Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ivan Mirchev Ivanov | -- | 2176 | 2023-08-03 13:44:08 | | | |
| 2 | Rita Xu | -308 word(s) | 1868 | 2023-08-04 04:32:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zvetkova, E.; Koytchev, E.; Ivanov, I.; Ranchev, S.; Antonov, A. Biomechanical, Healing and Therapeutic Effects of Stretching. Encyclopedia. Available online: https://encyclopedia.pub/entry/47615 (accessed on 08 February 2026).
Zvetkova E, Koytchev E, Ivanov I, Ranchev S, Antonov A. Biomechanical, Healing and Therapeutic Effects of Stretching. Encyclopedia. Available at: https://encyclopedia.pub/entry/47615. Accessed February 08, 2026.
Zvetkova, Elissaveta, Eugeni Koytchev, Ivan Ivanov, Sergey Ranchev, Antonio Antonov. "Biomechanical, Healing and Therapeutic Effects of Stretching" Encyclopedia, https://encyclopedia.pub/entry/47615 (accessed February 08, 2026).
Zvetkova, E., Koytchev, E., Ivanov, I., Ranchev, S., & Antonov, A. (2023, August 03). Biomechanical, Healing and Therapeutic Effects of Stretching. In Encyclopedia. https://encyclopedia.pub/entry/47615
Zvetkova, Elissaveta, et al. "Biomechanical, Healing and Therapeutic Effects of Stretching." Encyclopedia. Web. 03 August, 2023.
Copy Citation
Characterized in biomedical terms, stretching exercises have been defined as movements applied by external and/or internal forces to increase muscle and joint flexibility, decrease muscle stiffness, elevate the joint range of motion (ROM), increase the length of the “muscle–tendon” morpho-functional unit, and improve joint, muscle, and tendon movements, contraction, and relaxation.
stretching
static stretching (SS)
joint range of motion (ROM)
muscle stiffness
1. Introduction
In biomechanical terms, stretching has been characterized by Weerapong et al. as a movement applied by an external and/or internal force in order to increase muscle flexibility and to improve the joint range of motion (ROM) [1]. The aim of stretching in physical exercise is to increase the muscle–tendon unit length and to improve joint flexibility, as well as to decrease the risk of soft-tissue injuries [2][3][4][5][6][7].
2. Biomechanical Parameters, Healing, and Therapeutic Effects of Stretching
Interesting results arose from numerous recent investigations and various stretching programs applied in medical practice and sports. The biomechanical parameters and therapeutic effectiveness of stretching applications could modify joint, tendon, and muscle flexibility. For this purpose, different variables, such as collagen and elastin syntheses, fiber elongation and elasticity, energy absorption, etc., could be used as mechanobiological, cellular, and molecular biomarkers.
Many retrospective and prospective studies have been performed and stratified on the acute and chronic effects of stretching, both under physiological conditions and in pathological states. Progressive static stretching is effective during the prophylaxis of injuries in sports and exercise training [8][9][10]. The healing properties of stretching are of importance in the prophylaxis and treatment of joint injuries when also combined with other rehabilitation procedures (massage, heat/cold, warming up, etc.) [7][11][12][13][14]. Recent studies have reported a high effectiveness of stretching in the treatment and prevention of contractures and fasciitis, as well as useful methods for application in routine orthopedic and traumatological practice [5][15][16][17][18][19].
As a rehabilitation method, stretching has been applied to improve the biomechanical parameters of muscles, tendons, ligaments, fascia, and joints [4][6][7][9][20][21].
The viscoelastic responses of muscles, tendons, ligaments, fascia, and joints to slow stretching exercises could result in less passive tension, compared to faster procedures [22][23]. The faster the stretch, the higher the muscle stiffness will be [6]. Most stretching techniques (static, dynamic, ballistic, etc.) have been successfully implemented in clinical practice [2][4][24][25].
The effects of stretching on muscle and joint flexibility are closely related to the joint range of motion (ROM), whereby the increased range of motion induces the analgesic effects of stretching.
Various stretching techniques have been compared. Unfortunately, the current results of the chronic effects of static stretching (SS) exercises on the muscle strength, flexibility, joint ROM, and muscle power are still controversial [5][18][26].
3. Animal, Mathematical, and Computational Models of Stretching
More scientific information is needed for the creation of new, successful mathematical and computational models of stretching [27][28].
The mathematical and rheological models of the joint cavity capsule and intra-articular synovial fluid turnover (viscosity and permeation of hyaluronan, glycosaminoglycans (GAGs), and albumin) indicate cellular mechanisms of stretching and a role of the intercellular matrix as a selective molecular filter. The specific rheological properties of joint synovial fluid are altered in traumatic and post-traumatic pathological states (different arthroses, rheumatoid arthritis, osteoarthritis, etc.) [29].
In the treatment and prevention of sports injuries, as well as in the development of improved sports programs for injury prevention, static stretching (SS) is very important for the efficient rehabilitation of joints [3][30][31][32][33][34]. Based on the latest scientific findings, especially on the biomechanical contributions in this field, new preventive and therapeutic measures for avoiding stiffness and motion impairment in the joints can be adopted during the early stages of diseases [2][9][15].
The therapeutic effects of stretching have been established in a great number of experimental animal models (e.g., post-traumatic knee contractures in rat and rabbit models, which have significance for humans) [7][35][36][37][38]. Thus, it is possible to evaluate important data on cellular functions and the intracellular matrix components of joint cartilage, as well as information on the morphological structures and functions of joint capsules, both in healthy controls and in joints that have been modified in the processes of contractures (post-traumatic, myogenic, arthrogenic, etc.) [7][11][36][37][38][39]. Zhang et al. examined the effect of stretching combined with ultrashort wave diathermy on joint functions and clarified its cellular mechanisms in a rabbit knee contracture model [38]. Wang L. et al. [7] studied the effects of different static progressive stretching durations on the knee joint’s range of motion, collagen- and alpha-actin expressions in fibroblasts, inflammatory cell number, and fibrotic changes in the joint capsule (as the result of different static progressive stretching durations applied to a post-traumatic knee contracture in a rat model). The authors concluded that static progressive stretching could improve post-traumatic knee contractures by increasing the knee joint mobility.
Numerous animal models that simulate a “knee flexion contracture” and a few models of a “knee extension contracture” have been proposed [11]. The authors determined that the “aggravation of contractures” was correlated with the degree of “fibrosis response” of the joints, which is related to the activation of type I and type III collagen syntheses, as well as to the stimulation of pro-fibrotic gene expression in fibroblasts and chondroblasts. A proteomic analysis of the muscles and joint capsule was performed by the same study group [11]. The expression of transforming growth factor beta-1 (TGF-β-1) was also examined as a significant biomarker of changes in the synthesis and distribution of different collagen types (I–III) in the intercellular matrix. An important fact of clinical relevance is that “extension contracture models” better mimic fractures and the bed-associated immobilization of patients in traumatology than “flexion contracture models”.
The main question related to stretching biomechanics is: “Could chronic stretching change the joint–ligament–tendon–muscle mechanical properties?” The effects of stretching were reported for joint resistance and muscle and tendon stiffness, but a large heterogeneity was seen for most of the variables obtained [4]. The same authors analyzed 26 papers regarding longitudinal stretching (static, dynamic, and/or PNF) in humans of any age and with different health statuses. Structural and mechanical variables were evaluated for joints and muscle–tendon units: dynamic stretching, static stretching, flexibility, stiffness, mechanical joint properties, muscle morphology and functional activities, changes in the tendon characteristics, proprioceptive neuromuscular facilitation, etc. [6]. Adaptations to chronic stretching protocols shorter than 8 weeks seemed to occur mostly at the sensory level [6].
4. Biomechanical Effects of Static and Active Isometric Stretching Applied to the Human Knee Joint
The effects of stretching on muscle properties are clearly described in literature and depend on various factors, including stretching techniques, stretching time, retention time, rest time, and the time difference between the intervention and the measurement [28][40][41]. Most studies investigated the effects of static stretching on the passive properties of the muscle–tendon unit [42][43][44][45][46]. In a series of studies by Magnusson et al. [42][43][45][46][47], it was shown that static stretching for 90 s over five repetitions reduced muscle resistance, passive stiffness, peak torque, and stress relaxation. Another team of researchers [48][49] concluded that changes in the viscoelastic properties of the muscle–tendon unit depend more on the duration of stretching than on the number of stretches. An extension of static stretching time (from five to ten minutes) was shown to reduce tendon stiffness, as measured passively by ultrasonography [48][49]. The reduction in stiffness might be due to a change in the arrangement of collagen fibers in the tendon [48]. Stretching increased the range of motion of the femoral flexion and the outer rotation [21].
Isometric stretching is a type of static stretching associated with the resistance of muscle groups through isometric contractions of the stretched muscles. Due to the fact that this type of muscle stretching works in an isometric mode, the initiated muscle forces will affect the joints around which the muscles are located. The muscle forces produced by this type of stretching trigger processes within the joint itself. Cotofana et al. [50] demonstrated that the cartilage thickness decreased to 5.2% from a knee load with a force equal to 50% of the body weight. Herberthold et al. [51] evaluated the deformation at a force load equal to 150% of the body weight.
The experimental model and working hypothesis estimated that, as the result of active isometric stretching of the adjacent locomotor muscles, changes in the distance between the femur and the corresponding end of the tibia could be observed [28]. The changes in the distance between the two bones would, in turn, be conditioned by several factors: the magnitude of the isometric muscle tension during stretching; the duration and direction of the tension applied; the tendon’s biomechanical properties; and the biomechanical properties of the knee joint (shape, size, viscosity of the synovial fluid, and mechanical properties of the joint capsule elements).
Static investigations of knee joint stability are often directed to stretching exercises [28][52][53][54][55][56] and isometric back squats [55].
The study group’s quantitative estimation of the biomechanical processes in human knee joints during active isometric stretching was based on knee joint capsule ultrasound scanning during isometric stretching exercises [28][52][53][56]. During a right-lower-limb pose with a 140-degree femur–tibia angle, the distance between the tibia and femur bones forming the knee joint was measured using ultrasound scanning. The experimental model included an ultrasound examination of the knee joint after the isometric stretching of healthy men (n = 10). The changes (in millimeters) in the distances between the femur and tibia were measured with a portable ultrasound system (Vinno 6, China; Figure 1). The apparatus was used for the purposes of the study in the musculoskeletal mode and in real time with a scanning frequency for the linear transducer of 8 to 10 MHz. The system was able to work in three different upright positions, all with a femur–tibia angle of 140 degrees at rest. In two of the three upright positions, extra loads of 4 and 8 kg were applied vertically down to the lower right limb to induce isometric stretching. Three quantitative parameters—distance up (Dup), distance down (Down), and area (A, cm2)—were measured from the ultrasound pictures (Figure 1). They defined the two displacements (mm) and the area (cm2) between the intra-articular femur and tibia cartilage surfaces.
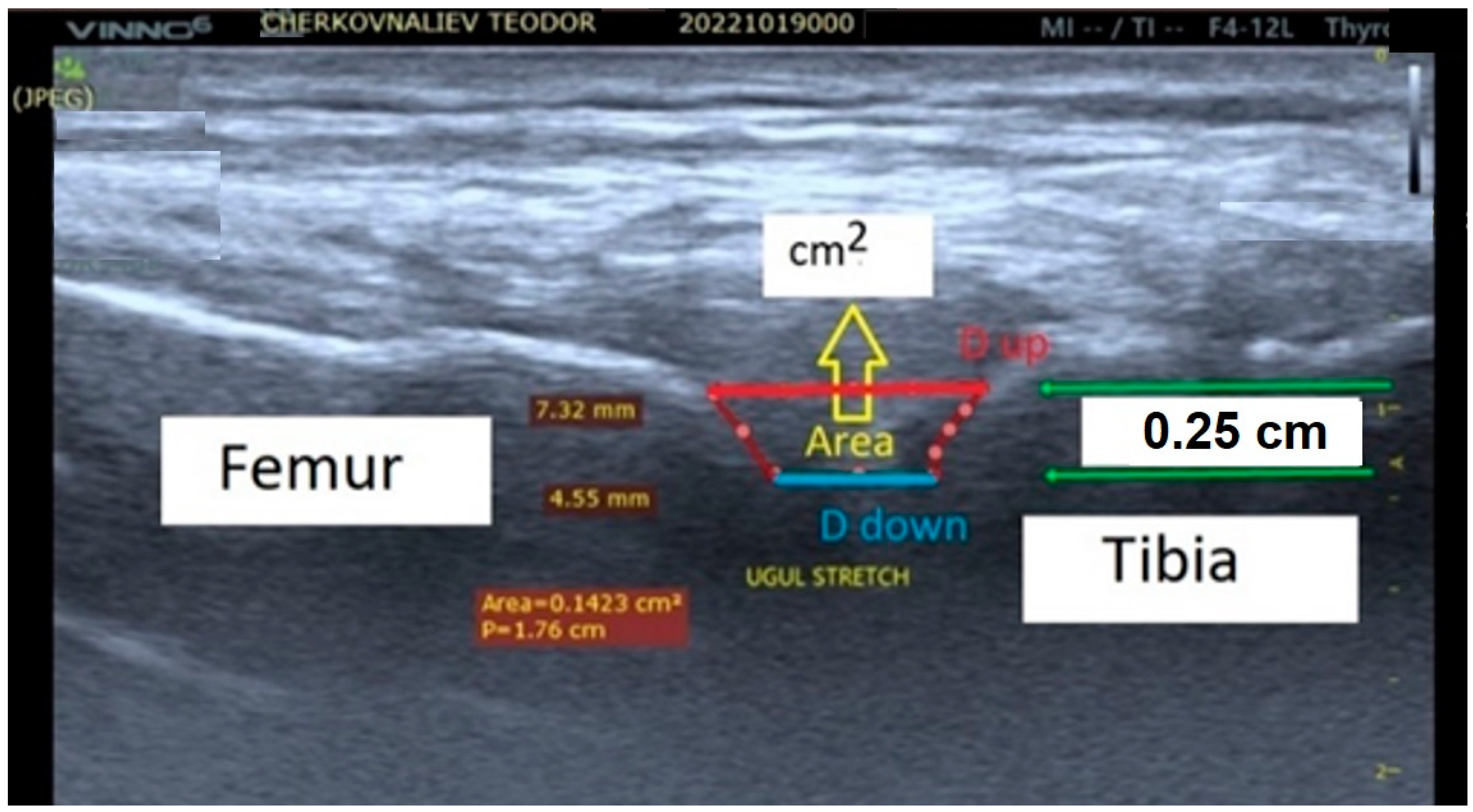
Figure 1. The screen view of the echography with measured distances between the femur and tibia bones in the knee joint of the participant. The distances Dup and Ddown between the femur and tibia for all participants at the reference position and at different loading levels were measured (in millimeters), with the depth space of the ultrasonographic scan equal to 0.25 cm. The next steps were used for improving the present experimental protocol with the addition of a “knee muff” for the stationary positioning of the ultrasound transducer toward the knee joint. The accuracy of the protocol was increased, with an error rate of less than 12%.
The results obtained for the change in the intra-articular geometry under a load and under stretching could serve as a quantitative assessment of the internal joint kinematics and might determine the joint mobility of individual participants in the stretching exercises [28][52][53][56].
The accuracy of the ultrasound pictures and measurements in the experimental model was limited by three main components (Figure 1) [56]. The first was related to the transducer accuracy characteristics. The second was the accuracy of the identity of the transducer–knee joint image position reproductions. The third component was the researcher’s skill at obtaining scanning pictures. The present preliminary experimental model accuracy was defined as the sum of the three components cited and was lower than 30%.
References
- Weerapong, P.; Hume, P.A.; Kolt, G.S. Stretching: Mechanisms and benefits for sport performance and injury prevention. Phys. Ther. Rev. 2004, 9, 189–206.
- Taylor, D.C.; Dalton, J.D., Jr.; Seaber, A.V.; Garrett, W.E., Jr. Viscoelastic properties of muscle-tendon units: The biomechanical effects of stretching. Am. J. Sports Med. 1990, 18, 300–309.
- Ferber, R.; Osternig, L.R.; Gravelle, D.C. Effect of PNF stretch techniques on knee flexor muscle EMG activity in older adults. J. Electromyogr. Kinesiol. 2002, 12, 391–397.
- Freitas, S.R.; Mendes, B.; Le Sant, G.; Andrade, R.J.; Nordez, A.; Milanovic, Z. Can chronic stretching change the muscle-tendon mechanical properties? A review. Scand. J. Med. Sci. Sports 2018, 28, 794–806.
- Arntz, F.; Markov, A.; Behm, D.G.; Behrens, M.; Negra, Y.; Nakamura, M.; Chaabene, H. Chronic Effects of Static Stretching Exercises on Muscle Strength and Power in Healthy Individuals Across the Lifespan: A Systematic Review with Multi-level Meta-analysis. Sports Med. 2023, 53, 723–745.
- Knudson, D. The biomechanics of stretching. J. Exerc. Sci. Physiother. 2006, 2, 3–12.
- Wang, L.; Cui, J.B.; Xie, H.M.; Zuo, X.Q.; He, J.L.; Jia, Z.S.; Zhang, L.N. Effects of Different Static Progressive Stretching Durations on Range of Motion, Myofibroblasts, and Collagen in a Posttraumatic Knee Contracture Rat Model. Phys. Ther. 2022, 102, pzab300.
- Salsich, G.B.; Mueller, M.J.; Sahrmann, S.A. Passive ankle stiffness in subjects with diabetes and peripheral neuropathy versus an age-matched comparison group. Phys. Ther. 2000, 80, 352–362.
- Sacco, I.C.; Sartor, C.D. From treatment to preventive actions: Improving function in patients with diabetic polyneuropathy. Diabetes/Metab. Res. Rev. 2016, 32, 206–212.
- Williams, D.B.; Brunt, D.; Tanenberg, R.J. Diabetic neuropathy is related to joint stiffness during late stance phase. J. Appl. Biomech. 2007, 23, 251–260.
- Zhang, R.; Zhang, Q.B.; Zhou, Y.; Zhang, R.; Wang, F. Possible mechanism of static progressive stretching combined with extracorporeal shock wave therapy in reducing knee joint contracture in rats based on MAPK/ERK pathway. Biomol. Biomed. 2023, 23, 277–286.
- Nakamura, M.; Konrad, A.; Kasahara, K.; Yoshida, R.; Murakami, Y.; Sato, S.; Wilke, J. The Combined Effect of Static Stretching and Foam Rolling With or Without Vibration on the Range of Motion, Muscle Performance, and Tissue Hardness of the Knee Extensor. J. Strength Cond. Res. 2022, 37, 322–327.
- Medeiros, D.M.; Cini, A.; Sbruzzi, G.; Lima, C.S. Influence of static stretching on hamstring flexibility in healthy young adults: Systematic review and meta-analysis. Physiother. Theory Pract. 2016, 32, 438–445.
- Fukaya, T.; Sato, S.; Yahata, K.; Yoshida, R.; Takeuchi, K.; Nakamura, M. Effects of stretching intensity on range of motion and muscle stiffness: A narrative review. J. Bodyw. Mov. Ther. 2022, 32, 68–76.
- Berrueta, L.; Muskaj, I.; Olenich, S.; Butler, T.; Badger, G.J.; Colas, R.A.; Spite, M.; Serhan, C.; Langevin, H.M. Stretching impacts inflammation resolution in connective tissue. J. Cell. Physiol. 2016, 231, 1621–1627.
- Król, M.; Kupnicka, P.; Bosiacki, M.; Chlubek, D. Mechanisms Underlying Anti-Inflammatory and Anti-Cancer Properties of Stretching—A Review. Int. J. Mol. Sci. 2022, 23, 10127.
- Su, H.; Chang, N.J.; Wu, W.L.; Guo, L.Y.; Chu, I.H. Acute effects of foam rolling, static stretching, and dynamic stretching during warm-ups on muscular flexibility and strength in young adults. J. Sport Rehabil. 2017, 26, 469–477.
- Cipriani, D.J.; Terry, M.E.; Haines, M.A.; Tabibnia, A.P.; Lyssanova, O. Effect of stretch frequency and sex on the rate of gain and rate of loss in muscle flexibility during a hamstring-stretching program: A randomized single-blind longitudinal study. J. Strength Cond. Res. 2012, 26, 2119–2129.
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst. Rev. 2017, 1, CD010802.
- Taylor, D. Physical activity is medicine for older adults. Postgrad. Med. J. 2014, 90, 26–32.
- Copeland, J. Stretching: Mechanisms and benefits for sport performance and injury prevention. New Zealand J. Physiother. 2005, 33, 68–69.
- Kataura, S.; Suzuki, S.; Matsuo, S.; Hatano, G.; Iwata, M.; Yokoi, K.; Tsuchida, W.; Banno, Y.; Asai, Y. Acute effects of the different intensity of static stretching on flexibility and isometric muscle force. J. Strength Cond. Res. 2017, 31, 3403–3410.
- Konrad, A.; Gad, M.; Tilp, M.J.S.J. Effect of PNF stretching training on the properties of human muscle and tendon structures. Scand. J. Med. Sci. Sports 2015, 25, 346–355.
- Hotta, K.; Behnke, B.J.; Arjmandi, B.; Ghosh, P.; Chen, B.; Brooks, R.; Maraj, J.J.; Elam, M.; Maher, P.; Kurien, D.; et al. Daily muscle stretching enhances blood flow, endothelial function, capillarity, vascular volume and connectivity in aged skeletal muscle. J. Physiol. 2018, 596, 1903–1917.
- Guissard, N.; Duchateau, J. Effect of static stretch training on neural and mechanical properties of the human plantar-flexor muscles. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2004, 29, 248–255.
- Middag, T.R.; Harmer, P. Active-isolated stretching is not more effective than static stretching for increasing hamstring ROM. Med. Sci. Sports Exerc. 2002, 34, S151.
- Stoichev, S.; Ivanov, I.; Ranchev, S.; Jotov, I. A review of the biomechanics of synovial joints with emphasize to static stretching exercise. Ser. Biomech. 2021, 35, 3–20.
- Ranchev, S.; Ivanov, I.; Iotov, I.; Stoytchev, S. On the biomechanical processes in human knee joint during active isometric stretching. Ser. Biomech. 2019, 33, 56–61.
- Davies, D.V. Synovial membrane and synovial fluid of joints. Lancet 1946, 248, 815–819.
- Bryanton, M.; Bilodeau, M. The role of thigh muscular efforts in limiting sit-to-stand capacity in healthy young and older adults. Aging Clin. Exp. Res. 2017, 29, 1211–1219.
- Hill, K.J.; Robinson, K.P.; Cuchna, J.W.; Hoch, M.C. Immediate effects of proprioceptive neuromuscular facilitation stretching programs compared with passive stretching programs for hamstring flexibility: A critically appraised topic. J. Sport Rehabil. 2017, 26, 567–572.
- Lin, W.C.; Lee, C.L.; Chang, N.J. Acute effects of dynamic stretching followed by vibration foam rolling on sports performance of badminton athletes. J. Sports Sci. Med. 2020, 19, 420.
- Kokkonen, J.; Nelson, A.G.; Eldredge, C.; Winchester, J.B. Chronic static stretching improves exercise performance. Med. Sci. Sports Exerc. 2007, 39, 1825–1831.
- Konrad, A.; Nakamura, M.; Paternoster, F.K.; Tilp, M.; Behm, D.G. A comparison of a single bout of stretching or foam rolling on range of motion in healthy adults. Eur. J. Appl. Physiol. 2022, 122, 1545–1557.
- Hagiwara, Y.; Ando, A.; Chimoto, E.; Tsuchiya, M.; Takahashi, I.; Sasano, Y.; Onoda, Y.; Suda, H.; Itoi, E. Expression of collagen types I and II on articular cartilage in a rat knee contracture model. Connect. Tissue Res. 2010, 51, 22–30.
- Hildebrand, K.A.; Zhang, M.; Germscheid, N.M.; Wang, C.; Hart, D.A. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta Orthop. 2008, 79, 116–125.
- Tokuda, K.; Yamanaka, Y.; Kosugi, K.; Nishimura, H.; Okada, Y.; Tsukamoto, M.; Tajima, T.; Suzuki, H.; Kawasaki, M.; Uchida, S.; et al. Development of a novel knee contracture mouse model by immobilization using external fixation. Connect. Tissue Res. 2022, 63, 169–182.
- Zhang, Q.B.; Zhou, Y.; Zhong, H.Z.; Liu, Y. Effect of stretching combined with ultrashort wave diathermy on joint function and its possible mechanism in a rabbit knee contracture model. Am. J. Phys. Med. Rehabil. 2018, 97, 357–363.
- Stoytchev, S.; Nikolov, S. Effects of flow-dependent and flow-independent viscoelastic mechanisms on the stress relaxation of articular cartilage. Ser. Biomech. 2023, 37, 43–50.
- McNair, P.; Stanley, S. Effect of passive stretching and jogging on the series muscle stiffness and range of motion of the ankle joint. Br. J. Sports Med. 1996, 30, 313–318.
- Magnusson, S. Passive properties of human skeletal muscle during stretch manoeuvres. MedSci. Sports Exerc. 1998, 8, 65–77.
- Magnusson, S.; Simonsen, E.; Aagaard, P.; Sorensen, H.; Kjaer, M. A mechanism for altered flexibility in human skeletal muscle. J. Physiol. 1996, 497, 291–298.
- Magnusson, S.; Simonsen, E.; Dyhre-Poulsen, P.; Aagaard, P.; Mohr, T.; Kjaer, M. Viscoelastic stressrelaxation during static stretch in human skeletal muscle in the absence of EMG activity. MedSci. Sports Exerc. 1996, 6, 323–328.
- McNair, P.J.; Dombroski, E.W.; Hewson, D.J.; Stanley, S.N. Stretching at the ankle joint: Viscoelastic responses to holds and continuous passive motion. Med. Sci. Sports Exerc. 2001, 33, 354–358.
- Magnusson, S.; Simonsen, E.; Aagaard, P.; Gleim, G.; McHugh, M.; Kjaer, M. Viscoelastic response to repeated static stretching in the human hamstring muscle. Scand. J. MedSci. Sports 1995, 5, 342–347.
- Magnusson, S.; Aagaard, P.; Larsson, B.; Kjaer, M. Passive energy absorption by human muscle-tendon unit is unaffected by increase in intramuscular temperature. J. Appl. Physiol. 2000, 88, 1215–1220.
- Magnusson, S.; Simonsen, E.; Aagaard, P.; Kjaer, M. Biomechanical responses to repeated stretches in human hamstring muscle in vivo. Am. J. Sports Med. 1996, 24, 622–628.
- Kubo, K.; Kanehisa, H.; Fukunaga, T. Is passive stiffness in human muscles related to the elasticity of tendon structures? Eur. J. Appl. Physiol. 2001, 85, 226–232.
- Kubo, K.; Kanehisa, H.; Fukunaga, T. Effects of resistance and stretching training programs on the viscoelastic properties of human tendon structures in vivo. J. Physiol. 2002, 538, 219–226.
- Cotofana, S.; Eckstein, F.; Wirth, W.; Souza, R.B.; Li, X.; Wyman, B.; Graverand, M.-P.H.-L.; Link, T.; Majumdar, S. In vivo measures of cartilage deformation: Patterns in healthy and osteoarthritic female knees using 3T MR imaging. Eur. Radiol. 2011, 21, 1127–1135.
- Herberhold, C.; Faber, S.; Stammberger, T.; Steinlechner, M.; Putz, R.; Englmeier, K.H.; Reiser, M.; Eckstein, F. In situ measurement of articular cartilage deformation in intact femoropatellar joints under static loading. J. Biomech. 1999, 32, 1287–1295.
- Ranchev, S.; Ivanov, I.M.; Yotov, I.; Stoytchev, S. Studies on paradox in the work of musculoskeletal system in isometric stretching. J. Appl. Sports Sci. 2020, 2, 80–90.
- Behm, D.G.; Alizadeh, S.; Daneshjoo, A.; Konrad, A. Potential Effects of Dynamic Stretching on Injury Incidence of Athletes: A Narrative Review of Risk Factors. Sports Med. 2023, 53, 1359–1373.
- Kuntz, A.B.; Chopp-Hurley, J.N.; Brenneman, E.C.; Karampatos, S.; Wiebenga, E.G.; Adachi, J.D.; Noseworthy, M.; Maly, M.R. Efficacy of a biomechanically-based yoga exercise program in knee osteoarthritis: A randomized controlled trial. PLoS ONE 2018, 13, e0195653.
- Trindade, T.B.; de Medeiros, J.A.; Dantas, P.M.S.; de Oliveira Neto, L.; Schwade, D.; de Brito Vieira, W.H.; Oliveira-Dantas, F.F. A comparison of muscle electromyographic activity during different angles of the back and front squat. Isokinet. Exerc. Sci. 2020, 28, 1–8.
- Raikova, R.; Ivanov, I.; Hristov, O.; Markova, N.; Trenev, L.; Angelova, S. Detailed investigation of the knee biomechanics during posture maintenance applying different static loading on the spine. Int. J. Bioautom. 2023, 27, 83.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
884
Revisions:
2 times
(View History)
Update Date:
04 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




