You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elisa Gamalero | -- | 2360 | 2023-08-04 07:06:35 | | | |
| 2 | Sirius Huang | -2 word(s) | 2358 | 2023-08-04 08:59:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gamalero, E.; Lingua, G.; Glick, B.R. Ethylene and ACC in Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/47653 (accessed on 28 December 2025).
Gamalero E, Lingua G, Glick BR. Ethylene and ACC in Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/47653. Accessed December 28, 2025.
Gamalero, Elisa, Guido Lingua, Bernard R. Glick. "Ethylene and ACC in Plants" Encyclopedia, https://encyclopedia.pub/entry/47653 (accessed December 28, 2025).
Gamalero, E., Lingua, G., & Glick, B.R. (2023, August 04). Ethylene and ACC in Plants. In Encyclopedia. https://encyclopedia.pub/entry/47653
Gamalero, Elisa, et al. "Ethylene and ACC in Plants." Encyclopedia. Web. 04 August, 2023.
Copy Citation
The molecule 1-aminocyclopropane-1-carboxylate (ACC) is the immediate precursor of the plant hormone ethylene in most seed plant species. Both 1-aminocyclopropane-1-carboxylate and ethylene can affect plant growth and development in a variety of ways.
ethylene
1-aminocyclopropane1-carboxylate
ACC deaminase
1. Introduction
Plant growth and development are regulated by several different phytohormones including cytokinins, gibberellins, auxins, salicylic acid, jasmonates, brassinosteroids, abscisic acid, strigolactones, and ethylene [1][2][3][4][5][6]. A key phytohormone, and of particular interest to this discussion, is ethylene (C2H4), a low molecular weight (28.05 g/mol) gaseous hydrocarbon that is produced in all higher plants and modulates a wide range of plant physiological and biochemical activities [7][8][9]. Thus, for example, “ethylene is involved in seed germination, tissue differentiation, formation of root and shoot primordia, root branching and elongation, lateral bud development, flowering initiation, anthocyanin synthesis, flower opening and senescence, fruit ripening and degreening, production of volatile organic compounds, …aroma formation in fruits, storage product hydrolysis, leaf senescence, leaf and fruit abscission, Rhizobia nodule formation, mycorrhizae-plant interaction, and (importantly) the response of plants to various biotic and abiotic stress” [7]. The impact of ethylene on a particular plant trait may be either stimulatory or inhibitory, and is a consequence of the genus and species of the plant, the age of the plant, and the soil conditions, including the presence of soil microbes, the weather, and the amount of ethylene that is produced. Moreover, depending upon these conditions, a very wide range of ethylene concentrations (~4000-fold) may exhibit biological activity [7][10]. In addition, in some plants under specific conditions, the presence of increasing ethylene may impact the synthesis of other phytohormones including abscisic acid, gibberellin, cytokinin, and auxin [11][12]. Thus, some of the biological activities largely attributed to ethylene may be the consequence of ethylene affecting the concentration of, or acting in concert with, other phytohormones.
2. Ethylene and ACC Biosynthesis in Plants
The biosynthesis of ethylene has been studied in detail in numerous higher plants and it appears that all of these plants utilize essentially the same mechanism to synthesize ethylene [13][14]. In addition to the plant biosynthesis of ethylene, some microorganisms can also synthesize ethylene, albeit using an entirely different biosynthetic pathway [15][16]. In plants, the biosynthesis of ethylene begins with the conversion of the relatively rare, but nonetheless very important, amino acid L-methionine into the compound S-adenosylmethionine (SAM) by the enzyme SAM synthase, which is encoded by a small multi-gene family (Figure 1).
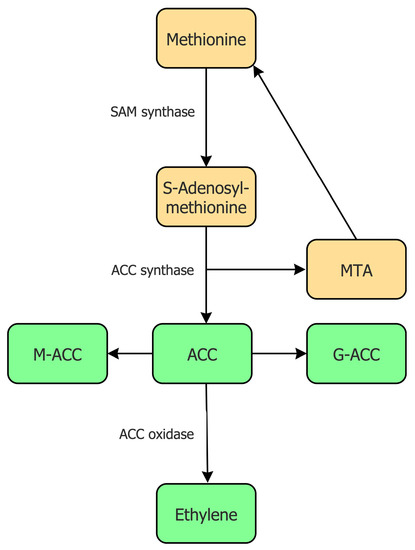
Figure 1. Overview of the biosynthetic pathway for the synthesis of ACC and ethylene in plants. Abbreviations: MTA, 5′-methylthioadenosine; M-ACC, 1-(malonyl)-ACC; G-ACC, 1-(glutamyl)-ACC. The enzymes catalyzing some of these reactions are shown to the left of the arrow indicating a catalyzed reaction.
Being at the junction of several biosynthetic pathways [17], SAM is moderately abundant within plant tissues. For the synthesis of ethylene, the compound SAM is converted into 1-aminocyclopropane-1-carboxylate (ACC) and 5′-methylthioadenosine (MTA) by the enzyme ACC synthase [18][19]. The MTA that is formed as a byproduct of this reaction is recycled to form the amino acid L-methionine. This allows the amount of L-methionine in a plant cell to remain relatively constant even during fairly high rates of ethylene production. Some scientists believe that the synthesis of ACC from SAM is the committal, or rate-limiting, step in the biosynthesis of ethylene [20][21]. There are several, nearly identical, ACC synthase enzymes present in a plant cell as a consequence of the fact that the genes that encode this enzyme are part of a multi-gene family. Moreover, considerable evidence suggests that the transcription of different genes that encode this enzyme are regulated under a range of different environmental or plant physiological conditions (e.g., [19]). When high levels of ACC synthesis are not required, the amount of ACC synthase in plant cells remains relatively low. The subsequent conversion of ACC to ethylene is catalyzed by the enzyme ACC oxidase [22][23][24], which is also generally present constitutively in most plant tissues at very low levels. Similar to ACC synthase genes, ACC oxidase genes are part of a multi-gene family with different isoforms of this enzyme being actively transcribed under different environmental or plant physiological conditions [25]. Thus, in many physiological conditions, both ACC synthase and ACC oxidase may be considered to be inducible enzymes. The fact that both enzymes are inducible is somewhat unusual in that in most metabolic pathways that have been studied only a single step is typically thought of as rate limiting. However, inducible ACC synthesis followed by inducible ethylene synthesis is consistent with the idea, posited below, that ACC synthesis and ethylene synthesis evolved separately from one another.
Plant cells often make more ACC than they require at any particular time. This enables them to rapidly respond to changing environmental conditions and to quickly synthesize ethylene from this storehouse of ACC. However, to remove some of the excessive ACC when it is not immediately needed, plant cells are able to convert ACC into inactive conjugated forms of this compound. For example, the conjugation of ACC with malonate or glutathione occurs as a consequence of the action of either the enzyme ACC N-malonyl transferase [26][27] or the enzyme gamma-glutamyl transpeptidase [28]. These reactions result in the production of either 1-(malonyl)-ACC (M-ACC) or 1-(glutamyl)-ACC (G-ACC), respectively (Figure 1). Kinetic studies have determined that the tightness of the enzyme ACC N-malonyl transferase binding to its substrate ACC is much lower than the tightness of the enzyme ACC oxidase for the same substrate. Thus, if these two enzymes are present in cells in similar amounts, the ACC will bind preferentially to ACC oxidase, and it will subsequently be converted into ethylene [13]. However, as indicated earlier, there is often an excess of ACC in the cell that needs to be removed, and it would be detrimental to the plant to convert all the available ACC into ethylene [29]. In this regard, the conjugation reactions remove ACC only when it is present in relatively high levels, i.e., there is more ACC than is required for the necessary ethylene synthesis.
3. Ethylene as a Signaling Molecule
Ethylene is one of the simplest signaling molecules with hormone-like behavior that is synthesized by plants. When it plays the role of a hormone, it regulates plant development (seed germination, cell elongation, fruit ripening, seed dispersal) and plant responses to environmental stresses such as soil pollution by metals, high salinity levels, low water availability, and sub-optimal temperatures, as well as pathogen (fungi, insects, nematodes) attack. The long history of research on plant ethylene, well described in the review paper written by Bakshi et al. [30], started in 1885 with the first observation by George Fahnestock reporting that illuminating gas used for lighting in both homes and streets negatively affected plant health and growth in a greenhouse in Philadelphia [31]. About fifteen years later, Dimitry Neljubow, a plant physiologist at the Botanical Institute of St. Petersburg University in Russia, identified ethylene as the active molecule in illuminating gas that affects plants [32]. Since then, a quite large number of papers have focused on the roles of ethylene in plants and on its biosynthetic pathways (see Section 2), and only more recently has attention been given to the perception of this gas through ethylene-binding sites and to the genes and proteins differentially expressed as a consequence of ethylene synthesis. All the major molecular elements involved in ethylene signaling pathways have been identified and described through a combination of molecular biology, cell biology, biochemistry, and genetic tools [10].
Based on the results of several studies, performed mainly on Arabidopsis thaliana, it has been hypothesized that the plant gene(s) dedicated to ethylene perception are derived from a cyanobacterium that transferred this coding DNA to the chloroplast genome [33][34][35]. According to one pioneering study, it has been estimated that about 4000 ethylene-binding sites are distributed through each tobacco leaf cell [36], mainly located in the endoplasmic reticulum membrane [37][38]. All of the ethylene receptors described in the literature show a hydrophobic N-terminal domain comprising the ethylene-binding domain [35][39][40], followed by a cytosolic domain, which contains ubiquitous sequences occurring in a plethora of other signaling molecules expressed by members of all the three kingdoms of life, playing a pivotal role in protein–protein interactions between the receptors [41][42][43][44]. At the base of the ethylene receptor is a protein homodimer that binds noncovalently with other homodimers leading to the formation of higher order homomeric and heteromeric complexes [43].
The binding of ethylene with its receptor is supported by a copper-based cofactor, which is required for ethylene-receptor functions, and is provided by the RAN1 (Responsive to Antagonist1) copper transporter [35]. According to the studies performed on A. thaliana, there are five ethylene receptors in this model plant that can be categorized into two clades, the first one containing ETR1 and ESR1 (Ethylene Response 1 and Ethylene Response Sensor 1, respectively) and the second represented by ETR2, ERS2, and EIN4 (Ethylene Response 2, Ethylene Response Sensor 2, and Ethylene Insensitive 4, respectively) [45]. A structural model of this ethylene receptor has been proposed by Schott-Verdugo et al. [46] and, very recently, the structure of the ethylene-binding domain of ETR1 has been elucidated by Azhar et al. [47]. Based on the fact that the functioning of the ethylene receptor is dependent on copper availability, it has been hypothesized that the whole mechanism of ethylene perception depends on an ancient copper transport mechanism that protects plant cells from the toxicity induced by high concentrations of this metal [48].
The gene CTR1 (CONSTITUTIVE TRIPLE RESPONSE 1), coding for a serine/threonine protein kinase, behaves as a negative regulator of ethylene responses, where ethylene response in plants is suppressed by its protein kinase activity [49]. The N-terminal regulatory domain is closely connected to ETR1. Although this association is required in order to ensure the kinase activity of CTR1, the mechanism by which CTR1 is activated by the ethylene receptors is still unknown [50][51][52][53].
The protein EIN2 (ETHYLENE-INSENSITIVE 2), located in the endoplasmic reticulum membrane, shows 12 trans membrane domains at its N terminus and a plant specific domain that is involved in the activation of the ethylene downstream response at the C terminus [54][55]. It has been shown that A. thaliana has a single-copy EIN2 gene whose sequence is conserved from the charophyte green algae to land plants [56]. Its role is to transfer the ethylene plant response to EIN3 (ETHYLENE-INSENSITIVE 3), which is in the nucleus; in fact, when ethylene is perceived by the plant, EIN2 is cleaved by an unknown protease at the C terminal portion and the remaining sequence can move to the nucleus [57][58].
Additionally, EIN2-C can bind to the EBF1/EBF2 RNAs and be sequestered in cytoplasmic granules composed of translationally repressed mRNAs and proteins related to mRNA decay, called processing bodies (P-bodies) [58][59]. Finally, EIN2 stabilizes the two transcriptional factors EIN3 and EIL1 (ETHYLENE-INSENSITIVE 3-like 1 protein, homologous to EIN3), proteins which are key transcriptional factors involved in the modulation of the ethylene response genes such as ETHYLENE RESPONSE FACTORS (ERFs) [60][61]. The identification of this pathway allowed scientists to describe how an ethylene signal goes from the site of perception at the ER membrane and then to the nucleus. The pathway of ethylene signaling is depicted in Figure 2.

Figure 2. When ethylene is absent (Case A), the receptors located in the endoplasmic reticulum membrane (in the diagram this is represented by ETHYLENE RESPONSE 1, ETR1) repress downstream ethylene responses. A serine–threonine protein kinase called CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1) phosphorylates EIN2 (ETHYLENE-INSENSITIVE 2) protein at the C terminal. In this way, EIN2 becomes targeted for degradation. In the nucleus, proteins EBF1 (EIN3 BINDING F-BOX1) and EBF2 (EIN3 BINDING F-BOX2) cooperate to activate the degradation of two transcription factors: EIN3 (ETHYLENE-INSENSITIVE 3) and EIL1 (ETHYLENE-INSENSITIVE 3–like 1). Altogether, these steps lead to inhibition of downstream ethylene signaling.
If ethylene is present (case B), it binds to the receptor and inactivates CTR1. This inactivation promotes the cleavage of the C terminus of EIN 2 protein.
The EIN2 C-terminal domain (EIN2-C), released upon cleavage, inhibits the translation of EBF1/EBF2 thus allowing accumulation of the EIN3- and EIN3-LIKE1 (EIL1)-transcription factors that activate the transcription of ERF1 (ETHYLENE RESPONSE FACTOR 1) and of many other genes involved in ethylene response. Altogether, the transcription of these genes then activates the plant ethylene response. Furthermore, EIN2-C can bind to the EBF1/EBF2 RNAs and become sequestered in processing bodies (P-bodies) in the cytoplasm.
The response of plants to ethylene is the same regardless of whether the ethylene is exogenously provided or endogenously synthesized.
4. ACC as a Signaling Molecule
Several studies have provided evidence that, in addition to acting as a precursor for the synthesis of ethylene, ACC itself can act as a hormonal signal [7][21][61][62][63][64][65][66][67]. The first indication of this possibility, using chemical inhibitors of ethylene biosynthesis or ethylene perception, was that ACC appeared to be a signaling molecule for root to shoot communication under conditions where ethylene perception was blocked [61]. In other experiments, ACC was shown to play a role in stomal development in A. thaliana [68]. In addition, other studies suggested that ACC plays a direct role in plant defenses against the fungal phytopathogen Verticillium dahliae [69]. For example, during periods of flooding, ACC, which is primarily synthesized in plant roots, is transported through the xylem to the shoots where, as a consequence of the availability of oxygen, the ACC is converted to ethylene [70][71]. It is possible that the precise role of ACC as a signaling molecule might be better defined, at least in some cases, by repeating some of the experiments that have previously been performed using chemical ethylene (synthesis or perception) inhibitors or ethylene biosynthesis mutants and instead include the presence of the enzyme ACC deaminase, which is an ACC rather than an ethylene inhibitor. Interestingly, it has been demonstrated that ACC behaves as a signal molecule involved in the recruitment of specific bacteria able to cleave ACC into ammonia and α-ketobutyrate, so that it shapes the rhizosphere microbiome. In turn, these bacteria reduce the stress levels in plants and this new physiological condition can subsequently modulate the composition of the plant-associated bacterial communities [72][73].
Finally, assuming that ACC is in fact a plant-signaling molecule, at least under certain circumstances, this is consistent with the possibility that ACC may have been a major signaling molecule in primitive plants prior to the development of ethylene and ethylene signaling, and prior to ACC becoming a precursor for the synthesis of ethylene in seed plants [74].
References
- Orozco-Mosqueda, M.C.; Santoyo, X.; Glick, B.R. Recent advances in the bacterial phytohormone modulation of plant growth. Plants 2023, 12, 606.
- Weyers, J.D.B.; Patterson, N.W. Plant hormones and the control of physiological processes. N. Phytol. 2001, 152, 375–407.
- Santaner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307.
- Sabagh, A.E.L.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068.
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J. Agric. Food Chem. 2021, 69, 3566–3584.
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stress. Plant Grow. Regulat. 2020, 90, 189–203.
- Abeles, F.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology, 2nd ed.; Academic Press: San Diego, CA, USA, 1992.
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Heidelberg/Berlin, Germany, 2020; 383p.
- Mattoo, A.K.; Suttle, J.C. The Plant Hormone Ethylene; CRC Press, Inc.: Boca Raton, FL, USA, 1991; 347p.
- Chang, C.; Qamp, A. How do plants respond to ethylene and what is its importance? BMC Biol. 2016, 14, 7.
- Jackson, M.B. Ethylene-promoted elongation: An adaptation to submergence stress. Ann. Bot. 2008, 101, 229–248.
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Tivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475.
- Pattyn, J.; Vaughn-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. N. Phytol. 2021, 229, 770–782.
- Fluhr, R.; Matoo, A.K.; Dilley, D.R. Ethylene—Biosynthesis and perception. Crit. Rev. Plant Sci. 2008, 15, 479–523.
- Freebairn, H.T.; Buddenhagen, I.W. Ethylene production by Pseudomonas solanacearum. Nature 1964, 202, 313–314.
- Eckert, C.; Xu, W.; Xiong, W.; Lynch, S.; Ungerer, J.; Tao, L.; Gill, R.; Maness, P.-C.; Yu, J. Ethylene-forming enzyme and bioethylene production. Biotechnol. Biofuels 2014, 7, 33.
- Shen, B.; Li, C.; Tarczynski, M.C. High free-methionine and decreased lignin content result from a mutation in the Arabidopsis S-adenosyl-L-methionine synthetase 3 gene. Plant J. 2002, 29, 371–380.
- Zarembinski, T.I.; Theologis, A. Ethylene biosynthesis and action: A case of conservation. Plant Mol. Biol. 1994, 26, 1579–1597.
- Eun, H.-D.; Ali, S.; Jung, H.; Kim, K.; Kim, W.-C. Profiling of ACC synthase gene (ACS11) expression in Arabidopsis induced by abiotic stresses. Appl. Biol. Chem. 2019, 62, 42.
- Harpaz-Saad, S.; Yoon, G.M.; Mattoo, A.; Kieber, J.J. The formation of ACC and competition between polyamides and ethylene for SAM. Ann. Plant Rev. 2012, 44, 53–81.
- Polko, J.K.; Kieber, J.J. 1-Aminocyclopropane 1-carboxylic acid and its emerging role as an ethylene-dependent growth regulator. Front. Plant Sci. 2019, 10, 1602.
- Kende, H. Enzymes of ethylene biosynthesis. Plant Physiol. 1989, 91, 1–4.
- Hamilton, A.J.; Lycett, G.W.; Grierson, D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 1990, 346, 284–287.
- Houben, M.; Van de Poel, B. 1-Aminocyclopropane-1-carboxylic acid oxidase (ACO): The enzyme that makes the plant hormone ethylene. Front. Plant Sci. 2019, 10, 695.
- Barry, C.S.; Blume, B.; Bouzayen, M.; Cooper, W.; Hamilton, A.J.; Grierson, D. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996, 9, 525–535.
- Peiser, G.; Yang, S.F. Evidence for 1-(malonylamino)cyclopropane-1-carboxylic acid being the major conjugate of aminocyclopropane-1-carboxylic acid in tomato fruit. Plant Physiol. 1998, 116, 1527–1532.
- Yang, S.F. The biosynthesis and metabolism of 1-(malonylamino) cyclopropane-1-carboxylic acid in relation to ethylene production. In Conjugated Plant Hormones: Structure, Metabolism, and Function; Schreiber, K., Schütte, H.R., Sembdner, G., Eds.; VEB Deutscher Verlag der Wissenschaaften: Berlin, Germany, 1987; pp. 92–101.
- Martin, M.N.; Cohen, J.D.; Saftner, R.A. A new 1-aminocyclopropane-1-carboxylic acid conjugating activity in tomato fruit. Plant Physiol. 1995, 109, 917–926.
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189.
- Bakshi, A.; Shemansky, J.M.; Chang, C.; Binder, B.M. History of research on the plant hormone ethylene. J. Plant Growth Regul. 2015, 34, 809–827.
- Fahnestock, G.W. Memoranda of the effects of carbureted hydrogen gas upon a collection of exotic plants. Proc. Acad. Nat. Sci. Phil. 1858, 9–10, 118–134.
- Neljubow, D. Uber die horizontale nutation der stengel von Pisum sativum und einiger anderen pflanzen. Beih. Bot. Zentralb. 1901, 10, 128–139.
- Bleecker, A.B. Ethylene perception and signaling: An evolutionary perspective. Trends Plant Sci. 1999, 4, 269–274.
- Mount, S.M.; Chang, C. Evidence for a plastid origin of plant ethylene receptor gene. Plant Physiol. 2002, 130, 10–14.
- Rodríguez, F.I.; Esch, J.J.; Hall, A.E.; Binder, B.M.; Schaller, G.E.; Bleecker, A.B. A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 1999, 283, 996–998.
- Sisler, E.C. Measurement of ethylene binding in plant tissue. Plant Physiol. 1979, 64, 538–542.
- Evans, D.E.; Bengochea, T.; Cairns, A.J.; Dodds, J.H.; Hall, M.A. Studies on ethylene binding by cell-free preparations from cotyledons of Phaseolus vulgaris L.: Subcellular localization. Plant Cell Environ. 1982, 5, 101–107.
- Evans, D.E.; Dodds, J.H.; Lloyd, P.C.; Apgwynn, I.; Hall, M.A. A study of the subcellular localization of an ethylene binding site in developing cotyledons of Phaseolus vulgaris L. by high resolution autoradiography. Planta 1982, 154, 48–52.
- O’Malley, R.C.; Rodriguez, F.I.; Esch, J.J.; Binder, B.M.; O’Donnell, P.; Klee, H.J.; Bleecker, A.B. Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J. 2005, 41, 651–659.
- Hall, A.E.; Findell, J.L.; Schaller, G.E.; Sisler, E.C.; Bleecker, A.B. Ethylene perception by the ERS1 protein in Arabidopsis. Plant Physiol. 2000, 123, 1449–1458.
- Xie, F.; Liu, Q.; Wen, C.-K. Receptor signal output mediated by the ETR1 N terminus is primarily subfamily I receptor dependent. Plant Physiol. 2006, 142, 492–508.
- Grefen, C.; Städele, K.; Růzicka, K.; Obrdlik, P.; Harter, K.; Horák, J. Subcellular localization and in vivo interactions of the Arabidopsis thaliana ethylene receptor family members. Mol. Plant 2008, 1, 308–320.
- Gao, Z.; Wen, C.-K.; Binder, B.M.; Chen, Y.-F.; Chang, J.; Chiang, Y.-H.; Kerris, R.J.; Chang, C.; Schaller, G.E. Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J. Biol. Chem. 2008, 283, 23801–23810.
- Ho, Y.S.; Burden, L.M.; Hurley, J.H. Structure of the GAF domain, a ubiquitous signaling motif and a new class of cyclic GMP receptor. EMBO J. 2000, 19, 5288–5299.
- Binder, B.M.; Rodriguez, F.I.; Bleecker, A.B. The copper transporter RAN1 is essential for biogenesis of ethylene receptors in Arabidopsis. J. Biol. Chem. 2010, 285, 37263–37270.
- Schott-Verdugo, S.; Müller, L.; Classen, E.; Gohlke, H.; Groth, G. Structural model of the ETR1 Ethylene Receptor Transmembrane Sensor Domain. Sci. Rep. 2019, 9, 8869.
- Azhar, B.J.; Abbas, S.; Aman, S.; Yamburenko, M.V.; Chen, W.; Müller, L.; Uzun, B.; Jewell, D.A.; Dong, J.; Shakeel, S.N.; et al. Basis for high-affinity ethylene binding by the ethylene receptor ETR1 of Arabidopsis. Proc. Natl. Acad. Sci. USA 2023, 120, e2215195120.
- Matoo, K.; Baker, J.E.; Moline, H.E. Induction by Copper Ions of Ethylene Production in Spirodela oligorrhiza: Evidence for a Pathway Independent of 1-Aminocyclopropane-1-carboxylic Acid. J. Plant Physiol. 1983, 123, 193–202.
- Roman, G.; Lubarsky, B.; Kieber, J.J.; Rothenberg, M.; Ecker, J.R. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: Five novel mutant loci integrated into a stress response pathway. Genetics 1995, 139, 1393–1409.
- Clark, K.L.; Larsen, P.B.; Wang, X.; Chang, C. Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc. Natl. Acad. Sci. USA 1998, 95, 5401–5406.
- Cancel, J.D.; Larsen, P.B. Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol. 2002, 129, 1557–1567.
- Gao, Z.; Chen, Y.F.; Randlett, M.D.; Zhao, X.C.; Findell, J.L.; Kieber, J.J.; Schaller, G.E. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J. Biol. Chem. 2003, 278, 34725–34732.
- Huang, Y.; Li, H.; Hutchison, C.E.; Laskey, J.; Kieber, J.J. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J. 2003, 33, 221–233.
- Alonso, J.M.; Hirayama, T.; Roman, G.; Nourizadeh, S.; Ecker, J.R. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 1999, 284, 2148–2152.
- Bisson, M.M.; Bleckmann, A.; Allekotte, S.; Groth, G. EIN2, the central regulator of ethylene signaling, is localized at the ER membrane where it interacts with the ethylene receptor ETR1. Biochem. J. 2009, 23, 1–6.
- Ju, C.; Van de Poel, B.; Cooper, E.D.; Thierer, J.H.; Gibbons, T.R.; Delwiche, C.F.; Chang, C. Conservation of ethylene as a plant hormone over 450 million years of evolution. Nat. Plants 2015, 1, 14004.
- Qiao, H.; Shen, Z.; Huang, S.S.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 2012, 338, 390–393.
- Li, W.; Ma, M.; Feng, Y.; Li, H.; Wang, Y.; Ma, Y.; Li, M.; An, F.; Guo, H. EIN2-directed translational regulation of ethylene signaling in Arabidopsis. Cell 2015, 163, 670–683.
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725.
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping ethylene response: The role of EIN3/EIL1 Transcription Factors. Front. Plant Sci. 2019, 10, 1030.
- Xu, S.L.; Rahman, A.; Baskin, T.I.; Kieber, J.J. Two leucine-rich repeat receptor kinases mediate signaling linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 2008, 20, 3065–3079.
- Yoon, G.M.; Kieber, J.J. 14-3-3 regulates 1-aminocyclopropane-1-carboxylate synthase protein turnover in Arabidopsis. Plant Cell 2013, 25, 1016–1028.
- Tsang, D.L.; Edmond, C.; Harrington, J.L.; Nuhse, T.S. Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol. 2011, 156, 596–604.
- Vanderstraeten, L.; Depaepe, T.; Bertrand, S.; Van Der Straeten, D. The ethylene precursor ACC affects early vegetative development independently of ethylene signaling. Front. Plant Sci. 2019, 10, 1591.
- Van de Poel, B.; Van Der Straeten, D. 1-Aminocyclopropane-1-carboxylic acid (ACC) in plants: More than just the precursor of ethylene! Front. Plant Sci. 2014, 5, 640.
- Mou, W.; Kao, Y.-T.; Michard, E.; Simon, A.A.; Li, D.; Wudick, M.M.; Lizzio, M.A.; Feijo, J.A.; Chang, C. Ethylene-independent signalling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat. Common. 2020, 11, 4082.
- Tsuchisaka, A.; Yu, G.; Jin, H.; Alonso, J.M.; Ecker, J.R.; Zhang, X.; Gao, S.; Theologis, A. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 2009, 183, 979–1003.
- Yin, J.; Zhang, X.; Zhang, G.; Wen, Y.; Liang, G.; Chen, X. Aminocyclopropane-1-carboxylic acid is a key regulator of guard mother cell terminal division in Arabidopsis thaliana. J. Exp. Bot. 2018, 70, 897–908.
- Tsolakidou, M.-D.; Pantelides, L.S.; Tzima, A.K.; Kang, S.; Paplomatas, E.J.; Tsaltas, D. Disruption and overexpression of the gene encoding acc (1-aminocyclopropane-1-carboxylic acid) deaminase in soil-borne fungal pathogen Verticillium dahlia revealed the role of ACC as a potential regulator of virulence and plant defense. Mol. Plant Microbe Interact. 2019, 32, 639–653.
- Bradford, K.J.; Yang, S.F. Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol. 1980, 65, 322–326.
- Else, M.A.; Jackson, M.B. Transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in the transpiration stream of tomato (Lycopersicon esculentum) in relation to foliar ethylene production and petiole epinasty. Aust. J. Plant Physiol. 1998, 25, 453–458.
- Chen, Y.; Bonkowski, M.; Shen, Y.; Griffiths, B.S.; Jiang, Y.; Wang, X.; Sun, B. Root ethylene mediates rhizosphere microbial community reconstruction when chemically detecting cyanide produced by neighbouring plants. Microbiome 2020, 8, 4.
- Nascimento, F.; Rossi, M.; Glick, B. Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) in plant-bacterial interactions. Front. Plant Sci. 2018, 9, 114.
- Li, D.; Mou, W.; Van de Poel, B.; Chang, C. Something old, something new: Conservation of the ethylene precursor 1-amino-cyclopropane-1-carboxylic acid as a signaling molecule. Curr. Opin. Plant Biol. 2022, 65, 102116.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
04 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




