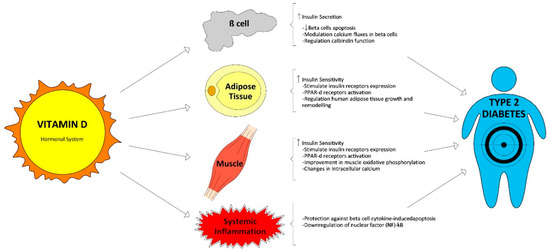
1. Vitamin D Physiology and Glucose Homeostasis
The term vitamin D includes vitamin D2 or ergocalciferol and vitamin D3 or cholecalciferol. The main metabolites of vitamin D, which differ in their hydroxylation patterns, are 25-hydroxyvitamin D or calcidiol (25(OH)D) and 1,25-dihydroxyvitamin D3 or calcitriol (1,25-(OH)2D3). In humans, the main sources of vitamin D come from the skin through the cutaneous synthesis of vitamin D3 and, to a lesser extent, from the intake of foods rich in vitamins D2 and D3 or supplements. Circulating vitamin D is bound to vitamin D binding protein (DBP), which transports it to the liver, there vitamin D25-hydroxylase converts it to 25(OH)D. This form of vitamin D is primarily converted to the most biologically active form, 1,25-(OH)2D in the kidneys. This transformation is done by the enzyme 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1). The presence of CYP27B1 in multiple tissues, which also express the vitamin D receptor, suggests that vitamin D could play an important function beyond bone metabolism.
Both in vitro and in vivo studies have reported that vitamin D may play an important role in the maintenance of pancreatic beta cell function [
10]. This effect could have different explanations. It could be induced by the activation of the vitamin D receptor (VDR) located in pancreatic beta cells. It was suggested by the study results that showed how mice without VDR have impaired insulin secretion [
11] and the addition of calcitriol to the culture medium stimulated pancreatic islets and resulted in an increased insulin secretion [
12].
Moreover, vitamin D could also influence insulin secretion by regulating calcium channel opening and closure. Calcitriol participates as a chemical messenger interacting with different receptors regulating calcium flux in beta cells. They are located on the phospholipid layers of plasma membranes. For this reason, calcium is essential for appropriate insulin secretion by pancreatic beta cells; and, therefore, insufficient vitamin D may alter normal insulin secretion through alterations in calcium flux in beta cells [
13,
14]. In relation to this, the regulation of the protein calbindin, a calcium-binding protein, by vitamin D may be another mechanism influencing insulin secretion. In addition, preclinical studies show that vitamin D can reduce the hyperactivity of the renin angiotensin system and, thus, improve the functioning of beta cells (Leung PS. Nutrients 2016 [
15]).
An adequate vitamin D level can also improve insulin resistance pathways associated with diabetes. It is caused mainly by alterations in calcium flux and concentration through the cell membranes of insulin-responsive tissues [
16]. The regulation of extracellular and intracellular calcium concentrations may promote dephosphorylation of glucose transporter-4 (GLUT-4) driving a reduced insulin-stimulated glucose transport [
14,
17]. The 1,25-(OH)
2D stimulates the expression of insulin receptors and, therefore, stimulates insulin sensitivity. In addition, calcitriol could also improve insulin sensitivity activating the peroxisome proliferator-activated delta receptor (PPAR-d), a transcription factor that regulates fatty acids metabolism in adipose tissue and the skeletal muscle. Another interesting study indicates that insulin resistance may also be reduced by the specific effects of calcitriol on hepatic lipid synthesis and glucose output, and on skeletal muscle (Leung PS. Nutrients 2016 [
15]).
Calcitriol has a central role in a wide variety of metabolic pathways by binding to the VDR, and the measurement of its substrate 25(OH)D is an important marker for health risks. This receptor is expressed in an assortment of cells, such as in the pancreatic beta cells of Langerhans, but also in liver, adipose tissue, and muscle cells [
18,
19]. The VDR and the 1α-hydroxylase, the enzyme catalyzing calcidiol to calcitriol conversion, are expressed in primary preadipocytes and recently differentiated adipocytes [
18]. Therefore, in vitro studies suggest that calcitriol regulates the growth of human adipose tissue and its remodeling. Moreover, fat tissue is a storage site for vitamin D [
19]. In contrast, a higher body mass index (BMI) is associated to lower vitamin D concentrations. Vitamin D, a fat-soluble hormone, is sequestered in the adipose tissue and, consequently, only small quantities are available for circulation [
20]. On the other hand, since the concentrations of 25(OH)D in serum and adipose tissue are closely related, obesity can reduce serum 25(OH)D through volumetric dilution and the distribution of 25(OH)D in larger fat volumes [
21].
Vitamin D could also shorten the effects of chronic inflammation, and it is well established that it plays a key role in the pathogenesis of T2D. Therefore, 1,25(OH)
2D can protect against cytokine-induced apoptosis of beta cells directly regulating the activity and expression of cytokines, with an improvement in insulin sensitivity [
22]. Moreover, vitamin D demonstrated the possibility of deactivating inflammatory cytokines associated with insulin resistance and promoting calbindin expression which involves protection from apoptosis [
23]. Finally, vitamin D also reduces the accumulation of advanced glycation products in experimental studies [
24]. These products are related with the development of T2D complications and have been involved with insulin resistance. Vitamin D functions related with glucose homeostasis are summarized in
Figure 1.
Figure 1. Vitamin D functions related with glucose homeostasis.
2. Vitamin D Status and Its Relationship with T2D in Cross-Sectional and Longitudinal Studies
Serum 25(OH)D concentrations have been noticed to be inversely associated with glucose homeostasis, insulin resistance, and beta cell function, and forecast lower risks of both metabolic syndrome and T2D [
25,
26,
27]. Numerous clinical studies have associated vitamin D inadequacy with the development of insulin resistance in different populations, not only in adults [
5,
28,
29] but also in children [
30,
31].
Consistently, higher baseline 25(OH)D levels have been found to predict better beta cell function and lower glucose levels in subjects at risk for T2D in longitudinal studies [
32]. Overall, data from observational studies strongly support an association between low vitamin D status and incidence of T2D [
33,
34,
35].
We discuss in this review the largest prospective articles and some meta-analyses. In 2013, Afzal et al. published the results of a prospective cohort study that included 9841 participants who were followed-up for 29 years. They found an odds ratio for the development of T2D of 1.5 (95% CI 1.33–1.70) between the lowest and the highest quartile of 25(OH)D [
34]. More recently, Park et al. measured 25(OH)D levels in a cohort of 903 adults without diabetes or prediabetes, these authors found an inverse dose-response association between 25(OH)D concentration and risk of diabetes. They proposed a target 25(OH)D of 50 ng/mL; higher than the levels previously suggested in other studies, in the attempt to influence and reduce the incidence rate of diabetes [
36]. These data are consistent with the levels published recently by Avila-Rubio et al. in postmenopausal women, the authors link values of 25(OH)D > 45 ng/dL in these women with better glycemic indexes measured by homeostasis model assessment (HOMA) [
37].
The multicenter EPIC-InterAct study measured plasma 25(OH)D metabolites: non-epimeric 25(OH)D
3, 3-epi-25(OH)D
3 and 25(OH)D
2. They identified that plasma non-epimeric 25(OH)D
3 (the major component of total 25(OH)D) was inversely associated with T2D, whereas 3-epi-25(OH)D
3 was positively associated with the incidence of T2D, and 25(OH)D
2 was not associated with T2D [
38].
Another large cohort was The Melbourne cohort, which included a sample of middle-aged Australians, the authors showed how vitamin D status was inversely associated with the risk of T2D and, apparently, this association cannot be explained by reverse causality [
39]. If the association was due to reverse causality, then a much stronger association would be expected to be observed in the first few years of follow-up.
The meta-analysis conducted by Song et al. included 21 observational studies with 76,220 subjects in total; the authors found a 38% lesser risk of developing T2D in the highest baseline reference category of 25(OH)D compared to the lowest one (95% CI 0.54–0.70) [
35].
Despite the consistency of these results, all these were observational studies and estimation of causality cannot be completely excluded because of residual confounding agents.
3. Vitamin D Supplementation and Risk of T2D: Randomized Trials and Meta-Analysis
In the last decade, more than ten well-designed, randomized trials evaluated the effect of vitamin D3 supplementation on glucose homeostasis in subjects at risk for T2D and showed inconsistent results. We have selected a set of studies that analyzed outcomes related to the objectives of this review. Table 1 summarizes the main results of these studies.
Table 1. Clinical trials investigating the association between vitamin D supplementation and risk of type 2 diabetes (T2D).
BMI, body mass index; FPG, fasting plasma glucose; 2hs PG, 2 h plasma glucose; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NAFLD, nonalcoholic fatty liver disease; T2D, type 2 diabetes.
In
Table 1 we describe the main findings of the largest trials. Sollid et al. [
40] conducted a randomized clinical trial with approximately 500 prediabetes subjects comparing vitamin D versus placebo for the prevention of T2D. They supplemented with 20,000 IU cholecalciferol weekly and after one year, no significant differences were reported between those receiving vitamin D and those taking placebo in any of the glycemic or inflammatory markers and blood pressure, regardless of baseline serum 25(OH)D concentrations.
Two years later, Forouhi et al. [
41] compared, in another large randomized trial including 340 prediabetes or at risk of developing T2D subjects, the effect of supplementation with cholecalciferol or ergocalciferol (both 100,000 IU/month) versus placebo, for four months. Prediabetes was estimated by the Cambridge Risk Score [
42]. Despite vitamin D supplementation, neither ergocalciferol or cholecalciferol, resulted in increased 25(OH)D
2 and 25(OH)D
3 concentrations. No differences in HbA1c concentration were found between groups. It is important to point out that only half of the subjects had concentrations of 25(OH)D < 50 nmol/L. This data could influence the results.
Their results are in accordance with previous findings by Davidson et al. [
43]. They supplemented a cohort of Latino and African Americans subjects with prediabetes and hypovitaminosis D at baseline for one year. They used a cholecalciferol dose sufficient to raise serum 25(OH)D levels into the upper-normal range versus placebo. They did not find any effect on insulin secretion or sensitivity, nor the proportion of subjects who developed T2D or whose oral glucose tolerance test became normal [
43].
However, it is possible to find some positive effect on glycemic markers in some studies. In this line, Gagnon et al. [
44] reported when they performed a post hoc analysis only including subjects with prediabetes, an improvement in insulin sensitivity indices was observed. Previously, they gave a supplement of calcium carbonate 1200 mg and cholecalciferol 2000–6000 UI daily to subjects with glucose intolerance or recently diagnosed diabetes, but they found no effect on insulin sensitivity or secretion, and beta cell function. So, despite most clinical randomized trials failing to show a favorable effect of vitamin D supplementation on glycemic control, insulin sensitivity indices, and incident T2D [
40,
41,
43,
44,
45,
46,
47,
48,
49] in subjects at risk for diabetes, there is some interesting evidence supporting a beneficial effect of vitamin D on beta cell function. In fact, Mitri et al. [
50] reported in 2011 a significant improvement in insulin secretion in 92 prediabetic subjects who were overweight or obese and at risk for T2D. They were supplemented with cholecalciferol 2000 IU daily and calcium carbonate versus placebo for four months. An important restriction of the above described studies is that they were not designed specifically to assess glycemic homeostasis and the results found correspond to post hoc analyses.
Although not being designed for this purpose, we would like to point out the results of the Vitamin D and Omega-3 Trial (VITAL), a large-scale trial that evaluated high-dose vitamin D supplementation. This study was designed to evaluate the effect of supplementation with vitamin D on incidence of invasive cancer or cardiovascular events versus placebo. Overall, no differences were found between groups. However, in Black Americans a potential beneficial effect was found in cancer mortality [
51].
A recent meta-analysis conducted by Rafiq et al. showed an inverse relationship; higher vitamin D concentrations were associated with lower BMI in T2D patients and non-diabetic subjects at risk for T2D. But this association was more pronounced in T2D patients. Moreover, the correlation was directly associated to the BMI quartiles, so the highest BMI quartile had the greatest correlations in both populations, both T2D and non-diabetic [
52].
Tang et al. [
53] published a meta-analysis and did not find an effect of vitamin D supplementation on the incidence of T2D. However, the authors suggested a possible dose-response effect of vitamin D supplementation to improve glucose and insulin metabolism among non-diabetic adults. They postulated a possible benefit of taking vitamin D supplements in higher doses for the primary prevention of T2D.
In summary, studies were very heterogeneous in terms of design, duration, and type of supplement administered and participants characteristics. It is noteworthy that adherence to the treatment would have played a major role in arguing these results.
Recently, the design of a new randomized clinical trial has been published and its results can be a determinant in clarifying many of the uncertainties that exist today. The D2d is a large randomized clinical trial (including participants from 22 sites across the U.S.) hypothesizing that supplementation with vitamin D
3 daily lowers risk of diabetes in adults with prediabetes [
54]. This trial meets people with a large spectrum of diabetes risk, more convenient for testing the underlying hypothesis. D2d trial results are expected to answer two important questions: whether vitamin D supplementation is useful to prevent T2D and how the 2010 expanded American Diabetes Association (ADA) criteria for prediabetes would impact the natural history of this state previous to diabetes.
4. New Thresholds for the Relationship between Vitamin D and T2D
An important question that has arisen is what 25(OH)D levels are necessary to influence glycemic homeostasis and the risk of developing T2D. Three recent studies have addressed this issue. Von Horst et al. found that optimal 25OHD concentrations for reducing IR were around 50 ng/dL in a randomized controlled study with 81 Asian women [
49]. Avila Rubio et al. [
37], in a study conducted in women with postmenopausal osteoporosis, suggested that the established goal of reaching a level of 25(OH)D > 30 ng/mL was insufficient to improve glucose metabolism in these population. The data from this study indicates that 25(OH)D > 45 ng/mL are necessary to achieve this goal. These data are consistent with a cohort study of 903 adults of 12 years of duration in non-diabetic population where reaching values of 25(OH)D > 50 ng/mL contributed to reach the maximum benefits to reduce the risk of incident diabetes [
36]. Therefore, it is important to establish what 25(OH)D values are necessary to achieve and, even more importantly, maintain all the potential benefits of vitamin D. The currently available studies do not allow us to answer this question with certainty.
This entry is adapted from the peer-reviewed paper 10.3390/nu11030642