Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kit-Leong Cheong | -- | 1987 | 2023-08-03 11:05:18 | | | |
| 2 | Alfred Zheng | Meta information modification | 1987 | 2023-08-04 04:08:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cheong, K.; Zhang, Y.; Li, Z.; Li, T.; Ou, Y.; Shen, J.; Zhong, S.; Tan, K. Polysaccharides as Feed Additives for Methane Mitigation. Encyclopedia. Available online: https://encyclopedia.pub/entry/47604 (accessed on 07 February 2026).
Cheong K, Zhang Y, Li Z, Li T, Ou Y, Shen J, et al. Polysaccharides as Feed Additives for Methane Mitigation. Encyclopedia. Available at: https://encyclopedia.pub/entry/47604. Accessed February 07, 2026.
Cheong, Kit-Leong, Yiyu Zhang, Zhuoting Li, Tongtong Li, Yiqing Ou, Jiayi Shen, Saiyi Zhong, Karsoon Tan. "Polysaccharides as Feed Additives for Methane Mitigation" Encyclopedia, https://encyclopedia.pub/entry/47604 (accessed February 07, 2026).
Cheong, K., Zhang, Y., Li, Z., Li, T., Ou, Y., Shen, J., Zhong, S., & Tan, K. (2023, August 03). Polysaccharides as Feed Additives for Methane Mitigation. In Encyclopedia. https://encyclopedia.pub/entry/47604
Cheong, Kit-Leong, et al. "Polysaccharides as Feed Additives for Methane Mitigation." Encyclopedia. Web. 03 August, 2023.
Copy Citation
Marine algal polysaccharides have emerged as a promising research avenue because of their abundance and sustainability. Polysaccharides, such as alginate, laminaran, and fucoidan, which are extracted from marine seaweeds, have demonstrated the potential to reduce methane emissions by influencing the microbial populations in the rumen.
polysaccharides
feed additives
ruminants
methane mitigation
1. Introduction
Global warming is a major environmental challenge that poses serious threats to the well-being of our planet and its inhabitants. This warming is mainly driven by an increase in atmospheric greenhouse gas concentrations, which trap heat in the Earth’s atmosphere and lead to rising temperatures. Methane, a potent contributor to global warming, has warming potential approximately 28 times greater than that of CO2 over a 100-year timescale [1]. Methane emissions result from both natural and human activities, including organic matter decomposition in wetlands, rice cultivation, landfilling, and the digestive processes of ruminants such as cows, sheep, and goats [2]. According to the Intergovernmental Panel on Climate Change, approximately 14.5% of global greenhouse gas emissions can be attributed to the agricultural sector, and enteric fermentation (i.e., methane emissions from livestock) is responsible for approximately 40% of these emissions [3]. As the global demand for meat and dairy products is expected to increase in the coming years, the problem of methane emissions from ruminants will intensify, making it imperative to reduce these emissions for environmental and economic reasons [4]. A range of strategies have been proposed, including dietary modifications, genetic selection, and improved herd management practices.
Methane emissions from ruminants result from enteric fermentation [5], which occurs in the digestive systems of animals, specifically in the rumen, a vast fermentation chamber that harbours a large number of microorganisms [6]. These microorganisms play a pivotal role in enabling ruminants to digest complex plant materials such as cellulose and hemicellulose, which cannot be broken down by monogastric animals such as pigs [7]. During enteric fermentation, the microorganisms in the rumen decompose feed materials and generate methane as a byproduct. Methane is released into the atmosphere via belching, contributing to increases in the concentrations of greenhouse gases and exacerbating warming global temperatures (Figure 1) [8]. Apart from the environmental impact, reducing methane emissions is crucial for the economic sustainability of animal agriculture, as numerous countries have established targets for the reduction of greenhouse gas emissions to meet international agreements and address climate change [9]. Additionally, reducing methane emissions from ruminants may lead to substantial improvements in animal health and productivity. Methane production results in energy depletion in animals, as it represents lost potential energy that could be used for growth or milk production [10]. By reducing methane emissions, more energy can be made available for production purposes, which may result in increased efficiency and profitability in animal agriculture.
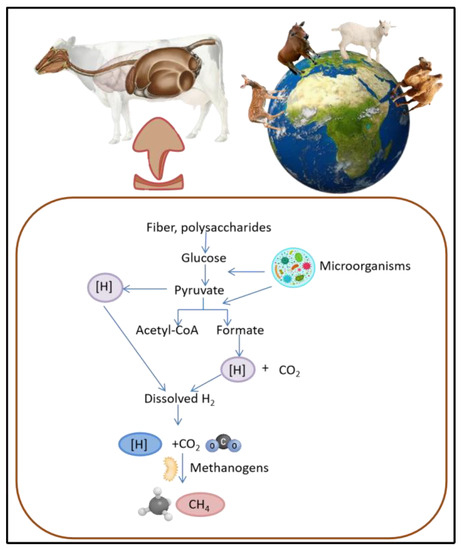
Figure 1. Pathway of methane emission in livestock. Methanogens use various organic compounds, including carbon dioxide and hydrogen, to produce methane gas as a byproduct, illustrating potential role of feed additives such as MAPs in mitigating methane emissions.
Macroalgae, popularly known as seaweed, are large multicellular algae primarily found in marine habitats. By contrast, microalgae are single-celled algae that inhabit both marine and freshwater environments [11]. Both macroalgae and microalgae contain various intricate polysaccharides, such as laminaran [12], fucoidan [13], alginate [14], carrageenan [15], and porphyran [16], which exhibit several biological properties, including immune modulation and antioxidant, antiviral, prebiotic, and antimicrobial effects [17]. Marine algal polysaccharides (MAPs) mitigate the risk of inflammatory disorders in ruminants, improve food digestion, and augment nutrient absorption, thereby decreasing the probability of pathogen proliferation in the digestive system and enhancing overall animal health [18][19].
As shown in Table 1, some feed additives have been used to reduce methane production. As feed supplements, MAPs are generally deemed ecologically sound and sustainable as they are sourced from renewable sources and do not pose the same hazards or create the same concerns as other feed additives, such as antibiotics. These compounds hold promise in modifying the microbial populations in the rumen and/or inhibiting specific enzymatic pathways involved in methane production, so they are plausible candidates for the reduction of methane emissions from ruminants.
Table 1. Estimates of methane reduction through the use of feed additives.
| Feed Additive | Animal/In Vitro | Treatment | Methane Reduction (%) | Reference |
|---|---|---|---|---|
| 3-Nitrooxypropanol | Cattle | 10 mg/kg dry matter | 39 | [20] |
| Corn oil, wheat starch, marine algae | Dairy cows and goats | 1.5% inclusion | 28 | [21] |
| Asparagopsis armata | Cows | 1% inclusion level | 47.2 | [22] |
| origanum oil, hydrolysable tannins, and tea saponin | Sheep | 40 mL/kg origanum oil | 30 | [23] |
| Grape marc | Dairy cows | 5.0 kg dry matter of grape marc and 10.0 kg dry matter of ryegrass | 15 | [24] |
| Nannochloropsis oceanica (polysaccharide) | In vitro | 2.5% incubation | 10 | [25] |
| Macrocystis pyrifera (polysaccharide) | In vitro | 0.25 g of each diet | 47.3 | [26] |
| Fucus vesiculosus (polyphenol and polysaccharides) | In vitro | inclusion rate of 20% in dry matter | 62.6 | [27] |
| Laminaria japonica | In vitro | inclusion rate of 20% in dry matter | 18.3 | [28] |
| Sunflower and marine oils | In vitro | 2.0% inclusion | 16 | [29] |
| Ulva sp. (ulvan) | In vitro | 25% incubation | 55 | [30] |
| Zonaria farlowii (high starch and protein) | In vitro | 5% inclusion | 11 | [31] |
2. Effect of MAPs and VFAs in Rumen Fermentation and Methane Production
In the field of ruminant nutrition, an essential component playing a pivotal role in rumen health and overall productivity is VFAs. VFAs are the main end products of microbial fermentation in the rumens of ruminants. They are primarily produced via the anaerobic breakdown of carbohydrates by ruminal microorganisms. These acids include C2 to C6 carboxylic acids, including acetic, propionic, butyric, isobutyric, valeric, isovaleric, and caproic acids [32][33]. These VFAs act as energy sources for host animals and are essential for rumen fermentation and digestion. Fibre digestion in ruminants is facilitated by the interplay between VFAs and the rumen microbial population [34]. The microorganisms involved in VFA production are summarised in Table 2.
Table 2. Microorganisms involved in the volatile fatty acid process.
| VFA | Microorganisms | Ref. |
|---|---|---|
| Acetic acid | Acetobacter pasteurianus, A. aceti, Acetobacterium wieringae, Acetomicrobium flavidum, Acetobacterium woodii, Clostridium formicaceticum, C. aceticum, C. thermoaceticum, Gluconobacter strains, Moorella thermoacetica, Streptococcus lactis, Thermoanaerobacter kivui | [35][36][37] |
| Propionic acid | Propionibacterium freudenreichii, P. shermanii, P. acidipropionici, P. thoenii, P. jensenii | [38] |
| Butyric acid | Clostridium barkeri, C. thermobutyricum, C. butyricum, C. acetobutylicum, C. beijerinckii, Butyribacterium sp., Butyrivibrio fibrisolvens, Eubacterium, Fusobacterium nucleatum, Sarcinalimosum, Clostridium tyrobutyricum | [39][40][41] |
| Isovaleric acid | Propionibacterium freudenreichii, Pseudomonas sp. strain VLB120 | [42] |
VFAs are vital components of ruminal ecosystems. A balance among VFAs is essential for optimal ruminal function and animal performance. The generation of VFAs in the rumen is closely linked to methane production [43]. The proper allocation of VFAs for different physiological processes ensures efficient energy use and supports growth, reproduction, milk production, and methane emissions [44]. Different factors, such as the diet composition, rumen microbial population, rumen pH, and management practices, can influence the production and composition of VFAs and methane emissions [45]. Understanding these factors allows nutritionists and producers to formulate diets and management strategies that promote the production of VFAs that are favourable for rumen health and animal productivity.
Methanogens use a diverse range of substrates during methane production, including formate, hydrogen, methanol, butanol, 2-propanol, 2-butanol, propanol, dimethyl sulphide, dimethylamine, and trimethylamine [46]. Anaerobic digesters predominantly harbour hydrogenotrophic methanogens, indicating that hydrogen acts as the primary substrate for methane generation [47]. However, competition for substrates occurs between VFAs and methanogens because higher concentrations of VFAs can compete with methanogens for available hydrogen [48]. MAPs generally either have no marked effect on or decrease the levels of VFAs in the rumen. For instance, when 4% brown seaweed byproducts were incorporated as feed additives, only minimal alterations in volatile fatty acid concentrations were observed after 24 h [49]. The inclusion of Sargassum horneri did not influence the overall production of VFAs, whereas the 4% incorporation of Ulva sp. resulted in a decline in total rumen VFA production. Notably, both marine algal species led to a reduction in the methane content in the rumen [50]. When Laminaria ochroleuca, Gigartina sp., and Gracilaria vermiculophylla were combined with corn silage, a negative effect on total VFA production was evident, which was accompanied by a decline in methane production [30].
The effects of marine algae on the content and composition of VFAs vary depending on the substrate used. The breakdown of intricate polysaccharides derived from marine algae and the subsequent generation of fatty acids rely on the involvement of diverse bacterial species [51]. Notably, the phylum Bacteroidetes is recognised for its wide range of carbohydrate-active enzymes (CAZymes), which are responsible for this process. CAZymes can be categorised as glycoside hydrolases (GHs), polysaccharide lyases (PLs), and carbohydrate esterases (CEs) based on their distinct enzymatic catalytic mechanisms. These CAZymes specifically target bonds within MAPs, leading to their cleavage into smaller sugar units that can then undergo further metabolism. For instance, the CAZyme-encoding genes associated with fucoidan degradation and the breakdown of fucoidan linkages are found in the families CE4, GH29, GH107, S1_17, and S1_25 [13][52]. Kalyani et al. discovered mrbExg5, an enzyme that demonstrates exo-β-1,3-glucanase activity toward β-1,3-linked glucooligosaccharides and laminaran. This glycoside bears a structural resemblance to a member of the GH5_44 family, which is prominently present in Pseudobutyrivibrio sp. ACV-2 is an isolate derived from the rumen of cows [53]. The presence of abundant VFAs reduces the accessibility of hydrogen to methanogens, consequently hindering their activity and ultimately leading to a decline in methane production.
In vitro fermentation and in vivo studies have demonstrated the multiple beneficial effects of VFAs on host health. For instance, the use of porphyran and its partially acid-hydrolysed derivatives derived from Porphyra haitanensis can increase the concentrations of acetate, propionate, isobutyrate, butyrate, isovalerate, and valerate in the rumen [54][55]. Acetate is a key VFA generated during the breakdown of fibrous materials in the rumen. Their primary function is to provide substantial energy to ruminants, thereby fulfilling their energy needs. Acetate is an easily accessible energy substrate for animals that facilitates the maintenance of essential physiological processes [56]. However, acetate, as a substrate for methanogenesis, contributes to elevated methane emissions [57]. Propionates are vital VFAs that play pivotal roles in ruminant nutrition and energy metabolism. They serve as key precursors for gluconeogenesis, a fundamental process in glucose synthesis [58]. Glucose is essential for various metabolic functions, including milk production in dairy cows [59]. Higher propionate levels are associated with enhanced milk yields, emphasising their importance as VFAs in lactating animals. Additionally, propionate production is negatively correlated with methane emissions because it competes with methanogens for available hydrogen [60]. Choi et al. examined the effects of incorporating dried Sargassum fusiforme on ruminal fermentation in vitro. The experiment involved testing four different doses of Sargassum fusiforme (1%, 3%, 5%, and 10% of the total ratio). The findings indicated that supplementation with Sargassum fusiforme resulted in elevated propionate production with a simultaneous reduction in methane production [61]. Therefore, increasing propionate production relative to acetate production has the potential to mitigate methane emissions in ruminants. Although butyrate is produced in smaller quantities than acetate and propionate, its importance remains: it acts as a valuable energy source for the rumen epithelium and contributes to rumen health and integrity [62]. Furthermore, butyrate actively participates in microbial protein synthesis, facilitating the production of essential amino acids that are necessary for the overall growth and development of animals [63].
VFAs also provide the benefit of lowering the fermentation pH to below 6.0, thereby inhibiting the proliferation of methanogenic microorganisms. The inhibitory growth of the ruminal methanogen Methanobrevibacter ruminantium was demonstrated when suspended with lauric acid and myristic acid in low-pH conditions (approximately pH 5–6). The results showed that the decline in methane formation may have been related to the decreased survival of Methanobrevibacter ruminantium via increased ATP efflux, potassium leakage, and an increasing degree of protonation [64]. VFAs may have direct or indirect toxic effects on protozoa-associated methanogens, which are the microorganisms responsible for methane production. Macroalgae and their secondary metabolites are effective in reducing methane production based on in vitro results. For example, Asparagopsis taxiformis reduces methane production by suppressing methanogenesis [65].
In summary, understanding the interplay between MAPs, VFAs, rumen fermentation, and methane emissions is crucial in developing sustainable strategies to reduce the environmental footprint of ruminant livestock. By manipulating the production and use of VFAs through dietary interventions, people can potentially reduce methane emissions without compromising animal health or productivity. Future research should focus on further elucidating the mechanisms by which VFAs influence methane production and exploring novel dietary strategies and additives that can optimise VFA profiles and mitigate methane emissions.
References
- Balcombe, P.; Speirs, J.F.; Brandon, N.P.; Hawkes, A.D. Methane emissions: Choosing the right climate metric and time horizon. Environ. Sci.-Proc. Imp. 2018, 20, 1323–1339.
- Bačėninaitė, D.; Džermeikaitė, K.; Antanaitis, R. Global warming and dairy cattle: How to control and reduce methane emission. Animals 2022, 12, 2687.
- Alex Thumba, D.; Lazarova-Molnar, S.; Niloofar, P. Comparative evaluation of data requirements and level of decision support provided by decision support tools for reducing livestock-related greenhouse gas emissions. J. Clean. Prod. 2022, 373, 133886.
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, s2–s16.
- Ramin, M.; Huhtanen, P. Development of equations for predicting methane emissions from ruminants. J. Dairy Sci. 2013, 96, 2476–2493.
- Xue, B.; Wang, L.Z.; Yan, T. Methane emission inventories for enteric fermentation and manure management of yak, buffalo and dairy and beef cattle in China from 1988 to 2009. Agric. Ecosyst. Environ. 2014, 195, 202–210.
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Ariaeenejad, S.; Ding, X.-Z.; Han, J.-L.; Salekdeh, G.H. Lignocellulose degradation by rumen bacterial communities: New insights from metagenome analyses. Environ. Res. 2023, 229, 115925.
- Black, J.L.; Davison, T.M.; Box, I. Methane emissions from ruminants in Australia: Mitigation potential and applicability of mitigation strategies. Animals 2021, 11, 951.
- Carlson, K.M.; Gerber, J.S.; Mueller, N.D.; Herrero, M.; MacDonald, G.K.; Brauman, K.A.; Havlik, P.; O’Connell, C.S.; Johnson, J.A.; Saatchi, S.; et al. Greenhouse gas emissions intensity of global croplands. Nat. Clim. Chang. 2017, 7, 63–68.
- Glasson, C.R.K.; Kinley, R.D.; de Nys, R.; King, N.; Adams, S.L.; Packer, M.A.; Svenson, J.; Eason, C.T.; Magnusson, M. Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 2022, 64, 102673.
- Barsanti, L.; Birindelli, L.; Gualtieri, P. Paramylon and other bioactive molecules in micro and macroalgae. Int. J. Mol. Sci. 2022, 23, 8301.
- Cheong, K.L.; Li, J.K.; Zhong, S. Preparation and structure characterization of high-value Laminaria digitata oligosaccharides. Front. Nutr. 2022, 9, 945804.
- Wang, M.; Veeraperumal, S.; Zhong, S.; Cheong, K.-L. Fucoidan-derived functional oligosaccharides: Recent developments, preparation, and potential applications. Foods 2023, 12, 878.
- Wang, M.; Chen, L.; Zhang, Z. Potential applications of alginate oligosaccharides for biomedicine—A mini review. Carbohydr. Polym. 2021, 271, 118408.
- Qiu, S.-M.; Aweya, J.J.; Liu, X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.-L. Bioactive polysaccharides from red seaweed as potent food supplements: A systematic review of their extraction, purification, and biological activities. Carbohydr. Polym. 2022, 275, 118696.
- Qiu, H.-M.; Veeraperumal, S.; Lv, J.-H.; Wu, T.-C.; Zhang, Z.-P.; Zeng, Q.-K.; Liu, Y.; Chen, X.-Q.; Aweya, J.J.; Cheong, K.-L. Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydr. Polym. 2020, 246, 116626.
- Yao, W.; Qiu, H.-M.; Cheong, K.-L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485.
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17.
- Allen, V.G.; Pond, K.R.; Saker, K.E.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Schmidt, R.E.; Fike, J.H.; Zhang, X.; et al. Tasco: Influence of a brown seaweed on antioxidants in forages and livestock—A review. J. Anim. Sci. 2001, 79, E21–E31.
- Dijkstra, J.; Bannink, A.; France, J.; Kebreab, E.; van Gastelen, S. Short communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J. Dairy Sci. 2018, 101, 9041–9047.
- Martin, C.; Coppa, M.; Fougère, H.; Bougouin, A.; Baumont, R.; Eugène, M.; Bernard, L. Diets supplemented with corn oil and wheat starch, marine algae, or hydrogenated palm oil modulate methane emissions similarly in dairy goats and cows, but not feeding behavior. Anim. Feed Sci. Technol. 2021, 272, 114783.
- Roque, B.M.; Salwen, J.K.; Kinley, R.; Kebreab, E. Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Clean. Prod. 2019, 234, 132–138.
- Zhang, F.; Li, B.; Ban, Z.; Liang, H.; Li, L.; Zhao, W.; Yan, X. Evaluation of origanum oil, hydrolysable tannins and tea saponin in mitigating ruminant methane: In vitro and in vivo methods. J. Anim. Physiol. Anim. Nutr. 2021, 105, 630–638.
- Moate, P.J.; Jacobs, J.L.; Hixson, J.L.; Deighton, M.H.; Hannah, M.C.; Morris, G.L.; Ribaux, B.E.; Wales, W.J.; Williams, S.R.O. Effects of feeding either red or white grape marc on milk production and methane emissions from early-lactation dairy cows. Animals 2020, 10, 976.
- Meehan, D.J.; Cabrita, A.R.J.; Silva, J.L.; Fonseca, A.J.M.; Maia, M.R.G. Effects of Chlorella vulgaris, Nannochloropsis oceanica and Tetraselmis sp. supplementation levels on in vitro rumen fermentation. Algal Res. 2021, 56, 102284.
- Lee-Rangel, H.A.; Roque-Jiménez, J.A.; Cifuentes-López, R.O.; Álvarez-Fuentes, G.; Cruz-Gómez, A.D.l.; Martínez-García, J.A.; Arévalo-Villalobos, J.I.; Chay-Canul, A.J. Evaluation of three marine algae on degradability, in vitro gas production, and CH4 and CO2 emissions by ruminants. Fermentation 2022, 8, 511.
- Pandey, D.; Hansen, H.H.; Dhakal, R.; Aryal, N.; Rai, S.P.; Sapkota, R.; Nielsen, M.O.; Novoa-Garrido, M.; Khanal, P. Interspecies and seasonal variations in macroalgae from the Nordic region: Chemical composition and impacts on rumen fermentation and microbiome assembly. J. Clean. Prod. 2022, 363, 132456.
- Ahmed, E.; Batbekh, B.; Fukuma, N.; Hanada, M.; Nishida, T. Evaluation of different brown seaweeds as feed and feed additives regarding rumen fermentation and methane mitigation. Fermentation 2022, 8, 504.
- Vargas, J.E.; Andrés, S.; Snelling, T.J.; López-Ferreras, L.; Yáñez-Ruíz, D.R.; García-Estrada, C.; López, S. Effect of sunflower and marine oils on ruminal microbiota, in vitro fermentation and digesta fatty acid profile. Front. Microbiol. 2017, 8, 1124.
- Maia, M.R.G.; Fonseca, A.J.M.; Oliveira, H.M.; Mendonça, C.; Cabrita, A.R.J. The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci. Rep. 2016, 6, 32321.
- Brooke, C.G.; Roque, B.M.; Shaw, C.; Najafi, N.; Gonzalez, M.; Pfefferlen, A.; De Anda, V.; Ginsburg, D.W.; Harden, M.C.; Nuzhdin, S.V.; et al. Methane reduction potential of two pacific coast macroalgae during in vitro ruminant fermentation. Front. Mar. Sci. 2020, 7, 561.
- Bhatia, S.K.; Yang, Y.-H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio/Technol. 2017, 16, 327–345.
- Dijkstra, J. Production and absorption of volatile fatty acids in the rumen. Livest. Prod. Sci. 1994, 39, 61–69.
- Zeineldin, M.; Barakat, R.; Elolimy, A.; Salem, A.Z.M.; Elghandour, M.M.Y.; Monroy, J.C. Synergetic action between the rumen microbiota and bovine health. Microb. Pathog. 2018, 124, 106–115.
- Merli, G.; Becci, A.; Amato, A.; Beolchini, F. Acetic acid bioproduction: The technological innovation change. Sci. Total Environ. 2021, 798, 149292.
- Wang, Z.; Yan, M.; Chen, X.; Li, D.; Qin, L.; Li, Z.; Yao, J.; Liang, X. Mixed culture of Saccharomyces cerevisiae and Acetobacter pasteurianus for acetic acid production. Biochem. Eng. J. 2013, 79, 41–45.
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557.
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458.
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786.
- Jang, Y.-S.; Im, J.A.; Choi, S.Y.; Lee, J.I.; Lee, S.Y. Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab. Eng. 2014, 23, 165–174.
- Baroi, G.N.; Baumann, I.; Westermann, P.; Gavala, H.N. Butyric acid fermentation from pretreated and hydrolysed wheat straw by an adapted Clostridium tyrobutyricum strain. Microb. Biotechnol. 2015, 8, 874–882.
- Lang, K.; Zierow, J.; Buehler, K.; Schmid, A. Metabolic engineering of Pseudomonas sp. strain VLB120 as platform biocatalyst for the production of isobutyric acid and other secondary metabolites. Microb. Cell Fact. 2014, 13, 2.
- Hill, J.; McSweeney, C.; Wright, A.-D.G.; Bishop-Hurley, G.; Kalantar-zadeh, K. Measuring methane production from ruminants. Trends Biotechnol. 2016, 34, 26–35.
- González, L.A.; Kyriazakis, I.; Tedeschi, L.O. Review: Precision nutrition of ruminants: Approaches, challenges and potential gains. Animal 2018, 12, s246–s261.
- Pragna, P.; Chauhan, S.S.; Sejian, V.; Leury, B.J.; Dunshea, F.R. Climate change and goat production: Enteric methane emission and its mitigation. Animals 2018, 8, 235.
- Amin, F.R.; Khalid, H.; El-Mashad, H.M.; Chen, C.; Liu, G.; Zhang, R. Functions of bacteria and archaea participating in the bioconversion of organic waste for methane production. Sci. Total Environ. 2021, 763, 143007.
- Menon, A.; Lyng, J.; Giannis, A. Higher bacterial diversity in two-phase thermophilic anaerobic digestion of food waste after micronutrient supplementation. Biomass Convers. Bior. 2023, 13, 5187–5195.
- Xu, F.; Shi, J.; Lv, W.; Yu, Z.; Li, Y. Comparison of different liquid anaerobic digestion effluents as inocula and nitrogen sources for solid-state batch anaerobic digestion of corn stover. Waste Manag. 2013, 33, 26–32.
- Hong, Z.S.; Kim, E.J.; Jin, Y.C.; Lee, J.S.; Choi, Y.J.; Lee, H.G. Effects of supplementing brown seaweed by-products in the diet of Holstein cows during transition on ruminal fermentation, growth performance and endocrine responses. Asian-Australas J. Anim. Sci. 2015, 28, 1296–1302.
- Park, K.Y.; Jo, Y.H.; Ghassemi Nejad, J.; Lee, J.C.; Lee, H.G. Evaluation of nutritional value of Ulva sp. and Sargassum horneri as potential eco-friendly ruminants feed. Algal Res. 2022, 65, 102706.
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354.
- Vickers, C.; Liu, F.; Abe, K.; Salama-Alber, O.; Jenkins, M.; Springate, C.M.K.; Burke, J.E.; Withers, S.G.; Boraston, A.B. Endo-fucoidan hydrolases from glycoside hydrolase family 107 (GH107) display structural and mechanistic similarities to α-l-fucosidases from GH29. J. Biol. Chem. 2018, 293, 18296–18308.
- Kalyani, D.C.; Reichenbach, T.; Aspeborg, H.; Divne, C. A homodimeric bacterial exo-β-1,3-glucanase derived from moose rumen microbiome shows a structural framework similar to yeast exo-β-1,3-glucanases. Enzyme Microb. Technol. 2021, 143, 109723.
- Xu, S.-Y.; Aweya, J.J.; Li, N.; Deng, R.-Y.; Chen, W.-Y.; Tang, J.; Cheong, K.-L. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019, 289, 177–186.
- Yu, B.; Wang, M.; Teng, B.; Veeraperumal, S.; Cheung, P.C.-K.; Zhong, S.; Cheong, K.-L. Partially acid-hydrolyzed porphyran improved dextran sulfate sodium-induced acute colitis by modulation of gut microbiota and enhancing the mucosal barrier. J. Agric. Food. Chem. 2023, 71, 7299–7311.
- Gäbel, G.; Aschenbach, J.R.; Müller, F. Transfer of energy substrates across the ruminal epithelium: Implications and limitations. Anim. Health Res. Rev. 2007, 3, 15–30.
- Hao, L.-P.; Lü, F.; He, P.-J.; Li, L.; Shao, L.-M. Predominant contribution of syntrophic acetate oxidation to thermophilic methane formation at high acetate concentrations. Environ. Sci. Technol. 2011, 45, 508–513.
- Abdalkareem Jasim, S.; Jade Catalan Opulencia, M.; Alexis Ramírez-Coronel, A.; Kamal Abdelbasset, W.; Hasan Abed, M.; Markov, A.; Raheem Lateef Al-Awsi, G.; Azamatovich Shamsiev, J.; Thaeer Hammid, A.; Nader Shalaby, M.; et al. The emerging role of microbiota-derived short-chain fatty acids in immunometabolism. Int. Immunopharmacol. 2022, 110, 108983.
- Bicalho, M.L.S.; Marques, E.C.; Gilbert, R.O.; Bicalho, R.C. The association of plasma glucose, BHBA, and NEFA with postpartum uterine diseases, fertility, and milk production of Holstein dairy cows. Theriogenology 2017, 88, 270–282.
- Wang, K.; Xiong, B.; Zhao, X. Could propionate formation be used to reduce enteric methane emission in ruminants? Sci. Total Environ. 2023, 855, 158867.
- Choi, Y.Y.; Lee, S.J.; Lee, Y.J.; Kim, H.S.; Eom, J.S.; Kim, S.C.; Kim, E.T.; Lee, S.S. New challenges for efficient usage of Sargassum fusiforme for ruminant production. Sci. Rep. 2020, 10, 19655.
- Cheong, K.-L.; Yu, B.; Chen, J.; Zhong, S. A comprehensive review of the cardioprotective effect of marine algae polysaccharide on the gut microbiota. Foods 2022, 11, 3550.
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090.
- Zhou, X.; Zeitz, J.O.; Meile, L.; Kreuzer, M.; Schwarm, A. Influence of pH and the degree of protonation on the inhibitory effect of fatty acids in the ruminal methanogen Methanobrevibacter ruminantium strain M1. J. Appl. Microbiol. 2015, 119, 1482–1493.
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; de Nys, R.; Tomkins, N. Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J. Appl. Phycol. 2016, 28, 1443–1452.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
653
Revisions:
2 times
(View History)
Update Date:
04 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




