizes into the hydrogel matrix, which are positioned randomly to promote an irregular orientation of chondrocytes [231]. For instance, alginate-grafted hyaluronic acid (Alg-HA) hydrogels and fragmented PLA fibers were fabricated through an aminolysis reaction to enhance hydrophobicity and cell-interaction capabilities. The nanofiber-reinforced hydrogels exhibited higher compression modulus and lower swelling index compared to Alg-HA hydrogels without PLA fibers. The orientation of the fibers in the hydrogel allowed for control over fracture and gel strength. Notably, the scaffold demonstrated excellent cytocompatibility, promoting chondrocyte proliferation and the production of glycosaminoglycans (GAGs) and other extracellular molecules [238]. Similarly, hydrogels composed of chitosan enriched with electrospun fibroin fibers exhibited improved compression and Young’s modulus properties compared to chitosan alone. These hybrid hydrogels hold promise for cartilage formation [239].
The incorporation of a porogenic agent in scaffold manufacturing for cartilage regeneration is a valuable strategy to enhance cell infiltration within porous fibers. Through electrospinning, highly porous PCL fibrous scaffolds were created using a combination of PCL and PEO fibers. The PEO fibers acted as a porogen, leading to the formation of macropores upon their subsequent removal through dissolution. In contrast to other conventional nanofiber systems with limited cellular infiltration, the electrospun scaffolds with enlarged pores facilitated the rapid and complete cellular infiltration in MSC cultures. The extent of cell infiltration increased with higher percentages of PEO incorporation. However, it is important to note that as the percentage of PEO increased, the scaffold exhibited greater deformation, indicating the significant influence of the 3D microenvironment on cellular processes. Despite this, the inclusion of PEO resulted in enhanced cell infiltration and the formation of a robust ECM [240].
The manufacturing of scaffolds can be diverse, involving the creation of 3D scaffolds through the combination of various techniques, such as crosslinking the fibers using chemical reactions after electrospinning. For instance, two-dimensional scaffolds of poly(l-lactide-co-ε-caprolactone)/silk fibroin (PLCL/SF) were fabricated via conjugated electrospinning. Subsequently, these scaffolds were crosslinked with chondroitin sulfate (CS) and transformed into 3D scaffolds through freeze-drying, resulting in improved mechanical and biological properties. The obtained scaffolds exhibited high porosity, rapid water absorption, and stable mechanical properties. Moreover, scaffolds with CS crosslinked fibers demonstrated enhanced cell seeding efficiency and chondroprotective effects compared to non-crosslinked scaffolds. In vivo tests conducted on a rabbit articular cartilage defect model indicated that the crosslinked scaffolds facilitated the formation of tissues resembling mature cartilage, leading to superior repair outcomes in the articular cartilage defect model. Additionally, the expression levels of proinflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor (TNF)-α, were reduced in the crosslinked scaffolds (Figure 8) [241].
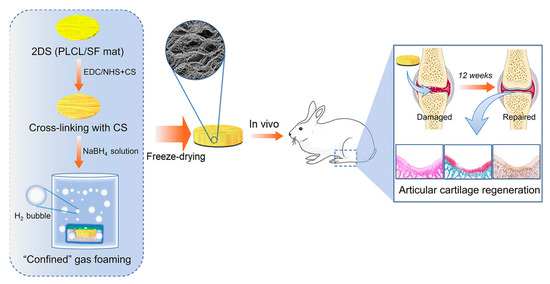
Figure 8. Three-dimensional (3D) scaffold obtained by post-processing of PLCL/SF electrospun 2D meshes for cartilage regeneration.
5.5. Tendon and Ligament Regeneration
Tendons and ligaments are composed of densely packed collagen fibers, and their regeneration and treatment of injuries, including inflammation, tears, and ruptures [220][242], pose a significant medical challenge. These injuries often involve the regeneration of tissue–tissue interfaces or the regeneration of soft tissues adjacent to hard tissues, such as tendon/ligament–bone, cartilage–bone, and muscle–tendon interfaces [243]. These interfaces are primarily located at the junctions between soft and hard matrices, exhibiting gradual variations in matrix composition, architecture, mineral content, and significant differences in mechanical properties [244].
Some common surgical treatments utilize autografts and allografts [245]. However, prostheses are an excellent alternative option due to their ability to promote favorable remodeling and lack of immune response [246]. Electrospun fiber-based scaffolds offer a promising alternative for the treatment and regeneration of damaged tendons and ligaments. These scaffolds resemble meshes composed of uniaxially aligned nanofibers, which can be easily manufactured using a rotary collector or parallel electrodes [247]. By designing specialized structures, electrospun scaffolds can facilitate the transmission of structural and mechanical stress between two mechanically distinct tissues. Scaffolds with randomly aligned nanofibers better mimic the graded structure at the tendon–bone interface [247], while aligned nanofibers emulate the structure of tendons. The random structure of the scaffolds imitates the less ordered collagen fibers found in bone [248].
One effective approach to replicate the required biomechanical properties for tendon or ligament scaffolds is through the hierarchical construction of electrospun scaffolds. For example, hierarchically constructed PLLA scaffolds bundle various fibers together to mimic the structure of tendons and ligaments using multiple layers of electrospun fibers. This method results in better replication of the epitenon membrane and the necessary biomechanical properties for tendon or ligament scaffolds [249].
Scaffolds have the ability to incorporate agents that stimulate tissue regeneration, such as tendon/ligament–bone interfaces and even MSCs derived from adipose tissue or induced pluripotent stem cells (iPSCs). These cells can undergo tenogenic differentiation when exposed to uniaxial nanofibers or when subjected to deforming stress within the scaffold. Although most tendon and ligamentous tissues exhibit highly anisotropic structures, 3D scaffolds with braided, woven, or knitted fiber networks are more favorable for regeneration and differentiation compared to meshes [250]. Furthermore, the inclusion of hydrogels in the nanofibrous matrix can benefit the encapsulation of biomolecules and cells. For instance, chitosan/HA hydrogel-coated unidirectional PCL nanofibers have been used for ligament regeneration [251].
Some scaffolds developed specifically for this purpose have consisted of multiple layers comprising PCL fibers and methacrylated gelatin. These layers were created through dual electrospinning and incorporated stem cells derived from human adipose tissue that were treated with TGF-β3 to promote differentiation into tenocytes. Subsequent PCR studies revealed a notable upregulation of tendon markers and tenascin-C, indicating that the cells encapsulated within the scaffold were still responsive to soluble tenogenic factors. Moreover, the construct allowed for the diffusion of exogenous biochemical signals through the porosity of the structure [252]. In another study, trichostatin A, a histone deacetylase inhibitor, was incorporated into PLLA fibers. Tests conducted on various tenocytes demonstrated that trichostatin A significantly regulated the expression of tendon markers, suggesting its potential use in promoting the growth of tenocytes and repairing tendon defects [253].
5.6. Vascular Regeneration
Tubular scaffolds, manufactured through multi-electrospinning using a rotating mandrel type collector as a template, hold potential applications in vascular grafts with diameters up to approximately 6 mm. These scaffolds exhibit favorable biomechanical properties and facilitate the orientation of fibers, promoting cell adhesion [254][255]. However, small diameter grafts present challenges compared to large diameter grafts, particularly in maintaining lumen patency [256]. Occlusion, resulting from acute thrombosis and intimal hyperplasia, can significantly affect their functionality [254][257]. Therefore, it is crucial to design grafts with non-thrombogenic surfaces and achieve endothelialization within electrospun grafts to prevent thrombosis.
Significant research efforts have been dedicated to the development of grafts with surfaces that inhibit thrombus formation and promote better integration and endothelialization. Several approaches have been employed to enhance graft performance, including the combination of layers with varying porosity and the incorporation of biomolecules into the nanofibers. For instance, heparin has been utilized as an anticoagulant agent [258], while vascular endothelial growth factors have been incorporated to stimulate vascularization [259], and miRNA delivery has been explored to modulate the phenotype of endothelial cells [260]. Additionally, other strategies involve the direct seeding of endothelial cells or endothelial progenitor cells onto electrospun grafts [261]. These approaches aim to maintain a smooth muscle cell (SMC) contractile phenotype in order to minimize intimal hyperplasia and promote favorable outcomes.
For example, tubular scaffolds composed of a double layer of polylactide fibers (outer layer) and silk fibroin gelatin fibers (inner layer) were manufactured through multi-electrospinning [262]. These scaffolds exhibited suitable mechanical properties for developing blood vessel substitutes. Subsequent morphological studies demonstrated enhanced migration, adherence, spread, and proliferation of 3T3 mouse fibroblasts and human umbilical vein endothelial cells (HUVEC) on the 3D scaffolds. This can be attributed to their higher porosity and surface area-to-volume ratio compared to culture plates. The cells formed a continuous monolayer of cellular tissue with a vascular network, without the presence of macrophages or lymphocytes, indicating a low risk of inflammatory reactions in subcutaneous implantations.
On the other hand, highly aligned tubular scaffolds made of PLLA/HA nanofibers exhibited anisotropic wetting, hemocompatibility, and mechanical properties that are suitable for vascular scaffolding. These scaffolds promoted a morphology aligned along the axis of the nanofibers, which is likely due to the coordinated mechanisms of vascular scaffolding, nanotopographic guidance, and biochemical cues. Importantly, the aligned HA/PLLA nanofibers were found to enhance cell proliferation and increase cell contractility compared to cells on the aligned PLLA nanofibers. These findings highlight the promoting role of the HA coating on highly aligned PLLA nanofibers in modulating smooth muscle cell (SMC) regeneration both in vitro and in vivo, as it facilitates the improved expression of contractile genes in SMCs [263].
Electrospun scaffolds can be enhanced with hydrogel properties by coating them with precursor solutions, as demonstrated by Geng et al. They developed tubular scaffolds using heparin-modified poly(ε-caprolactone) (PCLH), followed by coating with 4-arm-PEG-acrylate and D,L-1,4-dithiothreitol as a gelling solution, which solidified in situ. The gel not only strengthened the scaffold but also slowed down its degradation process. In an abdominal aorta rat model, the scaffold with the hydrogel coating showed a notable decrease in the incidence rate of aneurysms compared to scaffolds without the hydrogel coating. Furthermore, it exhibited excellent vascular regeneration with reduced calcification in the implanted grafts during the initial month, outperforming scaffolds without the hydrogel coating. Notably, the hydrogel encapsulated the fibers and enabled sustained release, leading to the appearance of contractile SMCs in vivo. This hydrogel coating endowed the scaffold with anticoagulation and anti-calcification properties as well as promoting the early onset of contractile SMCs. Therefore, hydrogel coatings significantly enhance the in vivo regenerative properties of electrospun scaffolds by providing multiple beneficial features (Figure 9) [264].
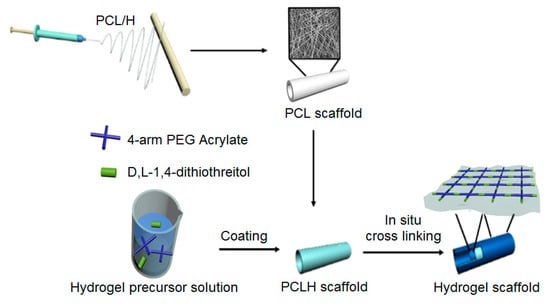
Figure 9. Hydrogel-coated, heparin-modified poly(ε-caprolactone) electrospun hybrid scaffold for vascular grafting.
5.7. Cardiac Regeneration
The walls of the heart consist of a multilayered structure with sufficient mechanical strength to facilitate contractions and relaxations of the myocardium. This strength is attributed to the perimysial fibers that contribute to the native interwoven structure of the myocardium [265]. Therefore, in the fabrication of scaffolds intended for myocardial regeneration, it is crucial to incorporate highly aligned fibers. These aligned fibers enable cell adhesion and alignment along their surface, promoting the development of tissue morphology similar to the native myocardium. Previous studies have primarily focused on 2D scaffolds for myocardial tissue regeneration, but their effectiveness has been limited [266]. However, a more efficient replication of the native cardiac tissue structure is achievable through the use of multilayer 3D electrospun scaffolds. These scaffolds allow for the controlled orientation and patterning of the layers, thereby improving the emulation of cardiac tissue structure and enhancing the restoration of myocardial functionality [267]. Furthermore, they offer the opportunity to incorporate biochemical, electrochemical, and topographic signals to further enhance the regenerative process [82].
Aligned and random electrospun PU scaffolds were evaluated in embryonic stem cell (ESC) differentiation [268]. The findings revealed that cells exhibited a rod-shaped morphology and an organized sarcomere structure on the aligned scaffolds, while the random fibers resulted in mixed cell shapes and a less organized sarcomere structure. Following differentiation into cardiomyocytes, cells on the aligned scaffolds formed end-to-end cell–cell junctions, whereas this was not observed on the random scaffolds. These results indicate that aligned scaffolds have a greater potential to induce cardiac maturation. Similar responses have been observed for other cell types, including MSCs [5], myoblasts [269], and neurons [270]. The alignment of cells on scaffolds can be enhanced by applying tension to the scaffolds during tissue culture, leading to the improved cardiac differentiation of MSCs. These findings suggest that a combination of scaffold design and mechanical properties can direct stem cell differentiation without the need for cell differentiation media.
Additional properties that have been incorporated into scaffolds for cardiac regeneration focus on piezoelectric characteristics, which can be achieved by using polymers such as PVDF, along with the incorporation of conductive agents like graphene particles [82] and carbon nanotubes [271]. For example, piezoelectric scaffolds were fabricated through the hybrid electrospinning of PVDF and gelatin with graphene oxide nanoparticles (PVDF-GO-CG), resulting in nanofibers with an average diameter of 379 ± 73 nm. The combination of the natural polymer and nanoparticles effectively modified the hydrophobicity and piezoelectric properties of PVDF. Subsequently, the scaffolds were evaluated using mouse embryonic cardiomyocytes. Cellular studies demonstrated that the cardiomyocytes exhibited an elongated morphology, resembling native myocardial tissue, and exhibited high viability without any toxic effects from the PVDF-GO-CG scaffold, as indicated by the average survival rate. Furthermore, the expression of connexin43 and troponin T genes increased by 41% and 35%, respectively, in the PVDF-GO-CG sample compared to the scaffolds without graphene oxide nanoparticles (Figure 10). This study highlights the practical potential of transmitting electrical signals and inducing the differentiation of embryonic cardiomyocytes into functional cardiac muscle using these piezoelectric scaffolds [82].
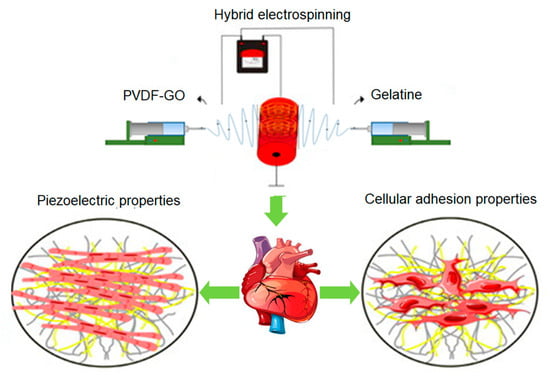
Figure 10. PVDF and gelatin hybrid piezoelectric electrospun scaffold with graphene oxide nanoparticles for cardiac regeneration.
References
- Tabata, Y. Tissue regeneration based on tissue engineering technology. Congenit. Anom. 2004, 44, 111–124.
- Cingolani, E.; Goldhaber, J.I.; Marbán, E. Next-generation pacemakers: From small devices to biological pacemakers. Nat. Rev. Cardiol. 2018, 15, 139–150.
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063.
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158.
- Guan, J.; Wang, F.; Li, Z.; Chen, J.; Guo, X.; Liao, J.; Moldovan, N.I. The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials 2011, 32, 5568–5580.
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738.
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. Part A 2017, 105, 2892–2905.
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell electrospinning: A unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 2006, 7, 3364–3369.
- Zussman, E.; Yarin, A.L.; Bazilevsky, A.V.; Avrahami, R.; Feldman, M. Electrospun polyacrylonitrile/poly (methyl methacrylate)-derived turbostratic carbon micro-/nanotubes. Adv. Mater. 2006, 18, 348–353.
- Qiao, Y.; Shi, C.; Wang, X.; Wang, P.; Zhang, Y.; Wang, D.; Qiao, R.; Wang, X.; Zhong, J. Electrospun Nanobelt-Shaped Polymer Membranes for Fast and High-Sensitivity Detection of Metal Ions. ACS Appl. Mater. Interfaces 2019, 11, 5401–5413.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415.
- Duan, G.; Jin, M.; Wang, F.; Greiner, A.; Agarwal, S.; Jiang, S. Core effect on mechanical properties of one dimensional electrospun core-sheath composite fibers. Compos. Commun. 2021, 25, 100773.
- Trinca, R.B.; Westin, C.B.; da Silva, J.A.F.; Moraes, Â.M. Electrospun multilayer chitosan scaffolds as potential wound dressings for skin lesions. Eur. Polym. J. 2017, 88, 161–170.
- Liu, J.; Zhang, F.; Hou, L.; Li, S.; Gao, Y.; Xin, Z.; Li, Q.; Xie, S.; Wang, N.; Zhao, Y. Synergistic engineering of 1D electrospun nanofibers and 2D nanosheets for sustainable applications. Sustain. Mater. Technol. 2020, 26, e00214.
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. WIREs Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626.
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602.
- Kong, Y.P.; Tu, C.H.; Donovan, P.J.; Yee, A.F. Expression of Oct4 in human embryonic stem cells is dependent on nanotopographical configuration. Acta Biomater. 2013, 9, 6369–6380.
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292.
- Zhong, H.; Huang, J.; Wu, J.; Du, J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2022, 15, 787–804.
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509.
- Dave, K.; Gomes, V.G. Interactions at scaffold interfaces: Effect of surface chemistry, structural attributes and bioaffinity. Mater. Sci. Eng. C 2019, 105, 110078.
- Chernozem, R.V.; Guselnikova, O.; Surmeneva, M.A.; Postnikov, P.S.; Abalymov, A.A.; Parakhonskiy, B.V.; De Roo, N.; Depla, D.; Skirtach, A.G.; Surmenev, R.A. Diazonium chemistry surface treatment of piezoelectric polyhydroxybutyrate scaffolds for enhanced osteoblastic cell growth. Appl. Mater. Today 2020, 20, 100758.
- Grafahrend, D.; Heffels, K.H.; Beer, M.V.; Gasteier, P.; Möller, M.; Boehm, G.; Dalton, P.D.; Groll, J. Degradable polyester scaffolds with controlled surface chemistry combining minimal protein adsorption with specific bioactivation. Nat. Mater. 2011, 10, 67–73.
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524.
- Poh, P.S.P.; Valainis, D.; Bhattacharya, K.; van Griensven, M.; Dondl, P. Optimization of Bone Scaffold Porosity Distributions. Sci. Rep. 2019, 9, 9170.
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011, 22, 661–666.
- Tran, T.T.; Hamid, Z.A.; Cheong, K.Y. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080.
- Fang, Z.; Starly, B.; Sun, W. Computer-aided characterization for effective mechanical properties of porous tissue scaffolds. CAD Comput. Aided Des. 2005, 37, 65–72.
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13.
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105.
- Frenkel, S.R.; Toolan, B.; Menche, D.; Pitman, M.I.; Pachence, J.M. Chondrocyte Transplantation Using a Collagen Bilayer Matrix for Cartilage Repair. J. Bone Jt. Surg. Br. 1997, 79, 831–836.
- Yap, J.X.; Leo, C.P.; Mohd Yasin, N.H.; Show, P.L.; Chu, D.T.; Singh, V.; Derek, C.J.C. Recent advances of natural biopolymeric culture scaffold: Synthesis and modification. Bioengineered 2022, 13, 2226–2247.
- Thomson, R.C.; Yaszemski, M.J.; Powers, J.M.; Mikos, A.G. Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J. Biomater. Sci. Polym. Ed. 1996, 7, 23–38.
- Vert, M.; Christel, P.; Chabot, F.; Leray, J. Bioresorbable plastic materials for bone surgery. In Macromolecular Materials; CRC Press: Boca Raton, FL, USA, 2018; pp. 119–142.
- Pulapura, S.; Li, C.; Kohn, J. Structure-property relationships for the design of polyiminocarbonates. Biomaterials 1990, 11, 666–678.
- Daniels, A.U.; Chang, M.K.O.; Andriano, K.P.; Heller, J. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J. Appl. Biomater. 1990, 1, 57–78.
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2015, 45, 659–670.
- Kaihara, S.; Borenstein, J.; Koka, R.; Lalan, S.; Ochoa, E.R.; Ravens, M.; Pien, H.; Cunningham, B.; Vacanti, J.P. Silicon Micromachining to Tissue Engineer Branched Vascular Channels for Liver Fabrication. Tissue Eng. 2000, 6, 105–117.
- Atala, A. Tissue Engineering in the Genitourinary System. In Synthetic Biodegradable Polymer Scaffolds; Atala, A., Mooney, D.J., Eds.; Birkhäuser Boston: Boston, MA, USA, 1997; pp. 149–164. ISBN 978-1-4612-4154-6.
- Nerem, R.M.; Braddon, L.G.; Seliktar, D.; Ziegler, T. Tissue Engineering and the Vascular System. In Synthetic Biodegradable Polymer Scaffolds; Atala, A., Mooney, D.J., Eds.; Birkhäuser Boston: Boston, MA, USA, 1997; pp. 165–185. ISBN 978-1-4612-4154-6.
- Yu, N.Y.C.; Schindeler, A.; Little, D.G.; Ruys, A.J. Biodegradable poly(α-hydroxy acid) polymer scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 285–295.
- Ginjupalli, K.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Nagaraja Upadhya, P. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535.
- Hertz, A.; Bruce, I.J. Inorganic materials for bone repair or replacement applications. Nanomedicine 2007, 2, 899–918.
- Ninan, N.; Muthiah, M.; Park, I.K.; Wong, T.W.; Thomas, S.; Grohens, Y. Natural polymer/inorganic material based hybrid scaffolds for skin wound healing. Polym. Rev. 2015, 55, 453–490.
- Terzaki, K.; Kissamitaki, M.; Skarmoutsou, A.; Fotakis, C.; Charitidis, C.A.; Farsari, M.; Vamvakaki, M.; Chatzinikolaidou, M. Pre-osteoblastic cell response on three-dimensional, organic-inorganic hybrid material scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2283–2294.
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785.
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001.
- Colombo, A.; Chieffo, A.; Frasheri, A.; Garbo, R.; Masotti-Centol, M.; Salvatella, N.; Dominguez, J.F.O.; Steffanon, L.; Tarantini, G.; Presbitero, P.; et al. Second-generation drug-eluting stent implantation followed by 6- Versus 12-month dual antiplatelet therapy. J. Am. Coll. Cardiol. 2014, 64, 2086–2097.
- Ong, A.T.L.; Serruys, P.W. Technology Insight: An overview of research in drug-eluting stents. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 647–658.
- Lee, S.; Hwang, G.; Kim, T.H.; Kwon, S.J.; Kim, J.U.; Koh, K.; Park, B.; Hong, H.; Yu, K.J.; Chae, H.; et al. On-Demand Drug Release from Gold Nanoturf for a Thermo- and Chemotherapeutic Esophageal Stent. ACS Nano 2018, 12, 6756–6766.
- Chen, H.; Ni, J.; Chen, J.; Xue, W.; Wang, J.; Na, H.; Zhu, J. Activation of corn cellulose with alcohols to improve its dissolvability in fabricating ultrafine fibers via electrospinning. Carbohydr. Polym. 2015, 123, 174–179.
- Padil, V.V.T.; Cheong, J.Y.; KP, A.; Makvandi, P.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Černík, M.; Kim, I.-D.; Varma, R.S. Electrospun fibers based on carbohydrate gum polymers and their multifaceted applications. Carbohydr. Polym. 2020, 247, 116705.
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110.
- Palanisamy, C.P.; Cui, B.; Zhang, H.; Gunasekaran, V.P.; Ariyo, A.L.; Jayaraman, S.; Rajagopal, P.; Long, Q. A critical review on starch-based electrospun nanofibrous scaffolds for wound healing application. Int. J. Biol. Macromol. 2022, 222, 1852–1860.
- Schiffman, J.D.; Blackford, A.C.; Wegst, U.G.K.; Schauer, C.L. Carbon black immobilized in electrospun chitosan membranes. Carbohydr. Polym. 2011, 84, 1252–1257.
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658.
- Stijnman, A.C.; Bodnar, I.; Hans Tromp, R. Electrospinning of food-grade polysaccharides. Food Hydrocoll. 2011, 25, 1393–1398.
- Vicini, S.; Castellano, M.; Mauri, M.; Marsano, E. Gelling process for sodium alginate: New technical approach by using calcium rich micro-spheres. Carbohydr. Polym. 2015, 134, 767–774.
- Zhang, H.; Jin, C.; Lv, S.; Ren, F.; Wang, J. Study on electrospinning of wheat gluten: A review. Food Res. Int. 2023, 169, 112851.
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26.
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. Part A 2021, 109, 1751–1764.
- Rodríguez-Zamora, P.; Peña-Juárez, M.C.; Cedillo-Servín, G.; Paloalto-Landon, A.; Ortega-García, I.; Maaza, M.; Vera-Graziano, R. Characterization of mechanically reinforced electrospun dextrin-polyethylene oxide sub-microfiber mats. Polym. Eng. Sci. 2019, 59, 1778–1786.
- Perumcherry, S.R.; Chennazhi, K.P.; Nair, S.V.; Menon, D.; Afeesh, R. A novel method for the fabrication of fibrin-based electrospun nanofibrous scaffold for tissue-engineering applications. Tissue Eng. Part C Methods 2011, 17, 1121–1130.
- Jiang, Q.; Reddy, N.; Yang, Y. Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomater. 2010, 6, 4042–4051.
- Arampatzis, A.S.; Giannakoula, K.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Kampasakali, E.; Willems, A.; Christofilos, D.; Kritis, A.; et al. Novel electrospun poly-hydroxybutyrate scaffolds as carriers for the wound healing agents alkannins and shikonins. Regen. Biomater. 2021, 8, rbab011.
- Zhuravleva, M.; Gilazieva, Z.; Grigoriev, T.E.; Shepelev, A.D.; Tenchurin, T.K.; Kamyshinsky, R.; Krasheninnikov, S.V.; Orlov, S.; Caralogli, G.; Archipova, S.; et al. In vitro assessment of electrospun polyamide-6 scaffolds for esophageal tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 253–268.
- Janmohammadi, M.; Nourbakhsh, M.S. Electrospun polycaprolactone scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 527–539.
- Ciarfaglia, N.; Laezza, A.; Lods, L.; Lonjon, A.; Pepe, A.; Bochicchio, B.; Ciarfaglia, N.; Laezza, A.; Lods, L.; Lonjon, A.; et al. Thermal and dynamic mechanical behavior of poly(lactic acid) (PLA)-based electrospun scaffolds for tissue engineering. J. Appl. Polym. Sci. 2021, 138, 51313.
- Zhang, H.; Liu, J. Electrospun poly(lactic-co-glycolic acid)/wool keratin fibrous composite scaffolds potential for bone tissue engineering applications. J. Bioact. Compat. Polym. 2013, 28, 141–153.
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 7.
- Boland, E.D.; Wnek, G.E.; Simpson, D.G.; Pawlowski, K.J.; Bowlin, G.L. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly(glycolic acid) electrospinning. J. Macromol. Sci. Pure Appl. Chem. 2001, 38, 1231–1243.
- Huang, A.; Peng, X.; Geng, L.; Zhang, L.; Huang, K.; Chen, B.; Gu, Z.; Kuang, T. Electrospun poly (butylene succinate)/cellulose nanocrystals bio-nanocomposite scaffolds for tissue engineering: Preparation, characterization and in vitro evaluation. Polym. Test. 2018, 71, 101–109.
- Griffin, J.; Delgado-Rivera, R.; Meiners, S.; Uhrich, K.E. Salicylic acid-derived poly(anhydride-ester) electrospun fibers designed for regenerating the peripheral nervous system. J. Biomed. Mater. Res. Part A 2011, 97, 230–242.
- Herwig, G.; Perez-Madrigal, M.M.; Dove, A.P. Customized Fading Scaffolds: Strong Polyorthoester Networks via Thiol-Ene Cross-linking for Cytocompatible Surface-Eroding Materials in 3D Printing. Biomacromolecules 2021, 22, 1472–1483.
- Choueka, J.; Charvet, J.L.; Koval, K.J.; Alexander, H.; James, K.S.; Hooper, K.A.; Kohn, J. Canine bone response to tyrosine-derived polycarbonates and poly (L-lactic acid). J. Biomed. Mater. Res. 1996, 31, 35–41.
- Ibim, S.E.M.; Uhrich, K.E.; Attawia, M.; Shastri, V.R.; El-Amin, S.F.; Bronson, R.; Langer, R.; Laurencin, C.T. Preliminary in vivo report on the osteocompatibility of poly(anhydride- co-imides) evaluated in a tibial model. J. Biomed. Mater. Res. 1998, 43, 374–379.
- Peter, S.J.; Lu, L.; Kim, D.J.; Mikos, A.G. Marrow stromal osteoblast function on a poly(propylene fumarate)/β-tricalcium phosphate biodegradable orthopaedic composite. Biomaterials 2000, 21, 1207–1213.
- Yousefzade, O.; Katsarava, R.; Puiggalí, J. Biomimetic hybrid systems for tissue engineering. Biomimetics 2020, 5, 49.
- Carampin, P.; Conconi, M.T.; Lora, S.; Menti, A.M.; Baiguera, S.; Bellini, S.; Grandi, C.; Parnigotto, P.P. Electrospun polyphosphazene nanofibers for in vitro rat endothelial cells proliferation. J. Biomed. Mater. Res. Part A 2007, 80, 661–668.
- Caracciolo, P.C.; Thomas, V.; Vohra, Y.K.; Buffa, F.; Abraham, G.A. Electrospinning of novel biodegradable poly(ester urethane)s and poly(ester urethane urea)s for soft tissue-engineering applications. J. Mater. Sci. Mater. Med. 2009, 20, 2129–2137.
- Gao, X.; Wen, M.; Liu, Y.; Hou, T.; An, M. Mechanical performance and cyocompatibility of PU/PLCL nanofibrous electrospun scaffolds for skin regeneration. Eng. Regen. 2022, 3, 53–58.
- Dorkhani, E.; Noorafkan, Y.; Salehi, Z.; Ghiass, M.A.; Tafti, S.H.A.; Heirani-Tabasi, A.; Tavafoghi, M. Design and fabrication of polyvinylidene fluoride-graphene oxide/gelatine nanofibrous scaffold for cardiac tissue engineering. J. Biomater. Sci. Polym. Ed. 2023, 34, 1195–1216.
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Process. 2018, 33, 479–498.
- Bera, B. Literature Review on Electrospinning Process (A Fascinating Fiber Fabrication Technique). Imp. J. Interdiscip. Res. 2016, 2, 972–984.
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410.
- Park, S.; Park, K.; Yoon, H.; Son, J.; Min, T.; Kim, G. Apparatus for preparing electrospun nanofibers: Designing an electrospinning process for nanofiber fabrication. Polym. Int. 2006, 55, 961–969.
- Yan, S.; Zhang, X.; Zhang, L.; Liu, H.; Wang, X.; Li, Q. Polymer scaffolds for vascular tissue engineering fabricated by combined electrospinning and hot embossing. Biomed. Mater. 2018, 13, 015003.
- Borojeni, I.A.; Gajewski, G.; Riahi, R.A. Application of Electrospun Nonwoven Fibers in Air Filters. Fibers 2022, 10, 15.
- Chen, Y.; Dong, X.; Shafiq, M.; Myles, G.; Radacsi, N.; Mo, X. Recent Advancements on Three-Dimensional Electrospun Nanofiber Scaffolds for Tissue Engineering. Adv. Fiber Mater. 2022, 4, 959–986.
- Jiang, S.; Helfricht, N.; Papastavrou, G.; Greiner, A.; Agarwal, S. Low-Density Self-Assembled Poly(N-Isopropyl Acrylamide) Sponges with Ultrahigh and Extremely Fast Water Uptake and Release. Macromol. Rapid Commun. 2018, 39, 1700838.
- Jiang, S.; Agarwal, S.; Greiner, A. Low-Density Open Cellular Sponges as Functional Materials. Angew. Chemie Int. Ed. 2017, 56, 15520–15538.
- Robinson, A.J.; Pérez-Nava, A.; Ali, S.C.; González-Campos, J.B.; Holloway, J.L.; Cosgriff-Hernandez, E.M. Comparative analysis of fiber alignment methods in electrospinning. Matter 2021, 4, 821–844.
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805.
- Thomas, V.; Dean, D.R.; Jose, M.V.; Mathew, B.; Chowdhury, S.; Vohra, Y.K. Nanostructured biocomposite scaffolds based on collagen coelectrospun with nanohydroxyapatite. Biomacromolecules 2007, 8, 631–637.
- Reneker, D.H.; Yarin, A.L.; Zussman, E.; Xu, H. Electrospinning of Nanofibers from Polymer Solutions and Melts. Adv. Appl. Mech. 2007, 41, 43–195, 345–346.
- Shim, I.K.; Suh, W.H.; Lee, S.Y.; Lee, S.H.; Heo, S.J.; Lee, M.C.; Lee, S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. Part A 2009, 90, 595–602.
- Fan, L.; Zhang, S.; Zhao, G.; Fu, Q. Constructing fibrillated skeleton with highly aligned boron nitride nanosheets confined in alumina fiber via electrospinning and sintering for thermally conductive composite. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106282.
- Shim, I.K.; Jung, M.R.; Kim, K.H.; Seol, Y.J.; Park, Y.J.; Park, W.H.; Lee, S.J. Novel three-dimensional scaffolds of poly (L-lactic acid) microfibers using electrospinning and mechanical expansion: Fabrication and bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 150–160.
- Navaneethan, B.; Chou, C.F. Self-Searching Writing of Human-Organ-Scale Three-Dimensional Topographic Scaffolds with Shape Memory by Silkworm-like Electrospun Autopilot Jet. ACS Appl. Mater. Interfaces 2022, 14, 42841–42851.
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720.
- Lee, J.K.Y.; Chen, N.; Peng, S.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84.
- Daming, Z.; Jiang, C. Electrospinning of three-dimensional nanofibrous tubes with controllable architectures. Nano Lett. 2008, 8, 3283–3287.
- Pensa, N.W.; Curry, A.S.; Bonvallet, P.P.; Bellis, N.F.; Rettig, K.M.; Reddy, M.S.; Eberhardt, A.W.; Bellis, S.L. 3D printed mesh reinforcements enhance the mechanical properties of electrospun scaffolds. Biomater. Res. 2019, 23, 22.
- Mayoral, I.; Bevilacqua, E.; Gómez, G.; Hmadcha, A.; González-Loscertales, I.; Reina, E.; Sotelo, J.; Domínguez, A.; Pérez-Alcántara, P.; Smani, Y.; et al. Tissue engineered in-vitro vascular patch fabrication using hybrid 3D printing and electrospinning. Mater. Today Bio 2022, 14, 100252.
- Centola, M.; Rainer, A.; Spadaccio, C.; De Porcellinis, S.; Genovese, J.A.; Trombetta, M. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2010, 2, 014102.
- Ki, C.S.; Kim, J.W.; Hyun, J.H.; Lee, K.H.; Hattori, M.; Rah, D.K.; Park, Y.H. Electrospun three-dimensional silk fibroin nanofibrous scaffold. J. Appl. Polym. Sci. 2007, 106, 3922–3928.
- Leong, M.F.; Rasheed, M.Z.; Lim, T.C.; Chian, K.S. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D,L-lactide) scaffold fabricated by cryogenic electrospinning technique. J. Biomed. Mater. Res. Part A 2009, 91, 231–240.
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous silica nanoparticles as a promising skin delivery system for methotrexate. Int. J. Pharm. 2017, 530, 239–248.
- Kim, T.G.; Chung, H.J.; Park, T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008, 4, 1611–1619.
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534.
- Klumpp, D.; Rudisile, M.; Kühnle, R.I.; Hess, A.; Bitto, F.F.; Arkudas, A.; Bleiziffer, O.; Boos, A.M.; Kneser, U.; Horch, R.E.; et al. Three-dimensional vascularization of electrospun PCL/collagen-blend nanofibrous scaffolds in vivo. J. Biomed. Mater. Res. Part A 2012, 100, 2302–2311.
- Sun, B.; Long, Y.Z.; Yu, F.; Li, M.M.; Zhang, H.D.; Li, W.J.; Xu, T.X. Self-assembly of a three-dimensional fibrous polymer sponge by electrospinning. Nanoscale 2012, 4, 2134–2137.
- Han, D.; Gouma, P.I. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 37–41.
- Xu, Y.; Patnaik, S.; Guo, X.; Li, Z.; Lo, W.; Butler, R.; Claude, A.; Liu, Z.; Zhang, G.; Liao, J.; et al. Cardiac differentiation of cardiosphere-derived cells in scaffolds mimicking morphology of the cardiac extracellular matrix. Acta Biomater. 2014, 10, 3449–3462.
- Weiss, D.J.; Bates, J.H.T.; Gilbert, T.; Liles, W.C.; Lutzko, C.; Rajagopal, J.; Prockop, D. Stem cells and cell therapies in lung biology and diseases: Conference report. Ann. Am. Thorac. Soc. 2013, 10, S25–S44.
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up. Sci. Rep. 2016, 6, 23761.
- Yeo, M.; Kim, G.H. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020, 107, 102–114.
- Mejía Suaza, M.L.; Moncada, M.E.; Ossa-Orozco, C.P. Characterization of Electrospun Silk Fibroin Scaffolds for Bone Tissue Engineering: A Review. TecnoLógicas 2020, 23, 33–51.
- Cui, W.; Zhou, Y.; Chang, J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci. Technol. Adv. Mater. 2010, 11, 014108.
- Liu, Z.; Ramakrishna, S.; Liu, X. Electrospinning and emerging healthcare and medicine possibilities. APL Bioeng. 2020, 4, 030901.
- Tanaka, E.M.; Ferretti, P. Considering the evolution of regeneration in the central nervous system. Nat. Rev. Neurosci. 2009, 10, 713–723.
- Bard, P. Anatomical organization of the central nervous system in relation to control of the heart and blood vessels. Physiol. Rev. Suppl. 1960, 4, 3–26.
- Popović, D.B.; Sinkjær, T. Diseases and Injuries of the Central Nervous System Leading to Sensory–Motor Impairment. In Introduction to Neural Engineering for Motor Rehabilitation; IEEE Press: Piscataway, NJ, USA, 2013; pp. 3–20. ISBN 9781118628522.
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. BioMed Res. Int. 2016, 2016, 3856262.
- Muheremu, A.; Ao, Q. Past, Present, and Future of Nerve Conduits in the Treatment of Peripheral Nerve Injury. BioMed Res. Int. 2015, 2015, 237507.
- Manktelow, R.T. Functioning Muscle Transplantation to the Upper Limb. Clin. Plast. Surg. 1984, 11, 65–71.
- Quan, Q.; Chang, B.; Meng, H.Y.; Liu, R.X.; Wang, Y.; Lu, S.B.; Peng, J.; Zhao, Q. Use of electrospinning to construct biomaterials for peripheral nerve regeneration. Rev. Neurosci. 2016, 27, 761–768.
- Panseri, S.; Cunha, C.; Lowery, J.; Del Carro, U.; Taraballi, F.; Amadio, S.; Vescovi, A.; Gelain, F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008, 8, 39.
- Li, Y.F.; Gregersen, H.; Nygaard, J.V.; Cheng, W.; Yu, Y.; Huang, Y.; Dong, M.; Besenbacher, F.; Chen, M. Ultraporous nanofeatured PCL-PEO microfibrous scaffolds enhance cell infiltration, colonization and myofibroblastic differentiation. Nanoscale 2015, 7, 14989–14995.
- Gregory, H.; Phillips, J.B. Materials for peripheral nerve repair constructs: Natural proteins or synthetic polymers? Neurochem. Int. 2021, 143, 104953.
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150.
- Hild, M.; Toskas, G.; Aibibu, D.; Wittenburg, G.; Meissner, H.; Cherif, C.; Hund, R.D. Chitosan/gelatin micro/nanofiber 3D composite scaffolds for regenerative medicine. Compos. Interfaces 2014, 21, 301–308.
- Jiang, T.; Carbone, E.J.; Lo, K.W.H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24.
- Entekhabi, E.; Haghbin Nazarpak, M.; Shafieian, M.; Mohammadi, H.; Firouzi, M.; Hassannejad, Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2021, 109, 300–312.
- Hurtado, A.; Cregg, J.M.; Wang, H.B.; Wendell, D.F.; Oudega, M.; Gilbert, R.J.; McDonald, J.W. Robust CNS regeneration after complete spinal cord transection using aligned poly-l-lactic acid microfibers. Biomaterials 2011, 32, 6068–6079.
- Taskin, M.B.; Xu, R.; Gregersen, H.; Nygaard, J.V.; Besenbacher, F.; Chen, M. Three-Dimensional Polydopamine Functionalized Coiled Microfibrous Scaffolds Enhance Human Mesenchymal Stem Cells Colonization and Mild Myofibroblastic Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 15864–15873.
- Kim, Y.T.; Haftel, V.K.; Kumar, S.; Bellamkonda, R.V. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials 2008, 29, 3117–3127.
- Huber, A.B.; Kolodkin, A.L.; Ginty, D.D.; Cloutier, J.F. Signaling at the growth cone: Ligand-receptor complexes and the control of axon growth and guidance. Annu. Rev. Neurosci. 2003, 26, 509–563.
- Li, X.; Li, M.; Sun, J.; Zhuang, Y.; Shi, J.; Guan, D.; Chen, Y.; Dai, J. Radially Aligned Electrospun Fibers with Continuous Gradient of SDF1α for the Guidance of Neural Stem Cells. Small 2016, 12, 5009–5018.
- Lee, D.J.; Fontaine, A.; Meng, X.; Park, D. Biomimetic Nerve Guidance Conduit Containing Intraluminal Microchannels with Aligned Nanofibers Markedly Facilitates in Nerve Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1403–1410.
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.Q.; Yang, Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials 2020, 255, 120164.
- Kim, T.G.; Shin, H.; Lim, D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012, 22, 2446–2468.
- Roldán, S.; Vargas, C.; Mejía, M.; Zapata, J.; Moncada, M. Tipos de Biomateriales Empleaos en la Ingenieria de Tejidos. In Ingeniería de Tejidos y Aplicaciones; Fondo Editorial ITM: Medellín, Colombia, 2016; ISBN 9789588743844.
- Heumann, R.; Lindholm, D.; Bandtlow, C.; Meyer, M.; Radeke, M.J.; Misko, T.P.; Shooter, E.; Thoenen, H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: Role of macrophages. Proc. Natl. Acad. Sci. USA 1987, 84, 8735–8739.
- Liu, H.M.; Yang, L.H.; Yang, Y.J. Schwann Cell Properties: 3. C-fos Expression, bFGF Production, Phagocytosis and Proliferation During Wallerian Degeneration. J. Neuropathol. Exp. Neurol. 1995, 54, 487–496.
- Wittmer, C.R.; Claudepierre, T.; Reber, M.; Wiedemann, P.; Garlick, J.A.; Kaplan, D.; Egles, C. Multifunctionalized electrospun silk fibers promote axon regeneration in the central nervous system. Adv. Funct. Mater. 2011, 21, 4232–4242.
- Dinis, T.M.; Elia, R.; Vidal, G.; Auffret, A.; Kaplan, D.L.; Egles, C. Method to form a fiber/growth factor dual-gradient along electrospun silk for nerve regeneration. ACS Appl. Mater. Interfaces 2014, 6, 16817–16826.
- Roman, J.A.; Reucroft, I.; Martin, R.A.; Hurtado, A.; Mao, H.Q. Local Release of Paclitaxel from Aligned, Electrospun Microfibers Promotes Axonal Extension. Adv. Healthc. Mater. 2016, 5, 2628–2635.
- Wu, Y.; Wang, L.; Guo, B.; Shao, Y.; Ma, P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31.
- Maurel, P.; Einheber, S.; Galinska, J.; Thaker, P.; Lam, I.; Rubin, M.B.; Scherer, S.S.; Murakami, Y.; Gutmann, D.H.; Salzer, J.L. Nectin-like proteins mediate axon-Schwann cell interactions along the internode and are essential for myelination. J. Cell Biol. 2007, 178, 861–874.
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539.
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025.
- Wang, W.; Itoh, S.; Konno, K.; Kikkawa, T.; Ichinose, S.; Sakai, K.; Ohkuma, T.; Watabe, K. Effects of Schwann cell alignment along the oriented electrospun chitosan nanofibers on nerve regeneration. J. Biomed. Mater. Res. Part A 2009, 91, 994–1005.
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed. Mater. 2011, 6, 025004.
- Wang, T.Y.; Forsythe, J.S.; Nisbet, D.R.; Parish, C.L. Promoting engraftment of transplanted neural stem cells/progenitors using biofunctionalised electrospun scaffolds. Biomaterials 2012, 33, 9188–9197.
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136.
- Losquadro, W.D. Anatomy of the Skin and the Pathogenesis of Nonmelanoma Skin Cancer. Facial Plast. Surg. Clin. N. Am. 2017, 25, 283–289.
- Teo, W.E.; He, W.; Ramakrishna, S. Electrospun scaffold tailored for tissue-specific extracellular matrix. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 918–929.
- Lin, H.Y.; Chen, H.H.; Chang, S.H.; Ni, T.S. Pectin-chitosan-PVA nanofibrous scaffold made by electrospinning and its potential use as a skin tissue scaffold. J. Biomater. Sci. Polym. Ed. 2013, 24, 470–484.
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107.
- Sun, L.; Gao, W.; Fu, X.; Shi, M.; Xie, W.; Zhang, W.; Zhao, F.; Chen, X. Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the basketweave pattern of collagen fibrils in native skin. Biomater. Sci. 2018, 6, 340–349.
- Sun, X.; Cheng, L.; Zhu, W.; Hu, C.; Jin, R.; Sun, B.; Shi, Y.; Zhang, Y.; Cui, W. Use of ginsenoside Rg3-loaded electrospun PLGA fibrous membranes as wound cover induces healing and inhibits hypertrophic scar formation of the skin. Colloids Surf. B Biointerfaces 2014, 115, 61–70.
- Sun, X.; Cheng, L.; Zhao, J.; Jin, R.; Sun, B.; Shi, Y.; Zhang, L.; Zhang, Y.; Cui, W. BFGF-grafted electrospun fibrous scaffolds via poly(dopamine) for skin wound healing. J. Mater. Chem. B 2014, 2, 3636–3645.
- Larjava, H.; Häkkinen, L.; Koivisto, L. Re-epithelialization of wounds. In Oral Wound Healing; Larjava, H., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 81–123.
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv. Compos. Hybrid Mater. 2022, 5, 1111–1125.
- Wang, L.; Yang, J.; Ran, B.; Yang, X.; Zheng, W.; Long, Y.; Jiang, X. Small Molecular TGF-β1-Inhibitor-Loaded Electrospun Fibrous Scaffolds for Preventing Hypertrophic Scars. ACS Appl. Mater. Interfaces 2017, 9, 32545–32553.
- Shin, S.H.; Purevdorj, O.; Castano, O.; Planell, J.A.; Kim, H.W. A short review: Recent advances in electrospinning for bone tissue regeneration. J. Tissue Eng. 2012, 3, 2041731412443530.
- Jang, J.H.; Castano, O.; Kim, H.W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083.
- Calvert, J.W.; Weiss, L.E.; Sundine, M.J. New frontiers in bone tissue engineering. Clin. Plast. Surg. 2003, 30, 641–648.
- Cornell, C.N. Osteoconductive materials and their role as substitutes for autogenous bone grafts. Orthop. Clin. N. Am. 1999, 30, 591–598.
- Di Martino, A.; Liverani, L.; Rainer, A.; Salvatore, G.; Trombetta, M.; Denaro, V. Electrospun scaffolds for bone tissue engineering. Musculoskelet. Surg. 2011, 95, 69–80.
- Pramanik, S.; Pingguan-Murphy, B.; Abu Osman, N.A. Progress of key strategies in development of electrospun scaffolds: Bone tissue. Sci. Technol. Adv. Mater. 2012, 13, 043002.
- Yu, Y.; Hua, S.; Yang, M.; Fu, Z.; Teng, S.; Niu, K.; Zhao, Q.; Yi, C. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv. 2016, 6, 110557–110565.
- Jain, S.; Krishna Meka, S.R.; Chatterjee, K. Curcumin eluting nanofibers augment osteogenesis toward phytochemical based bone tissue engineering. Biomed. Mater. 2016, 11, 055007.
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491.
- Allo, B.A.; Rizkalla, A.S.; Mequanint, K. Synthesis and electrospinning of ε-polycaprolactone-bioactive glass hybrid biomaterials via a sol-gel process. Langmuir 2010, 26, 18340–18348.
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore Size of Porous Hydroxyapatite as the Cell-Substratum Controls BMP-Induced Osteogenesis. J. Biochem. 1997, 121, 317–324.
- Kim, H.-W.; Lee, H.-H.; Knowles, J.C. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly(lactic acid) for bone regeneration. J. Biomed. Mater. Res. Part A 2006, 79A, 643–649.
- Barbon, S.; Contran, M.; Stocco, E.; Todros, S.; Macchi, V.; De Caro, R.; Porzionato, A. Enhanced biomechanical properties of polyvinyl alcohol-based hybrid scaffolds for cartilage tissue engineering. Processes 2021, 9, 730.
- Morejón, L.; Delgado, J.A.; Ribeiro, A.A.; de Oliveira, M.V.; Mendizábal, E.; García, I.; Alfonso, A.; Poh, P.; van Griensven, M.; Balmayor, E.R. Development, characterization and in vitro biological properties of scaffolds fabricated from calcium phosphate nanoparticles. Int. J. Mol. Sci. 2019, 20, 1790.
- Song, L.; Xie, X.; Lv, C.; ur Rehman Khan, A.; Sun, Y.; Li, R.; Yao, J.; El-Newehy, M.; El-Hamshary, H.; Morsi, Y.; et al. Electrospun biodegradable nanofibers loaded with epigallocatechin gallate for guided bone regeneration. Compos. Part B Eng. 2022, 238, 109920.
- Guo, B.; Glavas, L.; Albertsson, A.C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286.
- Alonso, L.M.; Torres, I.F.; Tamayo, Á.M.Z.; Lozano, O.E.L.; Ramos, I.D.; García-Menocal, J.Á.D.; Rios-Donato, N.; Mendizábal, E. Antibacterial effect of acrylic bone cements loaded with drugs of different action’s mechanism. J. Infect. Dev. Ctries 2019, 13, 487–495.
- Venugopal, J.R.; Low, S.; Choon, A.T.; Kumar, A.B.; Ramakrishna, S. Nanobioengineered electrospun composite nanofibers and osteoblasts for bone regeneration. Artif. Organs 2008, 32, 388–397.
- Morejón, L.; Mendizábal, A.E.; García-Menocal, J.A.D.; Ginebra, M.P.; Aparicio, C.; Mur, F.J.G.; Marsal, M.; Davidenko, N.; Ballesteros, M.E.; Planell, J.A. Static mechanical properties of hydroxyapatite (HA) powder-filled acrylic bone cements: Effect of type of HA powder. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 345–352.
- Caeiro, J.R.; González, P.; Guede, D. Biomechanics and bone (& II): Trials in different hierarchical levels of bone and alternative tools for the determination of bone strength. Rev. Osteoporos. Metab. Miner. 2013, 5, 99–108.
- Madhurakkat Perikamana, S.K.; Lee, J.; Ahmad, T.; Jeong, Y.; Kim, D.G.; Kim, K.; Shin, H. Effects of immobilized BMP-2 and nanofiber morphology on in vitro osteogenic differentiation of hMSCs and in vivo collagen assembly of regenerated bone. ACS Appl. Mater. Interfaces 2015, 7, 8798–8808.
- Ding, Y.; Li, W.; Müller, T.; Schubert, D.W.; Boccaccini, A.R.; Yao, Q.; Roether, J.A. Electrospun Polyhydroxybutyrate/Poly(ϵ-caprolactone)/58S Sol-Gel Bioactive Glass Hybrid Scaffolds with Highly Improved Osteogenic Potential for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17098–17108.
- Banimohamad-Shotorbani, B.; Rahmani Del Bakhshayesh, A.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The efficiency of PCL/HAp electrospun nanofibers in bone regeneration: A review. J. Med. Eng. Technol. 2021, 45, 511–531.
- Gao, X.; Song, J.; Ji, P.; Zhang, X.; Li, X.; Xu, X.; Wang, M.; Zhang, S.; Deng, Y.; Deng, F.; et al. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 3499–3515.
- Dhinasekaran, D.; Vimalraj, S.; Rajendran, A.R.; Saravanan, S.; Purushothaman, B.; Subramaniam, B. Bio-inspired multifunctional collagen/electrospun bioactive glass membranes for bone tissue engineering applications. Mater. Sci. Eng. C 2021, 126, 111856.
- Wang, L.; Qiu, Y.; Lv, H.; Si, Y.; Liu, L.; Zhang, Q.; Cao, J.; Yu, J.; Li, X.; Ding, B. 3D Superelastic Scaffolds Constructed from Flexible Inorganic Nanofibers with Self-Fitting Capability and Tailorable Gradient for Bone Regeneration. Adv. Funct. Mater. 2019, 29, 1901407.
- Sofi, H.S.; Ashraf, R.; Beigh, M.A.; Sheikh, F.A. Scaffolds Fabricated from Natural Polymers/Composites by Electrospinning for Bone Tissue Regeneration. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer: Singapore, 2018; pp. 49–78. ISBN 978-981-13-0950-2.
- Castilla-Casadiego, D.A.; Maldonado, M.; Sundaram, P.; Almodovar, J. “Green” electrospinning of a collagen/hydroxyapatite composite nanofibrous scaffold. MRS Commun. 2016, 6, 402–407.
- Schröder, H.C.; Wang, X.; Neufurth, M.; Wang, S.; Tan, R.; Müller, W.E.G. Inorganic Polymeric Materials for Injured Tissue Repair: Biocatalytic Formation and Exploitation. Biomedicines 2022, 10, 658.
- Wren, A.W. 45S5 Bioglass Based Scaffolds for Skeletal Repair. In Biocompatible Glasses: From Bone Regeneration to Cancer Treatment; Marchi, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 183–201. ISBN 978-3-319-44249-5.
- Müller, A.; Schleid, T.; Doser, M.; Müschenborn, N.; Tautzenberger, A.; Ignatius, A.; Clauß, B.; Buchmeiser, M.R. Calcium Cl/OH-apatite, Cl/OH-apatite/Al2O3 and Ca3(PO4)2 fibre nonwovens: Potential ceramic components for osteosynthesis. J. Eur. Ceram. Soc. 2014, 34, 3993–4000.
- Dong, S.; Sun, J.; Li, Y.; Li, J.; Cui, W.; Li, B. Electrospun nanofibrous scaffolds of poly (l-lactic acid)-dicalcium silicate composite via ultrasonic-aging technique for bone regeneration. Mater. Sci. Eng. C 2014, 35, 426–433.
- Sanaei-rad, P.; Jafarzadeh Kashi, T.S.; Seyedjafari, E.; Soleimani, M. Enhancement of stem cell differentiation to osteogenic lineage on hydroxyapatite-coated hybrid PLGA/gelatin nanofiber scaffolds. Biologicals 2016, 44, 511–516.
- Kim, H.; Che, L.; Ha, Y.; Ryu, W. Mechanically-reinforced electrospun composite silk fibroin nanofibers containing hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2014, 40, 324–335.
- Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q.; Li, Z. Greener synthesis of electrospun collagen/ hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215.
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322.
- Saveleva, M.S.; Ivanov, A.N.; Kurtukova, M.O.; Atkin, V.S.; Ivanova, A.G.; Lyubun, G.P.; Martyukova, A.V.; Cherevko, E.I.; Sargsyan, A.K.; Fedonnikov, A.S.; et al. Hybrid PCL/CaCO3 scaffolds with capabilities of carrying biologically active molecules: Synthesis, loading and in vivo applications. Mater. Sci. Eng. C 2018, 85, 57–67.
- Coverdale, B. Incorporation of Surfactants into Electrospun Scaffolds for Improved Bone Tissue Engineering Applications. Ph.D. Thesis, University of Manchester, Manchester, UK, 2016.
- Gualandi, C.; Celli, A.; Zucchelli, A.; Focarete, M.L. Nanohybrid Materials by Electrospinning. In Organic-Inorganic Hybrid Nanomaterials; Kalia, S., Haldorai, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 87–142. ISBN 978-3-319-13593-9.
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous poly(lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol. Biosci. 2008, 8, 328–338.
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440.
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263.
- McKeon-Fischer, K.D.; Freeman, J.W. Characterization of electrospun poly (L-lactide) and gold nanoparticle composite scaffolds for skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 560–568.
- Bahremandi-Toloue, E.; Mohammadalizadeh, Z.; Mukherjee, S.; Karbasi, S. Incorporation of inorganic bioceramics into electrospun scaffolds for tissue engineering applications: A review. Ceram. Int. 2022, 48, 8803–8837.
- Martins, A.; Chung, S.; Pedro, A.J.; Sousa, R.A.; Marques, A.P.; Reis, R.L.; Neves, N.M. Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J. Tissue Eng. Regen. Med. 2009, 3, 37–42.
- Cai, Y.Z.; Zhang, G.R.; Wang, L.L.; Jiang, Y.Z.; Ouyang, H.W.; Zou, X.H. Novel biodegradable three-dimensional macroporous scaffold using aligned electrospun nanofibrous yarns for bone tissue engineering. J. Biomed. Mater. Res. Part A 2012, 100, 1187–1194.
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69.
- Stockwell, R.A. The cell density of human articular and costal cartilage. J. Anat. 1967, 101, 753–763.
- Caplan, A.I. Cartilage. Sci. Am. 1984, 251, 84–97.
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468.
- Myers, E.R.; Lai, W.M.; Mow, V.C. A Continuum Theory and an Experiment for the Ion-Induced Swelling Behavior of Articular Cartilage. J. Biomech. Eng. 1984, 106, 151–158.
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Xue, B.; Gao, X.; Qin, M.; Wang, W.; Chen, B.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 620.
- Mahmood, H.; Eckold, D.; Stead, I.; Shepherd, D.E.T.; Espino, D.M.; Dearn, K.D. A method for the assessment of the coefficient of friction of articular cartilage and a replacement biomaterial. J. Mech. Behav. Biomed. Mater. 2020, 103, 103580.
- Liu, S.H.; Yang, R.S.; Al-Shaikh, R.; Lane, J.M. Collagen in tendon, ligament, and bone healing: A current review. Clin. Orthop. Relat. Res. 1995, 318, 265–278.
- Knutsen, G.; Drogset, J.O.; Engebretsen, L.; Grøntvedt, T.; Isaksen, V.; Ludvigsen, T.C.; Roberts, S.; Solheim, E.; Strand, T.; Johansen, O. A randomized trial comparing autologous chondrocyte implantation with microfracture: Findings at five years. J. Bone Jt. Surg. 2007, 89, 2105–2112.
- Vilela, C.A.; Correia, C.; Oliveira, J.M.; Sousa, R.A.; Espregueira-Mendes, J.; Reis, R.L. Cartilage Repair Using Hydrogels: A Critical Review of in Vivo Experimental Designs. ACS Biomater. Sci. Eng. 2015, 1, 726–739.
- Katti, A.; Shimpi, N.; Roy, S.; Lu, H.; Fabrizio, E.F.; Dass, A.; Capadona, L.A.; Leventis, N. Chemical, physical, and mechanical characterization of isocyanate cross-Linked amine-modified silica aerogels. Chem. Mater. 2006, 18, 285–296.
- Li, Y.; Guo, J.; Li, M.; Tang, Y.; Murugadoss, V.; Seok, I.; Yu, J.; Sun, L.; Sun, C.; Luo, Y. Recent Application of Cellulose Gel in Flexible Sensing-A Review. ES Food Agrofor. 2021, 4, 9–27.
- Bosworth, L.A.; Turner, L.A.; Cartmell, S.H. State of the art composites comprising electrospun fibres coupled with hydrogels: A review. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 322–335.
- Moroni, L.; Schotel, R.; Hamann, D.; de Wijn, J.R.; van Blitterswijk, C.A. 3D fiber-deposited electrospun integrated scaffolds enhance cartilage tissue formation. Adv. Funct. Mater. 2008, 18, 53–60.
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018, 66, 166–176.
- Wood, A.T.; Everett, D.; Budhwani, K.I.; Dickinson, B.; Thomas, V. Wet-laid soy fiber reinforced hydrogel scaffold: Fabrication, mechano-morphological and cell studies. Mater. Sci. Eng. C 2016, 63, 308–316.
- Butcher, A.L.; Offeddu, G.S.; Oyen, M.L. Nanofibrous hydrogel composites as mechanically robust tissue engineering scaffolds. Trends Biotechnol. 2014, 32, 564–570.
- Wise, J.K.; Yarin, A.L.; Megaridis, C.M.; Cho, M. Chondrogenic Differentiation of Human Mesenchymal Stem Cells on Oriented Nanofibrous Scaffolds: Engineering the Superficial Zone of Articular Cartilage. Tissue Eng. Part A 2008, 15, 913–921.
- Chen, W.; Xu, Y.; Liu, Y.; Wang, Z.; Li, Y.; Jiang, G.; Mo, X.; Zhou, G. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater. Des. 2019, 179, 107886.
- Zheng, R.; Duan, H.; Xue, J.; Liu, Y.; Feng, B.; Zhao, S.; Zhu, Y.; Liu, Y.; He, A.; Zhang, W.; et al. The influence of Gelatin/PCL ratio and 3-D construct shape of electrospun membranes on cartilage regeneration. Biomaterials 2014, 35, 152–164.
- Shin, H.J.; Lee, C.H.; Cho, I.H.; Kim, Y.J.; Lee, Y.J.; Kim, I.A.; Park, K.D.; Yui, N.; Shin, J.W. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: Mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J. Biomater. Sci. Polym. Ed. 2006, 17, 103–119.
- Chen, J.P.; Su, C.H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243.
- Martin, A.R.; Patel, J.M.; Locke, R.C.; Eby, M.R.; Saleh, K.S.; Davidson, M.D.; Sennett, M.L.; Zlotnick, H.M.; Chang, A.H.; Carey, J.L.; et al. Nanofibrous hyaluronic acid scaffolds delivering TGF-β3 and SDF-1α for articular cartilage repair in a large animal model. Acta Biomater. 2021, 126, 170–182.
- Cheng, G.; Davoudi, Z.; Xing, X.; Yu, X.; Cheng, X.; Li, Z.; Deng, H.; Wang, Q. Advanced Silk Fibroin Biomaterials for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2704–2715.
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011.
- Mohabatpour, F.; Karkhaneh, A.; Sharifi, A.M. A hydrogel/fiber composite scaffold for chondrocyte encapsulation in cartilage tissue regeneration. RSC Adv. 2016, 6, 83135–83145.
- De Mori, A.; Fernández, M.P.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 2018, 10, 285.
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358.
- Chen, Y.; Xu, W.; Shafiq, M.; Song, D.; Xie, X.; Yuan, Z.; EL-Newehy, M.; EL-Hamshary, H.; Morsi, Y.; Liu, Y.; et al. Chondroitin sulfate cross-linked three-dimensional tailored electrospun scaffolds for cartilage regeneration. Biomater. Adv. 2022, 134, 112643.
- Leong, N.L.; Kator, J.L.; Clemens, T.L.; James, A.; Enamoto-Iwamoto, M.; Jiang, J. Tendon and Ligament Healing and Current Approaches to Tendon and Ligament Regeneration. J. Orthop. Res. 2020, 38, 7–12.
- Suzuki, D.; Murakami, G.; Minoura, N. Crocodilian bone-tendon an bone-ligament interfaces. Ann. Anat. 2003, 185, 425–433.
- Lei, T.; Zhang, T.; Ju, W.; Chen, X.; Heng, B.C.; Shen, W.; Yin, Z. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact. Mater. 2021, 6, 2491–2510.
- Hasslund, S.; Jacobson, J.A.; Dadali, T.; Basile, P.; Ulrich-Vinther, M.; Søballe, K.; Schwarz, E.M.; O’Keefe, R.J.; Mitten, D.J.; Awad, H.A. Adhesions in a murine flexor tendon graft model: Autograft versus allograft reconstruction. J. Orthop. Res. 2008, 26, 824–833.
- Moshiri, A. Tendon and Ligament Tissue Engineering, Healing and Regenerative Medicine. J. Sports Med. Doping Stud. 2013, 3, 1000126.
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25.
- Sensini, A.; Massafra, G.; Gotti, C.; Zucchelli, A.; Cristofolini, L. Tissue Engineering for the Insertions of Tendons and Ligaments: An Overview of Electrospun Biomaterials and Structures. Front. Bioeng. Biotechnol. 2021, 9, 645544.
- Sensini, A.; Gualandi, C.; Focarete, M.L.; Belcari, J.; Zucchelli, A.; Boyle, L.; Reilly, G.C.; Kao, A.P.; Tozzi, G.; Cristofolini, L. Multiscale hierarchical bioresorbable scaffolds for the regeneration of tendons and ligaments. Biofabrication 2019, 11, 035026.
- Czaplewski, S.K.; Tsai, T.L.; Duenwald-Kuehl, S.E.; Vanderby, R.; Li, W.J. Tenogenic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells dictated by properties of braided submicron fibrous scaffolds. Biomaterials 2014, 35, 6907–6917.
- Deepthi, S.; Jeevitha, K.; Nivedhitha Sundaram, M.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid hydrogel coated poly(caprolactone) multiscale bilayer scaffold for ligament regeneration. Chem. Eng. J. 2015, 260, 478–485.
- Yang, G.; Lin, H.; Rothrauff, B.B.; Yu, S.; Tuan, R.S. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 2016, 35, 68–76.
- Zhang, C.; Wang, X.; Zhang, E.; Yang, L.; Yuan, H.; Tu, W.; Zhang, H.; Yin, Z.; Shen, W.; Chen, X.; et al. An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater. 2018, 66, 141–156.
- Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; et al. Hybrid small-diameter vascular grafts: Anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials 2016, 76, 359–370.
- Handa, R.; Sharma, S. Vascular Graft Failure Of Leg Arterial Bypasses—A Review. J. Hypertens. Cardiol. 2014, 1, 17–21.
- Niu, Y.; Galluzzi, M.; Fu, M.; Hu, J.; Xia, H. In vivo performance of electrospun tubular hyaluronic acid/collagen nanofibrous scaffolds for vascular reconstruction in the rabbit model. J. Nanobiotechnology 2021, 19, 349.
- Emechebe, G.A.; Obiweluozor, F.O.; Jeong, I.S.; Park, J.K.; Park, C.H.; Kim, C.S. Merging 3D printing with electrospun biodegradable small-caliber vascular grafts immobilized with VEGF. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102306.
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Sutherland, F.W.H.; Chello, M.; Trombetta, M.; Rainer, A. Preliminary in vivo evaluation of a hybrid armored vascular graft combining electrospinning and additive manufacturing techniques. Drug Target Insights 2016, 10, DTI.S35202.
- Lee, S.J.; Kim, M.E.; Nah, H.; Seok, J.M.; Jeong, M.H.; Park, K.; Kwon, I.K.; Lee, J.S.; Park, S.A. Vascular endothelial growth factor immobilized on mussel-inspired three-dimensional bilayered scaffold for artificial vascular graft application: In vitro and in vivo evaluations. J. Colloid Interface Sci. 2019, 537, 333–344.
- Zhou, F.; Jia, X.; Yang, Y.; Yang, Q.; Gao, C.; Hu, S.; Zhao, Y.; Fan, Y.; Yuan, X. Nanofiber-mediated microRNA-126 delivery to vascular endothelial cells for blood vessel regeneration. Acta Biomater. 2016, 43, 303–313.
- Braghirolli, D.I.; Helfer, V.E.; Chagastelles, P.C.; Dalberto, T.P.; Gamba, D.; Pranke, P. Electrospun scaffolds functionalized with heparin and vascular endothelial growth factor increase the proliferation of endothelial progenitor cells. Biomed. Mater. 2017, 12, 025003.
- Wang, S.; Zhang, Y.; Wang, H.; Yin, G.; Dong, Z. Fabrication and properties of the electrospun polylactide/silk fibroin-gelatin composite tubular scaffold. Biomacromolecules 2009, 10, 2240–2244.
- Yuan, H.; Qin, J.; Xie, J.; Li, B.; Yu, Z.; Peng, Z.; Yi, B.; Lou, X.; Lu, X.; Zhang, Y. Highly aligned core-shell structured nanofibers for promoting phenotypic expression of vSMCs for vascular regeneration. Nanoscale 2016, 8, 16307–16322.
- Geng, X.; Xu, Z.Q.; Tu, C.Z.; Peng, J.; Jin, X.; Ye, L.; Zhang, A.Y.; Gu, Y.Q.; Feng, Z.G. Hydrogel Complex Electrospun Scaffolds and Their Multiple Functions in in Situ Vascular Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 2373–2384.
- Ren, J.; Xu, Q.; Chen, X.; Li, W.; Guo, K.; Zhao, Y.; Wang, Q.; Zhang, Z.; Peng, H.; Li, Y.G. Superaligned Carbon Nanotubes Guide Oriented Cell Growth and Promote Electrophysiological Homogeneity for Synthetic Cardiac Tissues. Adv. Mater. 2017, 29, 1702713.
- Jin, G.; He, R.; Sha, B.; Li, W.; Qing, H.; Teng, R.; Xu, F. Electrospun three-dimensional aligned nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C 2018, 92, 995–1005.
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738.
- Parrag, I.C.; Zandstra, P.W.; Woodhouse, K.A. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol. Bioeng. 2012, 109, 813–822.
- Sirivisoot, S.; Harrison, B.S. Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. Int. J. Nanomed. 2011, 6, 2483–2497.
- Carlberg, B.; Axell, M.Z.; Nannmark, U.; Liu, J.; Kuhn, H.G. Electrospun polyurethane scaffolds for proliferation and neuronal differentiation of human embryonic stem cells. Biomed. Mater. 2009, 4, 045004.
- Genchi, G.G.; Sinibaldi, E.; Ceseracciu, L.; Labardi, M.; Marino, A.; Marras, S.; De Simoni, G.; Mattoli, V.; Ciofani, G. Ultrasound-activated piezoelectric P(VDF-TrFE)/boron nitride nanotube composite films promote differentiation of human SaOS-2 osteoblast-like cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2421–2432.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
08 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




