1. Background
Acute respiratory distress syndrome is a complex and heterogeneous disorder, and multiple primary events can result in acute lung injury and ARDS
[1][2]. These patients can have different levels of hypoxemia, different distributions of opacities on chest radiographs, and different types of extra-pulmonary organ dysfunction. The pathologic changes in the lung depend on the primary injury, the development of inflammatory responses, and the timeframe and trajectory of acute events. The goal of precision medicine and personalized care involves the identification of the underlying endotype and the subsequent phenotype, which in turn potentially provides a more focused treatment plan. Ideally, this occurs during the first 48 h after admission. However, this approach is difficult in patients with ARDS, given the rapid evolution of lung injury and the limited time to collect specialized diagnostic information and to make decisions regarding the best treatment.
Patients with acute respiratory distress syndrome have a mortality rate of 30–46%
[3]. The Berlin definition requires exposure to a known risk factor or worsening of the respiratory symptoms within one week and bilateral infiltrates on imaging that are not completely explained by cardiogenic pulmonary edema
[4]. Its severity is classified as mild, moderate, or severe based on the degree of hypoxemia as measured by PaO
2/FiO
2, while on a minimum of 5 cm H
2O of positive end-expiratory pressure (PEEP). The injury in ARDS involves the alveolar epithelium and or the capillary endothelium
[1]. This injury results in increased permeability of alveolar capillary barriers, decreases alveolar fluid clearance, disrupts surfactant function in alveoli, and activates both inflammation and coagulation in the lung. Epithelial injury biomarkers, such as the receptor for the advanced glycation end products (RAGE) and surfactant protein D (SP-D), are increased in patients with direct lung injuries, and endothelial injury biomarkers, such as angiopoietin 2 (Ang-2), are increased in patients with indirect lung injuries, e.g., associated with pancreatitis
[5][6].
The initial stage in ARDS is an exudative phase, characterized by activation of the innate immune cells, which damage endothelial and epithelial cells and by the accumulation of interstitial and alveolar fluid. The next stage involves repair processes to restore cellular barrier function; the final fibrotic stage occurs in some ARDS patients who develop irreversible fibrosis. The factors that drive this process through distinct ARDS stages are unknown, and treatment responses in ARDS likely depend on the time frame for the injury and ongoing acute responses. The pathologic changes in ARDS include diffuse alveolar damage (DAD) with destruction of alveolar structures, the development of hyaline membranes, the infiltration of leukocytes, and the deposition of fibrin; these histologic changes are present in post-mortem studies in approximately 45% of patients who meet the current clinical definition of ARDS
[7][8]. Pneumonia without hyaline membranes or DAD is the second most common histologic finding in autopsy studies. However, classification based on the primary source and location of the injury may oversimplify the complexity of this syndrome, since ICU patients are frequently exposed to multiple possible insults, including volutrauma and barotrauma
[9][10][11].
The complex pathophysiology and the lack of established drug therapy for patients with ARDS has led investigators to try to analyze this syndrome using precision medicine.
2. Precision Medicine
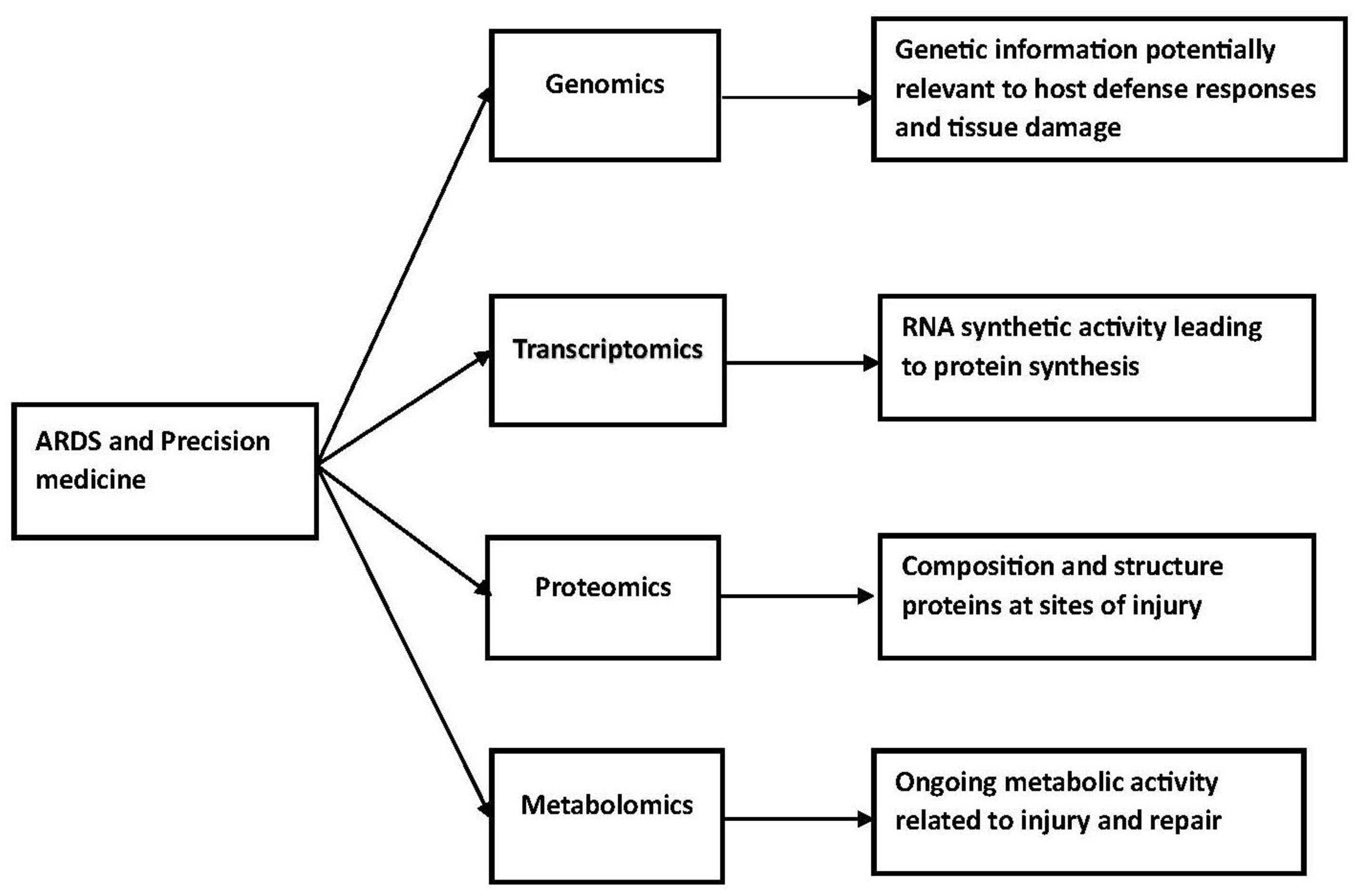
The essential elements necessary for precision medicine include phenotyping, endophenotyping, and genomic profiling
[12]. The sequence of events starts with a genomic network, which results in transcription and the development of proteins. These proteins provide the basis for metabolism. Eventually, whole organ effects, including a psychosocial network, and clinical phenotypes develop. This process can be modified at the post-genomic level by modification of translation and metabolic events. In addition, there can be epigenomic modifications and environmental exposures and microbiome interactions likely contribute to this process. The trajectory for these events will depend on whether the disorder is an acute medical disorder or a chronic medical disorder. Intervention will require a comprehensive understanding of the fundamental cause(s) of a clinical disorder, which could be at the genomic level, the transcription level, the proteomic level, or the metabolomic level. Proteomics is a complete evaluation of the function and structure proteins, including interactions, function, structure, and cellular activities. This can provide information about their basic function and alterations in protein function associated with disease. Metabolomics is the large-scale study of small molecules commonly known as metabolites within cells, body fluids, tissues, and organisms. This provides insight into the metabolic activity of cells during injury and repair.
Battaglini et al. have reviewed personalized medicine using all omics approaches in acute respiratory distress to identify biological subtypes
[13]. Their review considered genomics, transcriptomics, proteomics, and metabolomics. These studies have complex technological requirements. At a more simple but fundamentally important level, investigators need to decide which tissue or fluid to study. Studies in patients with acute lung disease could use bronchoalveolar fluid, plasma, lung biopsies, and exhaled condensates. These studies could identify genes that predispose patients to acute severe lung injury, changes in protein synthesis or catabolism associated with severe injury, or metabolic pathways associated with injury and repair. This information could potentially lead to the development of drugs, which inhibit the relevant biological process or enhance repair.
Precision medicine has the potential to improve outcomes by matching treatment to individuals or subgroups of patients most likely to benefit from a particular treatment
[1]. The introduction of precision medicine into the management of ARDS requires a comprehensive understanding of the complex heterogeneity of this syndrome and identifying methods to predict treatment responses in individual patients. These clinical trials will likely involve individualized strategies based on levels of biomarkers, suggesting a particular dominant (or important) pathophysiologic mechanism of injury. However, multiple difficulties limit the use of recently identified biomarkers of epithelial and endothelial injury (RAGE and Ang-2), vascular leak (BAL albumin), and the innate inflammatory response (IL-6, IL-8, and tumor necrosis factor alpha) in animal studies and in pilot studies in patients with ARDS
[6][14]. Most of these biomarkers were identified during pre-clinical studies and are not usually measured during routine clinical practice. These pre-clinical results frequently depend on the analysis of bronchoalveolar lavage (BAL) samples; however, ARDS research in ICUs often depends on plasma samples, which do not fully reflect the inflammatory response in the lungs. In addition, the variable fluid recovery during BAL limits the interpretation of biomarker concentrations. An additional barrier to the use of precision medicine in ARDS includes the rapid time course of this disease process. This syndrome evolves over hours to days, patients are often too sick to undergo biopsies or other invasive diagnostic testing, and test results are necessarily delayed to allow laboratory processing. For diagnostic tests to help identify ARDS phenotypes, clinicians must have the results available quickly.
The difficulties associated with developing a precision medicine approach in the management of acute respiratory distress syndrome are numerous and substantial. Most studies to date have focused on the clinical characterization of these patients and the development of clinical trials based on the pathophysiologic characteristics of this disorder. In patients with ARDS, this information will need to be available very early in the hospital course, and effective intervention will need to take place within the first several days of patient management (Figure 1).
Figure 1. ARDS and precision medicine.
3. Possible Phenotypes in ARDS
Based on the results of the ARDSNet and Low tidal Volume and elevated End-expiratory volume to Obviate Lung Injury trials, two ARDS phenotypes were identified, a phenotype called “hyper-inflammatory”, defined by higher plasma concentrations of inflammatory cytokines, lower serum bicarbonate levels, and higher vasopressor requirements, and a phenotype called “hypo-inflammatory”, defined by lower concentrations of inflammatory cytokines, higher serum bicarbonate levels, and lower vasopressor requirements
[15]. Post-hoc analysis of additional ARDS randomized controlled trials has demonstrated that these phenotypes have different responses to randomized treatments, results that were obscured in the original clinical trials that analyzed all ARDS patients as a single group. Patients with the hyper-inflammatory phenotype had lower mortality with a higher PEEP strategy, liberal fluid management, and simvastatin, whereas patients with the hypo-inflammatory phenotype either did not respond or had higher mortality with same treatments
[16].
In addition to the biochemical heterogeneity in the injuries in ARDS, there are also significant anatomic and physiologic differences that contribute to the complexity of this disorder. Different patient-specific characteristics, such as increased body mass indices, and different injury specific characteristics, such as the distribution of pulmonary opacities, can substantially change lung mechanics and may affect the optimal mechanical ventilation strategy. Consequently, additional research has led to the classification of radiographic subtypes of ARDS, defined as non-focal/diffuse and focal/lobar. Non-focal/diffuse ARDS occurs more frequently after systemic insults that indirectly cause lung injury, is associated with lower levels of RAGE (which reflects epithelial damage), and causes worse lung compliance and higher mortality. The identification of the distinct radiographic phenotypes led to trials, which attempted to personalize mechanical ventilation strategies with the hypothesis that individuals with focal/lobar ARDS have an increased volume of normal lung parenchyma, and thus would tolerate higher tidal volumes than those with non-focal/diffuse ARDS, but this trail did not find any change in mortality using radiographic criteria to adjust PEEP
[17].
Bos and Ware reviewed the causes, pathophysiology and phenotypes in patients with ARDS in 2022
[18][19]. They discussed subphenotypes based on cause, biological subphenotypes, radiological subphenotypes, and physiological subphenotypes and noted that mechanical ventilation can cause lung injury and inflammation. The development of barotrauma, volutrauma, and biotrauma associated with mechanical ventilation could change the individual patient’s phenotype and make it more difficult to study outcomes. In a second review, Bos et al. considered the various types of alveolar injury in ARDS and the biomarkers available to identify these injuries
[10][18]. They suggested that the predominant patterns include epithelial and/or endothelial injury, protein-rich pulmonary edema, and systemic or alveolar inflammatory responses. The most readily available biomarkers would involve bronchoalveolar lavage studies, and analysis of this fluid could identify increased total protein concentrations, increased neutrophil numbers, and increased cytokine levels. Whether or not this information would lead to classifications that predict therapeutic responses is uncertain.
Alipanah et al. also reviewed the possible classification of patients with ARDS using phenotypes
[20]. Potential phenotypes would include clinical phenotypes based on potential causes and radiographic presentations, physiologic phenotypes based on PaO
2/FiO
2 ratios, and biological phenotypes based on biomarkers reflecting injury and/or inflammation. Classification using these parameters may eventually determine clinically important phenotypes, but this research is limited by the lack of prospective validation. Gorman et al. reviewed ARDS and discussed the diagnosis, outcomes, long-term sequelae, and management
[21]. They noted that long-term outcomes in survivors often included physical, mental, and cognitive deficits. Therefore, treatment protocols may need to evaluate both short-term benefits and long-term benefits and consider the possibility that there are survivor phenotypes. Beitler et al. published a position paper on advancing precision medicine for ARDS based on the workshops sponsored by the United States National Heart, Lung, and Blood Institute
[22]. They noted that multi-center observational cohort studies were needed to collect information relevant to establishing phenotypes in this syndrome and that rapid diagnostic assays were essential to provide predictive and prognostic enrichment in any trial. Finally, they noted that platform trials with a master protocol with or without adaptive features could accelerate the identification of treatment-responsive subgroups.
The idea of ARDS classification based on phenotypes may have great promise but realistically is not available at present. Heijen et al. analyzed inflammatory biomarkers in mini-BAL fluid aspirations from patients with ARDS and the key features of the lung microbiome in this fluid
[23]. Patients were classified into three separate groups with two subphenotypes in each group. One phenotype was based on cluster analysis and included patients that were “uninflamed” or “reactive” lung injury. The second phenotype was based on latent class analysis and included patients who were either hypo-inflammatory or hyper-inflammatory. The third phenotype was based on etiology and included patients with either direct injury to the lung or indirect injury to the lung. There were no significant differences in these biomarkers or the microbiome in the subphenotypes in these three classifications. These results suggest that the phenotype classifications used in this study do not adequately reflect alveolar inflammatory responses. This greatly complicates efforts to use systemic biomarkers to identify phenotypes. In effect, most clinicians depend on clinical classifications to initiate treatment of patients with ARDS.
4. Clinical Classifications
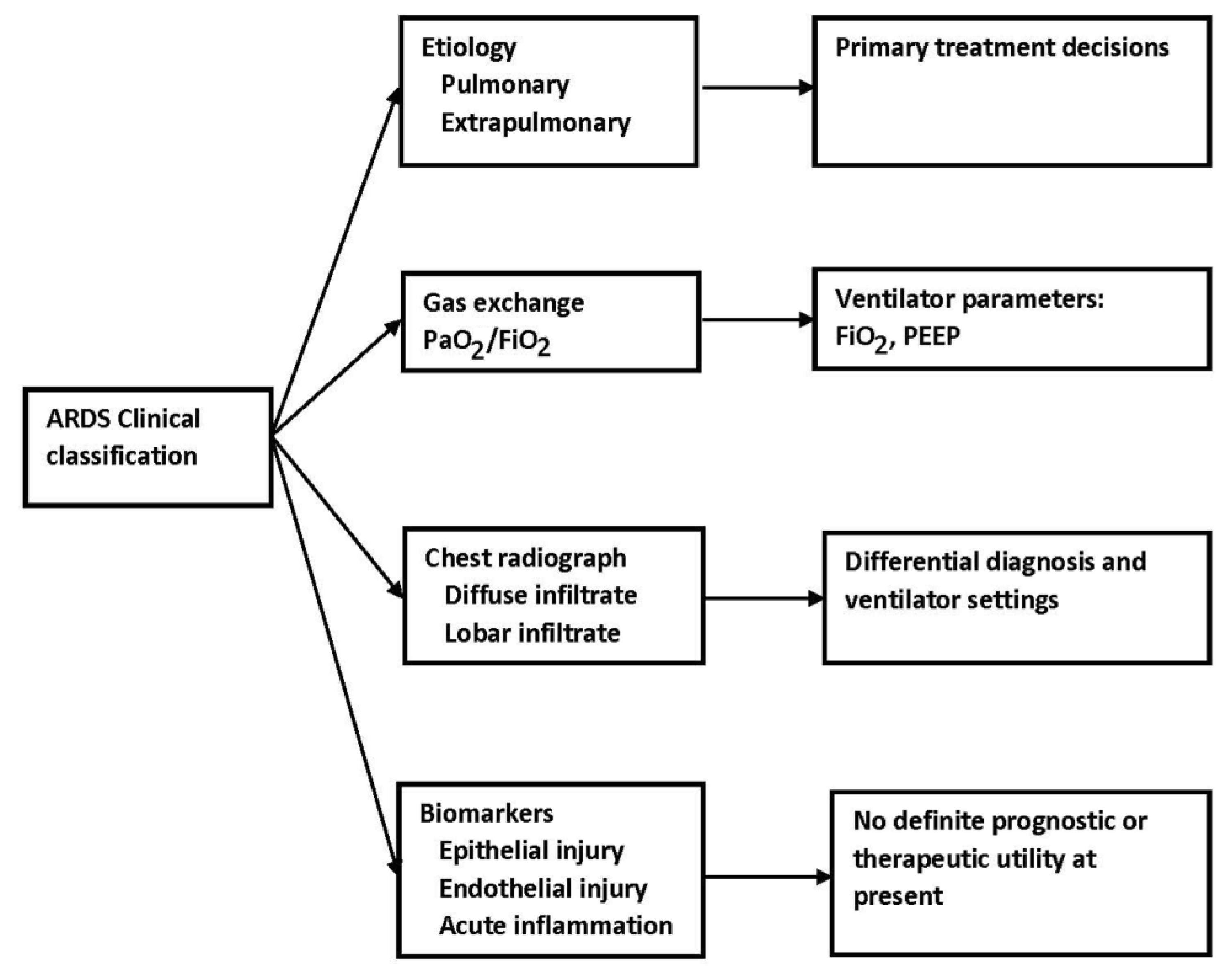
The most obvious clinical calcifications include the primary disease process causing or associated with ARDS, which is usually classified as either pulmonary or extrapulmonary, the degree of hypoxemia based on a PaO2/FiO2 ratios, and the extent of lung involvement based on radiographic images, both plain chest radiographs and computed tomography. These classifications have the potential to lead to personalized care and often form the basis for trial design discussed below.
Chest radiographs are readily available and provide important information in critically ill patients but, of course, cannot identify the exact pathologic change(s) in individual patients. Chiumello et al. reviewed lung imaging techniques in patients with ARDS. Computed tomography provides a better description of the distribution of infiltrates and the density of infiltrates
[24][25]. However, this technique requires the transfer of the patient to the radiology suite. Bedside ultrasonography is readily available and may have better accuracy than bedside chest X-rays in certain situations, such as pleural effusions. Other techniques that have been studied in ARDS patients include positron emission tomography, electrical impedance tomography, and magnetic resonance imaging. Chest radiographs, computed tomography, lung ultrasound, and electrical impedance tomography can be used to evaluate lung recruitment during PEEP trials. Patients with diffuse lung involvement on CT have higher mortality rates than patients with lobar or patchy involvement
[24]. An increase in involvement during the early phase of ARDS is also associated with increased mortality. In addition, information collected on one day may not be accurate the following days because of the rapid progression of the inflammatory process and fluid accumulation.
Advances in technology have provided additional important information about the lung involvement and ARDS. Electrical impedance tomography uses a device with 16–32 electrodes to inject small amounts of electrical current around the chest
[26]. The conduction of these currents is quantified to measure the level of tissue resistance. An air-filled lung has electrical resistance and increased impedance. A lung with a significant amount of extracellular water serves as a better conductor and has reduced impedance. These signals can be reconstructed into a digital matrix to generate a cross-sectional map of lung impedance through the respiratory cycle. Consequently, this device provides real-time information about lung ventilation. This information has been used to identify areas of lung overdistention and lung collapse. It can also be used to determine lung recruitment during PEEP trials and during prone positioning
[27]. However, there is limited information demonstrating that the use of electrical impedance tomography managed ventilator adjustments changes outcomes.
In summary, bedside techniques available to evaluate the patient’s lung include chest radiographs, ultrasonography, and electrical impedance tomography. Ultrasonography can be used to characterize the patient’s lung disease and to evaluate the effects of changes in PEEP levels on lung recruitment. It also provides information about the presence of pneumothorax and/or pleural effusions. These techniques offered the opportunity for personalized care or the adjustment of care based on individual characteristics (Figure 2).
Figure 2. ARDS and clinical classification.
5. Clinical Trial Outcomes
The outcomes in large well-designed treatment trials illustrate the difficulty in studying these patients. The first trial to report a mortality benefit in ARDS was the Acute Respiratory Distress Syndrome Network (ARDSNet) trial, which reported an 8.8% decrease in mortality (31% vs. 39.8%) in patients treated with low tidal volume ventilation (6 mL/kg vs. 12 mL/kg of ideal body weight)
[28]. The Fluids and Catheters Treatment Trial (FACTT) demonstrated an increase in ventilator-free days but no change in mortality in patients treated with a conservative fluid management protocol, and the Proning Severe ARDS Patients (PROSEVA) trial reported decreased mortality with prone positioning in ARDS patients with a PaO
2/FiO
2 less than 150 mmHg
[29][30]. By selectively enrolling patients with worse oxygenation (PaO
2/FiO
2 < 150 mmHg), the PROSEVA trial used a prognostic enrichment protocol, which selected patients with a higher likelihood of experiencing the primary study outcome. This increases the likelihood of detecting a difference in the outcome of interest with smaller sample sizes for a given effect size and provides a logical approach to develop inclusion criteria for clinical trials.
In contrast to the better outcomes based on optimal supportive care for ARDS patients, clinical trials of drug treatment have often had negative outcomes. The Dexamethasone Treatment for the Acute Respiratory Distress Syndrome: A Multicentre, Randomised Controlled Trial demonstrated that corticosteroid administration decreased mortality in patients who were treated early in their disease course; however, the Late Steroid Rescue study reported more adverse outcomes when corticosteroids were administered after 14 days of unresolving ARDS
[31][32]. These two trials demonstrate that time-sensitive therapeutic windows likely influence the outcomes of drug trials and indicate the need for additional research to precisely define the stages of ARDS to optimize treatment.
The heterogeneity of ARDS pathophysiology and outcomes is also affected by differences in the treatment of these patients in ICUs. The 2000 ARDSNet trial reported decreased mortality with low tidal volume ventilation, whereas The Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure study, published in 2016, reported that more than 33% of patients with ARDS were managed with a tidal volume of >8 mL/kg of ideal body weight
[33]. There are several possible factors contributing to this undesirable variability in care, and these include delayed recognition of ARDS and overestimation of the necessary tidal volume in patients. In addition to the incomplete use of proven therapies, there are likely to be other variations in other treatment approaches in ARDS that have not yet been identified, and differences in available critical care resources could contribute to heterogeneity in care in ARDS patients. The management of large multi-center trials will require strict inclusion criteria, practical treatment protocols, and frequent communication among the investigators throughout the trial.
6. Trial Design
During the COVID-19 pandemic, critical care investigators responded to the need for new treatment by establishing large platform trials, such as I-SPY COVID (a multi-center phase 2 adaptive platform trial designed to rapidly screen potential therapeutics for severe COVID-19), the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) suite of trials, and Randomised Evaluation of COVID-19 Therapy (RECOVERY)
[34][35]. These studies identified the beneficial effect of dexamethasone, baricitinib, and tocilizumab in COVID-19 ARDS and the lack of benefit with hydroxychloroquine and ivermectin. These trials had a different platform design, in which multiple potential therapies were tested simultaneously against a single control group, which can significantly increase trial efficiency compared to traditional randomized controlled trials that usually tests one therapy at a time. Of course, these studies require large patient numbers and complex study designs and cannot depend on precision medicine, at least at present. Adaptive trial design has the potential to improve outcomes in complex clinical disorders
[36].
7. Summary
In summary, investigators have identified several subgroups of ARDS, based on the heterogeneous aspects of this disease process, including host factors, etiology and timing of injury, radiographic injury patterns, and disease severity. Certain phenotypes have different responses to drug treatment when evaluated retrospectively, which revealed the important finding that the uniform use of a drug in ARDS, without concurrent endophenotyping, may limit the ability of investigators both to detect benefits in certain phenotypes and to detect harm in others. More research is needed to understand the effect of anatomic and physiologic heterogeneity of ARDS due to both patient-specific factors and disease-specific factors, since these differences could have important effects on lung mechanics and ventilation strategies and could form the basis for precision medicine.