
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manuel Muñoz-Torres | -- | 2922 | 2023-08-07 12:55:04 | | | |
| 2 | Peter Tang | + 4 word(s) | 2926 | 2023-08-08 03:10:09 | | |
Video Upload Options
The management and early treatment of type 2 diabetes (T2D) are essential to prevent further complications involving loss of quality of life and premature death. It is unclear whether vitamin D deficiency might be contributing to an increased T2D risk. A vast body of evidence associates vitamin D deficiency and T2D. This relationship could be mediated by the direct and indirect effects of vitamin D on glucose homeostasis such as insulin secretion, insulin sensitivity, and systemic inflammation.
1. Vitamin D Physiology and Glucose Homeostasis
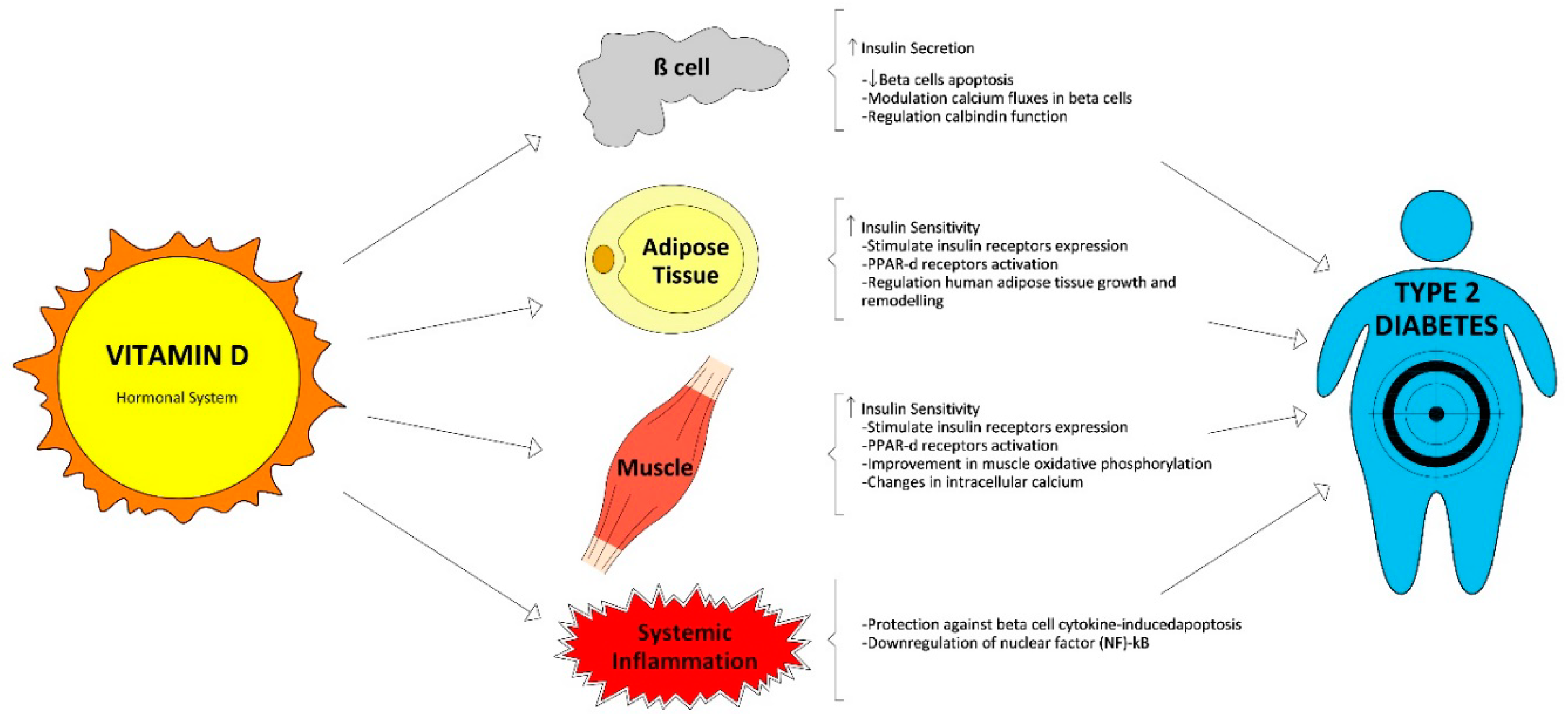
2. Vitamin D Status and Its Relationship with T2D in Cross-Sectional and Longitudinal Studies
3. Vitamin D Supplementation and Risk of T2D: Randomized Trials and Meta-Analysis
|
Study, Year |
Country |
Population Characteristics (Mean age/age range (years)) |
Type of Treatment |
N |
Duration (months) |
Main Outcomes |
|---|---|---|---|---|---|---|
|
LeBlanc et al. D2d Research Group [32] 2018 |
US |
Prediabetes (59) |
4000 IU D3/day vs. placebo |
2423 |
36 |
Not yet published |
|
Forouhi et al. [33] 2016 |
UK |
Prediabetes IFG or IGT or positive diabetes risk score (53) |
100,000 IU D2/month or 100,000 IU D3/month vs. placebo |
340 |
4 |
No effect on HbA1c Improve pulse wave velocity (arterial stiffness) |
|
Wagner et al. [34] 2016 |
Sweden |
Prediabetes or diet-controlled T2D (67.3) |
30,000 IU D3/week vs. placebo |
44 |
2 |
No difference in insulin secretion/sensitivity, beta cell function and glucose tolerance |
|
Tuomainen et al. [35] 2015 |
Finland |
Prediabetes (65.7) |
40 μg/day D3 or 80 μg/day D3 vs. placebo |
68 |
5 |
No difference in glucose homeostasis indicators |
|
Gagnon et al. [36] 2014 |
Australia |
25(OH)D ≤ 22ng/ml at risk of T2D (54) |
1200 mg calcium carbonate and 2000–6000 IU D3 day to target vs. placebo |
95 |
6 |
No difference in insulin secretion/sensitivity and beta cell function. A post-hoc analysis (only prediabetes patients) showed a significant beneficial effect on insulin sensitivity |
|
Sollid et al. [37] 2014 |
Norway |
Prediabetes IFG or IGT (62.1) |
20,000 IU D3/week vs. placebo |
511 |
12 |
No difference in insulin secretion/sensitivity or glucose metabolism, blood pressure or lipid status |
|
Oosterwerff et al. [38] 2014 |
Netherlands |
Overweight, vitamin D deficient subjects with prediabetes (20–65) |
Calcium carbonate 500 mg (all) and 1200 IU D3/day vs. placebo |
130 |
4 |
No difference in insulin sensitivity or in beta cell function. A post hoc analysis (without diabetes patients at baseline), showed a significant increase in the insulinogenic index when 25(OH)D ≥ 60 nmol/L |
|
Salehpour et al. [39] 2013 |
Iran |
Healthy, overweight/obese women (38) |
25 μg D3/daily vs. placebo |
77 |
4 |
No effect in glycemic indices (glucose, insulin, HbA1c and HOMA-IR) |
|
Belenchia et al. [40] 2013 |
US |
Obese adolescents (14.1) |
4000 IU D3/day vs. placebo |
35 |
6 |
Significant effect in fasting insulin, HOMA-IR and leptin-to-adiponectin ratio |
|
Davidson et al. [41] 2013 |
US |
Prediabetes and hypovitaminosis D (52) |
D3 to target serum 25OHD level of 65–90 ng/mL vs. placebo |
109 |
12 |
No difference on insulin secretion/sensitivity or the development of diabetes or returning to normal glucose tolerance |
|
Mitri et al. [42] 2011 |
US |
Prediabetes. At risk for T2D (57) |
2000 IU D3/daily vs. calcium carbonate 800 mg/day |
92 |
4 |
Significant effect in beta cell function and improvement in insulin secretion |
|
von Hurst et al. [43] 2010 |
New Zealand |
Insulin resistance, At risk for T2D 25(OH)D < 20 ng/mL (23–68) |
4000 IU D3/day vs. placebo |
81 |
6.5 |
No difference in FPG, HOMA2%B; C-peptide Significant effect on HOMA IR and insulin |
|
Jorde et al. [44] 2010 |
Norway |
Overweight/Obese; At risk for T2D (21–70) |
500 mg calcium/day plus D3, 40,000 IU/week or D3 20,000 IU/week |
438 |
48 |
No difference in HbA1c, FPG, 2hs PG and HOMA-IR |
4. New Thresholds for the Relationship between Vitamin D and T2D
References
- Cade, C.; Norman, A.W. Rapid normalization/stimulation by 1,25- dihydroxyvitamin d3 of insulin secretion and glucose tolerance in the vitamin d-deficient rat. Endocrinology 1987, 120, 1490–1497.
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003, 17, 509–511.
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; Van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776.
- Bland, R.; Markovic, D.; Hills, C.E.; Hughes, S.V.; Chan, S.L.F.; Squires, P.E.; Hewison, M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 121–125.
- Reusch, J.E.; Begum, N.; Sussman, K.E.; Draznin, B. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology 1991, 129, 3269–3273.
- Leung, P.S. The Potential Protective Action of Vitamin D in Hepatic Insulin Resistance and Pancreatic Islet Dysfunction in Type 2 Diabetes Mellitus. Nutrients 2016, 8, 147.
- Wright, D.C.; Hucker, K.A.; Holloszy, J.O.; Han, D.H. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 2004, 53, 330–335.
- Draznin, B. Cytosolic calcium and insulin resistance. Am. J. Kidney Dis. 1993, 21, 32–38.
- Nimitphong, H.; Holick, M.F.; Fried, S.K.; Lee, M.-J. 25-hydroxyvitamin D(3) and 1,25-dihydroxyvitamin D(3) promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 2012, 7, e52171.
- Blum, M.; Dolnikowski, G.; Seyoum, E.; Harris, S.S.; Booth, S.L.; Peterson, J.; Saltzman, E.; Dawson-Hughes, B. Vitamin D(3) in fat tissue. Endocrine 2008, 33, 90–94.
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue—The effect of obesity and diet-induced weight loss. Int. J. Obes. 2013, 37, 651–657.
- Hyppönen, E.; Boucher, B.J. Adiposity, vitamin D requirements, and clinical implications for obesity-related metabolic abnormalities. Nutr. Rev. 2018, 76, 678–692.
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151.
- Christakos, S.; Liu, Y. Biological actions and mechanism of action of calbindin in the process of apoptosis. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 401–404.
- Salum, E.; Kals, J.; Kampus, P.; Salum, T.; Zilmer, K.; Aunapuu, M.; Arend, A.; Eha, J.; Zilmer, M. Vitamin D reduces deposition of advanced glycation end-products in the aortic wall and systemic oxidative stress in diabetic rats. Diabetes Res. Clin. Pract. 2013, 100, 243–249.
- Forouhi, N.G.; Luan, J.; Cooper, A.; Boucher, B.J.; Wareham, N.J. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: The Medical Research Council Ely Prospective Study 1990-2000. Diabetes 2008, 57, 2619–2625.
- Kayaniyil, S.; Vieth, R.; Retnakaran, R.; Knight, J.A.; Qi, Y.; Gerstein, H.C.; Perkins, B.A.; Harris, S.B.; Zinman, B.; Hanley, A.J. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010, 33, 1379–1381.
- Maki, K.C.; Fulgoni, V.L., 3rd; Keast, D.R.; Rains, T.M.; Park, K.M.; Rubin, M.R. Vitamin D intake and status are associated with lower prevalence of metabolic syndrome in U.S. adults: National Health and Nutrition Examination Surveys 2003–2006. Metab. Syndr. Relat. Disord. 2012, 10, 363–372.
- Chiu, K.C.; Chu, A.; Go, V.L.W.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825.
- Gannage-Yared, M.-H.; Chedid, R.; Khalife, S.; Azzi, E.; Zoghbi, F.; Halaby, G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur. J. Endocrinol. 2009, 160, 965–971.
- Scragg, R.; Holdaway, I.; Singh, V.; Metcalf, P.; Baker, J.; Dryson, E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res. Clin. Pract. 1995, 27, 181–188.
- Olson, M.L.; Maalouf, N.M.; Oden, J.D.; White, P.C.; Hutchison, M.R. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J. Clin. Endocrinol. Metab. 2012, 97, 279–285.
- Parikh, S.; Guo, D.-H.; Pollock, N.K.; Petty, K.; Bhagatwala, J.; Gutin, B.; Houk, C.; Zhu, H.; Dong, Y. Circulating 25-hydroxyvitamin D concentrations are correlated with cardiometabolic risk among American black and white adolescents living in a year-round sunny climate. Diabetes Care 2012, 35, 1133–1138.
- Kayaniyil, S.; Retnakaran, R.; Harris, S.B.; Vieth, R.; Knight, J.A.; Gerstein, H.C.; Perkins, B.A.; Zinman, B.; Hanley, A.J. Prospective associations of vitamin D with beta-cell function and glycemia: The PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes 2011, 60, 2947–2953.
- Forouhi, N.G.; Ye, Z.; Rickard, A.P.; Khaw, K.T.; Luben, R.; Langenberg, C.; Wareham, N.J. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: Results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia 2012, 55, 2173–2182.
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Low 25-hydroxyvitamin D and risk of type 2 diabetes: A prospective cohort study and metaanalysis. Clin. Chem. 2013, 59, 381–391.
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2013, 36, 1422–1428.
- Park, S.K.; Garland, C.F.; Gorham, E.D.; BuDoff, L.; Barrett-Connor, E. Plasma 25-hydroxyvitamin D concentration and risk of type 2 diabetes and pre-diabetes: 12-year cohort study. PLoS ONE 2018, 13, e0193070.
- Avila-Rubio, V.; Garcia-Fontana, B.; Novo-Rodriguez, C.; Cantero-Hinojosa, J.; Reyes-Garcia, R.; Munoz-Torres, M. Higher Levels of Serum 25-Hydroxyvitamin D Are Related to Improved Glucose Homeostasis in Women with Postmenopausal Osteoporosis. J. Women’s Health 2018, 27, 1007–1015.
- Zheng, J.-S.; Imamura, F.; Sharp, S.J.; van der Schouw, Y.T.; Sluijs, I.; Gundersen, T.E.; Ardanaz, E.; Boeing, H.; Bonet, C.; Gómez, J.H.; et al. Association of plasma vitamin D metabolites with incident type 2 diabetes: EPIC-InterAct case-cohort study. J. Clin. Endocrinol. Metab. 2019, 104, 1293–1303.
- Heath, A.K.; Williamson, E.J.; Hodge, A.M.; Ebeling, P.R.; Eyles, D.W.; Kvaskoff, D.; O’Dea, K.; Giles, G.G.; English, D.R. Vitamin D status and the risk of type 2 diabetes: The Melbourne Collaborative Cohort Study. Diabetes Res. Clin. Pract. 2018, 149, 179–187.
- LeBlanc, E.S.; Pratley, R.E.; Dawson-Hughes, B.; Staten, M.A.; Sheehan, P.R.; Lewis, M.R.; Peters, A.; Kim, S.H.; Chatterjee, R.; Aroda, V.R.; et al. Baseline Characteristics of the Vitamin D and Type 2 Diabetes (D2d) Study: A Contemporary Prediabetes Cohort That Will Inform Diabetes Prevention Efforts. Diabetes Care 2018, 41, 1590–1599.
- Forouhi, N.G.; Menon, R.K.; Sharp, S.J.; Mannan, N.; Timms, P.M.; Martineau, A.R.; Rickard, A.P.; Boucher, B.J.; Chowdhury, T.A.; Griffiths, C.J.; et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2016, 18, 392–400.
- Wagner, H.; Alvarsson, M.; Mannheimer, B.; Degerblad, M.; Ostenson, C.-G. No Effect of High-Dose Vitamin D Treatment on beta-Cell Function, Insulin Sensitivity, or Glucose Homeostasis in Subjects With Abnormal Glucose Tolerance: A Randomized Clinical Trial. Diabetes Care 2016, 39, 345–352.
- Tuomainen, T.-P.; Virtanen, J.K.; Voutilainen, S.; Nurmi, T.; Mursu, J.; de Mello, V.D.F.; Schwab, U.; Hakumaki, M.; Pulkki, K.; Uusitupa, M. Glucose Metabolism Effects of Vitamin D in Prediabetes: The VitDmet Randomized Placebo-Controlled Supplementation Study. J. Diabetes Res. 2015, 2015, 672653.
- Gagnon, C.; Daly, R.M.; Carpentier, A.; Lu, Z.X.; Shore-Lorenti, C.; Sikaris, K.; Jean, S.; Ebeling, P.R. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and beta-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: A pilot randomized, placebo-controlled trial. PLoS ONE 2014, 9, e109607.
- Sollid, S.T.; Hutchinson, M.Y.S.; Fuskevag, O.M.; Figenschau, Y.; Joakimsen, R.M.; Schirmer, H.; Njolstad, I.; Svartberg, J.; Kamycheva, E.; Jorde, R. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care 2014, 37, 2123–2131.
- Oosterwerff, M.M.; Eekhoff, E.M.; Van Schoor, N.M.; Boeke, A.J.P.; Nanayakkara, P.; Meijnen, R.; Knol, D.L.; Kramer, M.H.; Lips, P. Effect of moderate-dose vitamin D supplementation on insulin sensitivity in vitamin D-deficient non-Western immigrants in the Netherlands: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 152–160.
- Salehpour, A.; Shidfar, F.; Hosseinpanah, F.; Vafa, M.; Razaghi, M.; Amiri, F. Does vitamin D3 supplementation improve glucose homeostasis in overweight or obese women? A double-blind, randomized, placebo-controlled clinical trial. Diabet. Med. 2013, 30, 1477–1481.
- Belenchia, A.M.; Tosh, A.K.; Hillman, L.S.; Peterson, C.A. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 774–781.
- Davidson, M.B.; Duran, P.; Lee, M.L.; Friedman, T.C. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care 2013, 36, 260–266.
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 486–494.
- Von Hurst, P.R.; Stonehouse, W.; Coad, J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—A randomised, placebo-controlled trial. Br. J. Nutr. 2010, 103, 549.
- Jorde, R.; Sneve, M.; Torjesen, P.; Figenschau, Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J. Intern. Med. 2010, 267, 462–472.
- Griffin, S.J.; Little, P.S.; Hales, C.N.; Kinmonth, A.L.; Wareham, N.J. Diabetes risk score: Towards earlier detection of type 2 diabetes in general practice. Diabetes. Metab. Res. Rev. 2000, 16, 164–171.
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2018, 380, 33–44.
- Rafiq, S.; Jeppesen, P.B. Body mass index, vitamin d, and type 2 diabetes: A systematic review and meta-analysis. Nutrients 2018, 10, 1182.
- Tang, H.; Li, D.; Li, Y.; Zhang, X.; Song, Y.; Li, X. Effects of Vitamin D Supplementation on Glucose and Insulin Homeostasis and Incident Diabetes among Nondiabetic Adults: A Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2018, 2018, 7908764.




