Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sanaa Bardaweel | -- | 1446 | 2023-08-04 07:46:15 | | | |
| 2 | Conner Chen | Meta information modification | 1446 | 2023-08-07 05:21:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rashid, Z.A.; Bardaweel, S.K. Structure and Function of Matrix Metalloproteinase-9. Encyclopedia. Available online: https://encyclopedia.pub/entry/47657 (accessed on 08 February 2026).
Rashid ZA, Bardaweel SK. Structure and Function of Matrix Metalloproteinase-9. Encyclopedia. Available at: https://encyclopedia.pub/entry/47657. Accessed February 08, 2026.
Rashid, Zainab Ahmed, Sanaa K. Bardaweel. "Structure and Function of Matrix Metalloproteinase-9" Encyclopedia, https://encyclopedia.pub/entry/47657 (accessed February 08, 2026).
Rashid, Z.A., & Bardaweel, S.K. (2023, August 04). Structure and Function of Matrix Metalloproteinase-9. In Encyclopedia. https://encyclopedia.pub/entry/47657
Rashid, Zainab Ahmed and Sanaa K. Bardaweel. "Structure and Function of Matrix Metalloproteinase-9." Encyclopedia. Web. 04 August, 2023.
Copy Citation
Matrix metalloproteinases (MMPs) belong to a family of zinc-dependent proteolytic metalloenzymes. MMP-9, a member of the gelatinase B family, is characterized as one of the most intricate MMPs. The crucial involvement of MMP-9 in extracellular matrix (ECM) remodeling underscores its significant correlation with each stage of cancer pathogenesis and progression. The design and synthesis of MMP-9 inhibitors is a potentially attractive research area.
MMP-9
matrix metalloproteinase
inhibitors
1. Introduction
Cancer is an abnormal cell growth that accounts for a significant portion of global deaths. It is considered the second major cause of death after heart disease [1]. Interestingly, anticancer drug development research focuses on over-expressed molecules that have critical roles in cell survival and proliferation. For instance, the extracellular matrix (ECM) has a major role in cancer-related functions, such as cell cycle regulation, survival, and apoptosis [2]. ECM consists of several molecules, such as proteoglycans, glycosaminoglycans, structural proteins (collagen and elastin), adhesion proteins (fibronectin and laminin), and proteases called matrix metalloproteases (MMPs) [3]. Supportive evidence is available on the key role of the extracellular proteinases (MMPs) as potential modulators of cell–cell and cell–ECM communication, which controls essential tissue homeostasis [4].
MMPs belong to a family of zinc-dependent endopeptidases that consists of 23 members. They participate in several biological and physiological processes and are highly regulated by cytokines, hormones, and growth factors [5]. MMPs have been classified according to their sub-cellular distribution and selectivity for ECM components. The first group includes collagenases (MMP-1, MMP-8, MMP-13, and MMP-18) that are responsible for the degradation of essential components in the bone called helical fibrillar collagen. The second group includes gelatinases (MMP-2 and MMP-9), which are essential in angiogenesis and neurogenesis. Additionally, stromelysins (MMP-3, MMP-10, and MMP-11) and matrilysins (MMP-7 and MMP-26) digest segments of the ECM [3][6]. MMPs are classified by domain structure into eight groups, five of which are secreted and three of which are membrane-associated [3].
Matrix metalloproteinase-9 (MMP-9) is one of the most complex MMPs that belongs to the gelatinase B family [7]. It is mainly located in the hippocampus, cerebellum, and cerebral cortex [8]. It is secreted in two forms, either as zymogens or as an inactive enzyme, from endothelial cells, leukocytes fibroblasts, neutrophils, and macrophages. During granulocyte differentiation, bone marrow is the main site of MMP-9 synthesis [9]. MMP-9 is engaged in several pathophysiological processes, such as extracellular matrix (ECM) degradation, tissue remodeling, and normal tissue turnover. The contribution of MMP-9 in the progression of several diseases was reported in extracranial arteriovenous malformations (AVMs) [10], rheumatoid arthritis [11][12], several neurological diseases and inflammatory processes [13][14], cancer [15][16][17], and ischemic stroke [18][19]. The suppression of MMP-9 activity is achieved by the binding of matrix metalloproteinase inhibitors (MMPIs) to the zinc (Zn2+) ion at the catalytic site [20]. It is still not clear how MMPIs suppress cancer growth. Several reports suggest that MMPIs may inhibit cell proliferation by inducing apoptosis through the release of ligands, such as TNFa and TRAIL (Tumor necrosis factor-related apoptosis-inducing ligand), from their membrane-bound inactive form [21].
In recent decades, numerous studies revealed the critical role of MMP-9 in cancer development and progression [18][22][23]. This gelatinase revealed a key role in tumorigenesis through the regulation of several processes, such as the survival of cancer cells, migration, stimulation of immune response, and generation of cancer microenvironment. Consequently, it has become a potentially attractive target for antitumor therapy. Several MMP-9 inhibitors have been synthesized and evaluated for biological activities [24][25][26]. However, the high homology of MMP-9 with other members of MMPs makes the development of effective, selective, and safe MMP-9 inhibitors extremely challenging.
2. Structure and Function
In humans, MMP-9 can be synthesized by and released from neutrophils, macrophages, fibroblasts, and endothelial cells [27]. It is synthesized as a pre-proenzyme and then transferred into the extracellular environment in the form of a pro-MMP-9 enzyme. The activated MMP-9 is produced by the protease-mediated cleavage of the pro-MMP-9 enzyme [27][28]. MMP-3 is an example of those proteases that activate MMP-9 through the removal of the N-terminal pro-peptide region [29]. The removal of the N-terminal pro-peptide significantly disrupts MMP latency.
The MMP-9 gene is located on chromosome 20q13.12, which contains 13 exons and 12 introns. This protein is composed of the following domains: signal peptide domain, pro-peptide region, catalytic domain, hemopexin-like domain, and hinge region [27][30][31] (Figure 1). The signal peptide domain is composed of 17–29 amino acids and is responsible for the secretion of MMP-9 [32]. The pro-peptide domain contains 77–87 amino acids. The amino acid sequence of the pro-domain is PRCGXPD. A cystein switch is vital to MMP-9 activation [33][34]. One cysteine residue (cys99) of pro-MMP-9 interacts with the catalytic zinc ion of this protein. This binding is essential for MMP latency [15]. Consequently, MMP-9 activity is suppressed by preventing the binding of water molecules to the zinc ion in the catalytic domain [35]. Another important domain that is responsible for the proteolytic activity of MMP-9 is the catalytic domain. It is structurally spherical and composed of 170 amino acids [36][37]. It contains an essential consensus zinc-binding sequence (HEXXHXXGXXH) for it is potential activity [38]. For structural integrity and specific activity, two zinc ions are available in the catalytic domain maintaining catalytic and structural roles [35]. Additionally, for enzyme stability, five calcium ions are placed in the catalytic domain [32][39]. Sub-domains are found in the catalytic domain, including the N-terminal domain and the C-terminal domain, which are separated by a shallow catalytic cleft and linked with a U loop [39]. The MMP-9 activation process through the coordination with the catalytic zinc ion requires water molecules in addition to three histidine amino acid residues (His218, His222, and His228) [39]. Six binding pockets are present in the catalytic cleft. Three of them are located on the left side (S1, S2, and S3 pockets). On the other hand, S1′, S2′, and S3′ pockets are located on the right side of the catalytic zinc ion [40]. Substrate selectivity is highly dependent on the depth, length, and amino acid sequence of the S1 pocket that varies among different MMPs. MMP-9 is characterized by an intermediate S1 pocket [41]. Comparable size, position in the catalytic domain, and exposure to the solvent of the S1 pocket were shown in both MMP-2 and MMP-9. The only difference was shown in the residues 425–431 that form a loop in MMP-9 but is absent in MMP-2 [19][42][43]. The fibronectin domain is unique to MMP-2 and MMP-9. This domain is composed of three fibronectin type II motifs, inserted into the metalloproteinase domain [35][44][45]. It is considered an essential modulator of gelatin, laminin, and collagens type I and IV recognition, binding, and degradation [40][46][47]. The hemopexin domain is an ellipsoidal-shaped domain, composed of 210 amino acids [30]. The wild-type enzyme contains four blades connected through a disulfide bond between the first and fourth blades [35]. It is an important domain to bind to tissue metalloproteinase inhibitors [15]. The Hinge region is characterized by its flexibility, which confers the mobility between the hemopexin-like domain and the catalytic domain that is essential for enzyme activity [27][48].
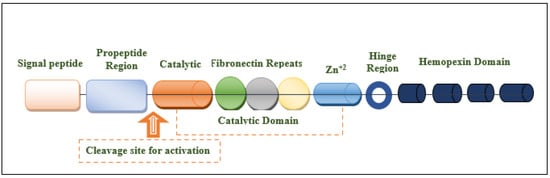
Figure 1. Structural illustration of the domain structures and motifs of MMP-9.
Extracellular matrix (ECM) remolding is one of the main functions of MMP-9. It involves the proteolytic cleavage of the most important MMP-9 substrates, including gelatin, elastin, and collagen [49]. In addition, other substrates are specifically cleaved by MMP-9, such as plasma membrane proteins, extracellular proteins, and intracellular proteins [50][51].
Due to this proteolytic cleavage ability of the MMP-9, it is engaged in various biological processes, such as the alteration of cell–cell and cell-ECM interactions [52][53]. Additionally, as collagen type IV is the main constituent in the basement membrane, MMP-9 plays an essential role in its degradation [54][55]. Consequently, tumor cell invasion and metastases are generally enhanced because of basement membrane destruction.
Angiogenesis is essential for tumor cell growth and development. MMP-9 endorses angiogenesis by degrading the basement membrane and ECM component. Consequently, endothelial cell migrates to produce new blood vessels. On the other hand, MMPs may prevent the mechanism of angiogenesis. It causes the release of angiostatin as a result of the degradation of plasminogen and cleaves the collagen XVIII to produce endostatin. MMP-9 helps in the degradation of plasminogen to produce angiostatin, which increases apoptosis in tumor cells [56].
MMP-9 has both pro- and anti-apoptotic activity. The pro-apoptotic activity is due to the alteration of ECM composition. Conversely, the anti-apoptotic activities are due to the cleavage of Fas ligand, activation of protein kinase B, or threonine kinase AKT [56][57].
MMP-9 has a very important role in various neurological and neurodegenerative diseases through ECM degradation, disruption of blood–brain barrier (BBB), and inflammation [58]. In addition, it is also associated with the pathogenesis of epilepsy by reducing synaptic plasticity and the formation of epileptic foci [7].
Moreover, MMP-9 has an important role in acute and chronic inflammatory diseases because it has both pro- and anti-inflammatory effects. It enhances leukocyte influx and BBB permeability, which promotes inflammation. Interestingly, MMP-9 inhibitors may be helpful in some autoimmune diseases, such as rheumatoid arthritis [59].
References
- Centers for Disease Control and Prevention. National Centers for Health Statistics National Centers for Health Statistics. Available online: http://www.cdc.gov/nchs/fastats/cancer.htm (accessed on 9 July 2023).
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep. 2014, 15, 1243–1253.
- Cui, N.; Hu, M.; Khalil, R.A. Chapter One—Biochemical and Biological Attributes of Matrix Metalloproteinases. In Progress in Molecular Biology and Translational Science; Khalil, R.A., Ed.; Matrix Metalloproteinases and Tissue Remodeling in Health and Disease: Cardiovascular Remodeling; Academic Press: Cambridge, MA, USA, 2017; Volume 147, pp. 1–73.
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67.
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Hitesh; Kaur, G.; Pathak, A. Seesaw of Matrix Metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35.
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183.
- Bronisz, E.; Kurkowska-Jastrzębska, I. Matrix Metalloproteinase 9 in Epilepsy: The Role of Neuroinflammation in Seizure Development. Mediat. Inflamm. 2016, 2016, 7369020.
- Sellner, J.; Leib, S.L. In Bacterial Meningitis Cortical Brain Damage Is Associated with Changes in Parenchymal MMP-9/TIMP-1 Ratio and Increased Collagen Type IV Degradation. Neurobiol. Dis. 2006, 21, 647–656.
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix Metalloproteinase-9: Many Shades of Function in Cardiovascular Disease. Physiology 2013, 28, 391–403.
- Wei, T.; Zhang, H.; Cetin, N.; Miller, E.; Moak, T.; Suen, J.Y.; Richter, G.T. Elevated Expression of Matrix Metalloproteinase-9 Not Matrix Metalloproteinase-2 Contributes to Progression of Extracranial Arteriovenous Malformation. Sci. Rep. 2016, 6, 24378.
- Szeremeta, A.; Jura-Półtorak, A.; Zoń-Giebel, A.; Olczyk, K.; Komosińska-Vassev, K. TNF-α Inhibitors in Combination with MTX Reduce Circulating Levels of Heparan Sulfate/Heparin and Endothelial Dysfunction Biomarkers (SVCAM-1, MCP-1, MMP-9 and ADMA) in Women with Rheumatoid Arthritis. J. Clin. Med. 2022, 11, 4213.
- Grillet, B.; Yu, K.; Ugarte-Berzal, E.; Janssens, R.; Pereira, R.V.S.; Boon, L.; Martens, E.; Berghmans, N.; Ronsse, I.; Van Aelst, I.; et al. Proteoform Analysis of Matrix Metalloproteinase-9/Gelatinase B and Discovery of Its Citrullination in Rheumatoid Arthritis Synovial Fluids. Front. Immunol. 2021, 12, 763832.
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in Translation: From Molecule to Brain Physiology, Pathology, and Therapy. J. Neurochem. 2016, 139 (Suppl. 2), 91–114.
- Hannocks, M.-J.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The Gelatinases, MMP-2, and MMP-9, as Fine Tuners of Neuroinflammatory Processes. Matrix Biol. 2019, 75–76, 102–113.
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249.
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The Relationship between MMP-2 and MMP-9 Expression Levels with Breast Cancer Incidence and Prognosis. Oncol. Lett. 2017, 14, 5865–5870.
- Akter, H.; Park, M.; Kwon, O.-S.; Song, E.J.; Park, W.-S.; Kang, M.-J. Activation of Matrix Metalloproteinase-9 (MMP-9) by Neurotensin Promotes Cell Invasion and Migration through ERK Pathway in Gastric Cancer. Tumour Biol. 2015, 36, 6053–6062.
- Zhong, C.; Yang, J.; Xu, T.; Xu, T.; Peng, Y.; Wang, A.; Wang, J.; Peng, H.; Li, Q.; Ju, Z.; et al. Serum Matrix Metalloproteinase-9 Levels and Prognosis of Acute Ischemic Stroke. Neurology 2017, 89, 805–812.
- Buraczynska, K.; Kurzepa, J.; Ksiazek, A.; Buraczynska, M.; Rejdak, K. Matrix Metalloproteinase-9 (MMP-9) Gene Polymorphism in Stroke Patients. Neuromol. Med. 2015, 17, 385–390.
- Amin, S.A.; Adhikari, N.; Jha, T. Is Dual Inhibition of Metalloenzymes HDAC-8 and MMP-2 a Potential Pharmacological Target to Combat Hematological Malignancies? Pharmacol. Res. 2017, 122, 8–19.
- Nyormoi, O.; Mills, L.; Bar-Eli, M. An MMP-2/MMP-9 Inhibitor, 5a, Enhances Apoptosis Induced by Ligands of the TNF Receptor Superfamily in Cancer Cells. Cell Death Differ. 2003, 10, 558–569.
- Bayramoglu, A.; Gunes, H.V.; Metintas, M.; Değirmenci, I.; Mutlu, F.; Alataş, F. The Association of MMP-9 Enzyme Activity, MMP-9 C1562T Polymorphism, and MMP-2 and -9 and TIMP-1, -2, -3, and -4 Gene Expression in Lung Cancer. Genet. Test. Mol. Biomark. 2009, 13, 671–678.
- Zhang, X.; Jin, G.; Li, J.; Zhang, L. Association between Four MMP-9 Polymorphisms and Breast Cancer Risk: A Meta-Analysis. Med. Sci. Monit. 2015, 21, 1115–1123.
- Scannevin, R.H.; Alexander, R.; Haarlander, T.M.; Burke, S.L.; Singer, M.; Huo, C.; Zhang, Y.-M.; Maguire, D.; Spurlino, J.; Deckman, I.; et al. Discovery of a Highly Selective Chemical Inhibitor of Matrix Metalloproteinase-9 (MMP-9) That Allosterically Inhibits Zymogen Activation. J. Biol. Chem. 2017, 292, 17963–17974.
- Behrends, M.; Wagner, S.; Kopka, K.; Schober, O.; Schäfers, M.; Kumbhar, S.; Waller, M.; Haufe, G. New Matrix Metalloproteinase Inhibitors Based on γ-Fluorinated α-Aminocarboxylic and α-Aminohydroxamic Acids. Bioorg. Med. Chem. 2015, 23, 3809–3818.
- Hugenberg, V.; Breyholz, H.-J.; Riemann, B.; Hermann, S.; Schober, O.; Schäfers, M.; Gangadharmath, U.; Mocharla, V.; Kolb, H.; Walsh, J.; et al. A New Class of Highly Potent Matrix Metalloproteinase Inhibitors Based on Triazole-Substituted Hydroxamates: (Radio)Synthesis and in Vitro and First in Vivo Evaluation. J. Med. Chem. 2012, 55, 4714–4727.
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9): The next Decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272.
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A Delicate Balance: Role of MMP-9 in Brain Development and Pathophysiology of Neurodevelopmental Disorders. Front. Cell. Neurosci. 2015, 9, 280.
- Geurts, N.; Becker-Pauly, C.; Martens, E.; Proost, P.; Van den Steen, P.E.; Stöcker, W.; Opdenakker, G. Meprins Process Matrix Metalloproteinase-9 (MMP-9)/Gelatinase B and Enhance the Activation Kinetics by MMP-3. FEBS Lett. 2012, 586, 4264–4269.
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494.
- Bruschi, F.; Pinto, B. The Significance of Matrix Metalloproteinases in Parasitic Infections Involving the Central Nervous System. Pathogens 2013, 2, 105–129.
- Djuric, T.; Živković, M. Overview of MMP Biology and Gene Associations in Human Diseases. In The Role of Matrix Metalloproteinase in Human Body Pathologies; Intechopen: London, UK, 2017; ISBN 978-953-51-3717-7.
- Van Wart, H.E.; Birkedal-Hansen, H. The Cysteine Switch: A Principle of Regulation of Metalloproteinase Activity with Potential Applicability to the Entire Matrix Metalloproteinase Gene Family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582.
- Sela-Passwell, N.; Rosenblum, G.; Shoham, T.; Sagi, I. Structural and Functional Bases for Allosteric Control of MMP Activities: Can It Pave the Path for Selective Inhibition? Biochim. Biophys. Acta 2010, 1803, 29–38.
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573.
- Klein, G.; Vellenga, E.; Fraaije, M.W.; Kamps, W.A.; de Bont, E.S.J.M. The Possible Role of Matrix Metalloproteinase (MMP)-2 and MMP-9 in Cancer, e.g., Acute Leukemia. Crit. Rev. Oncol. Hematol. 2004, 50, 87–100.
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015, 2, 26–34.
- Itoh, Y. Membrane-Type Matrix Metalloproteinases: Their Functions and Regulations. Matrix Biol. 2015, 44–46, 207–223.
- Adhikari, N.; Mukherjee, A.; Saha, A.; Jha, T. Arylsulfonamides and Selectivity of Matrix Metalloproteinase-2: An Overview. Eur. J. Med. Chem. 2017, 129, 72–109.
- Jacobsen, J.A.; Major Jourden, J.L.; Miller, M.T.; Cohen, S.M. To Bind Zinc or Not to Bind Zinc: An Examination of Innovative Approaches to Improved Metalloproteinase Inhibition. Biochim. Biophys. Acta 2010, 1803, 72–94.
- Adhikari, N.; Amin, S.A.; Jha, T. Collagenases and Gelatinases and Their Inhibitors as Anticancer Agents. In Cancer-Leading Proteases; Academic Press: Cambridge, MA, USA, 2020; pp. 265–294. ISBN 978-0-12-818168-3.
- Tandon, A.; Sinha, S. Structural Insights into the Binding of MMP9 Inhibitors. Bioinformation 2011, 5, 310–314.
- Messah, A.; Darmiati, S.; Rumende, C.; Soemarwoto, R.; Prihartono, J.; Prihartono, J.; Fadilah, F.; Prawiningrum, A. Prediction of MMP-9 Polymorphism Impacts on MDR-TB by Molecular Simulation and Network Interaction. Pharmacogn. J. 2022, 14, 833–841.
- Rundhaug, J.E. Matrix Metalloproteinases, Angiogenesis, and Cancer: Commentary Re: A. C. Lockhart et al., Reduction of Wound Angiogenesis in Patients Treated with BMS-275291, a Broad Spectrum Matrix Metalloproteinase Inhibitor. Clin. Cancer Res. 2003, 9, 551–554.
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix Metalloproteinases: Fold and Function of Their Catalytic Domains. Biochim. Biophys. Acta 2010, 1803, 20–28.
- O’Farrell, T.J.; Pourmotabbed, T. The Fibronectin-like Domain Is Required for the Type V and XI Collagenolytic Activity of Gelatinase B. Arch. Biochem. Biophys. 1998, 354, 24–30.
- Lauer-Fields, J.L.; Whitehead, J.K.; Li, S.; Hammer, R.P.; Brew, K.; Fields, G.B. Selective Modulation of Matrix Metalloproteinase 9 (MMP-9) Functions via Exosite Inhibition. J. Biol. Chem. 2008, 283, 20087–20095.
- Rahman, F. Production and Purification of Recombinant C-Terminal Truncated Pro-Matrix Metalloprotease-9. Master’s Thesis, UiT Norges Arktiske Universitet, Tromsø, Norway, 2016.
- Farina, A.R.; Mackay, A.R. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers 2014, 6, 240–296.
- Vaisar, T.; Kassim, S.Y.; Gomez, I.G.; Green, P.S.; Hargarten, S.; Gough, P.J.; Parks, W.C.; Wilson, C.L.; Raines, E.W.; Heinecke, J.W. MMP-9 Sheds the Beta2 Integrin Subunit (CD18) from Macrophages. Mol. Cell. Proteom. 2009, 8, 1044–1060.
- Hill, J.W.; Poddar, R.; Thompson, J.F.; Rosenberg, G.A.; Yang, Y. Intranuclear Matrix Metalloproteinases Promote DNA Damage and Apoptosis Induced by Oxygen-Glucose Deprivation in Neurons. Neuroscience 2012, 220, 277–290.
- Dwivedi, A.; Slater, S.C.; George, S.J. MMP-9 and -12 Cause N-Cadherin Shedding and Thereby Beta-Catenin Signalling and Vascular Smooth Muscle Cell Proliferation. Cardiovasc. Res. 2009, 81, 178–186.
- Hsu, C.-C.; Huang, S.-F.; Wang, J.-S.; Chu, W.-K.; Nien, J.-E.; Chen, W.-S.; Chow, S.-E. Interplay of N-Cadherin and Matrix Metalloproteinase 9 Enhances Human Nasopharyngeal Carcinoma Cell Invasion. BMC Cancer 2016, 16, 800.
- Hou, H.; Zhang, G.; Wang, H.; Gong, H.; Wang, C.; Zhang, X. High Matrix Metalloproteinase-9 Expression Induces Angiogenesis and Basement Membrane Degradation in Stroke-Prone Spontaneously Hypertensive Rats after Cerebral Infarction. Neural Regen. Res. 2014, 9, 1154–1162.
- Misko, A.; Ferguson, T.; Notterpek, L. Matrix Metalloproteinase Mediated Degradation of Basement Membrane Proteins in Trembler J Neuropathy Nerves. J. Neurochem. 2002, 83, 885–894.
- Chen, Y.; Wang, W.; Liu, F.; Tang, L.; Tang, R.; Li, W. Apoptotic Effect of Mtrix Metalloproteinases 9 in the Development of Diabetic Retinopathy. Int. J. Clin. Exp. Pathol. 2015, 8, 10452–10459.
- Gu, Z.; Cui, J.; Brown, S.; Fridman, R.; Mobashery, S.; Strongin, A.Y.; Lipton, S.A. A Highly Specific Inhibitor of Matrix Metalloproteinase-9 Rescues Laminin from Proteolysis and Neurons from Apoptosis in Transient Focal Cerebral Ischemia. J. Neurosci. 2005, 25, 6401–6408.
- Tokito, A.; Jougasaki, M. Matrix Metalloproteinases in Non-Neoplastic Disorders. Int. J. Mol. Sci. 2016, 17, 1178.
- Vandooren, J.; Knoops, S.; Buzzo, J.L.A.; Boon, L.; Martens, E.; Opdenakker, G.; Kolaczkowska, E. Differential Inhibition of Activity, Activation and Gene Expression of MMP-9 in THP-1 Cells by Azithromycin and Minocycline versus Bortezomib: A Comparative Study. PLoS ONE 2017, 12, e0174853.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
07 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




