Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vladimir Nizhnichenko | -- | 2231 | 2023-08-01 11:49:36 | | | |
| 2 | Fanny Huang | Meta information modification | 2231 | 2023-08-02 03:07:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dolmatov, I.Y.; Nizhnichenko, V.A. Proteins Modifying Extracellular Matrix of Echinoderms. Encyclopedia. Available online: https://encyclopedia.pub/entry/47485 (accessed on 07 February 2026).
Dolmatov IY, Nizhnichenko VA. Proteins Modifying Extracellular Matrix of Echinoderms. Encyclopedia. Available at: https://encyclopedia.pub/entry/47485. Accessed February 07, 2026.
Dolmatov, Igor Yu., Vladimir A. Nizhnichenko. "Proteins Modifying Extracellular Matrix of Echinoderms" Encyclopedia, https://encyclopedia.pub/entry/47485 (accessed February 07, 2026).
Dolmatov, I.Y., & Nizhnichenko, V.A. (2023, August 01). Proteins Modifying Extracellular Matrix of Echinoderms. In Encyclopedia. https://encyclopedia.pub/entry/47485
Dolmatov, Igor Yu. and Vladimir A. Nizhnichenko. "Proteins Modifying Extracellular Matrix of Echinoderms." Encyclopedia. Web. 01 August, 2023.
Copy Citation
The extracellular matrix (ECM), the most important innovation in the evolution of Metazoa, made it possible to form and maintain multicellularity. In extant animals, connective tissue performs a wide variety of functions, from conducting cell–cell signals to creating support structures. In echinoderms, the ECM constitutes a substantial portion of tissue. Its composition, structure, and renewal play an important role in the physiology of these animals.
echinoderms
extracellular matrix (ECM)
collagen
1. Introduction
The extracellular matrix (ECM), the most important innovation in the evolution of Metazoa, made it possible to form and maintain multicellularity [1]. In extant animals, connective tissue performs a wide variety of functions, from conducting cell–cell signals to creating support structures. In echinoderms, the ECM constitutes a substantial portion of tissue. Its composition, structure, and renewal play an important role in the physiology of these animals. The echinoderm connective tissue is capable of changing its mechanical properties. For this reason, it is referred to as mutable collagenous tissue (MCT) [2], or catch connective tissue [3]. Echinoderms use this ability for maintaining a posture (the catch state) [4][5], in case of autotomy [6][7], and in asexual reproduction [8][9][10][11]. Nevertheless, to date, the mechanisms changing the ECM strength and the substances involved are incompletely known [12][13].
The echinoderm connective tissue consists of proteins and polysaccharides, which are mostly homologous to those of other animals, especially vertebrates [14]. Its major part is composed of various types of collagens, glycoproteins, and proteoglycans. Although echinoderms and vertebrates have descended from a common ancestor and both belong to the Deuterostomia, they differ significantly in their connective tissue composition. In particular, echinoderms lack the tropoelastin gene and, accordingly, the ECM does not contain elastin. Unlike many other ECM proteins, elastin emerged within the vertebrate group and is absent from agnathans and lower chordates, as well as from invertebrates [15]. An assumption has been made that the tropoelastin gene was formed on the basis of the fibrillin gene [16].
One of the major mechanisms of origin and evolution of connective tissue proteins is the domain shuffling of pre-existing domains [1]. In this regard, identifying ECM proteins of non-model species often poses a challenge, since their domain composition may differ from the “typical” one. Examples of such proteins are tenascins and fibronectins. These play an important role in the structural integrity of ECM in vertebrates [17][18]. Echinoderms have proteins that contain domains characteristic of tenascin and fibronectin such as FBG, EGF, TILa, and FN3 [19][20]. However, all these domains are ancient in origin and are found in a variety of animals. As a combination typical of tenascins and fibronectins, these are observed only in chordates [1][21].
ECM components are undoubtedly involved in the mechanisms changing the MCT properties. In this regard, addressing the question as to how the echinoderm ECM and its associated “adhesome” has evolved as a system and what the differences are from the vertebrate ECM is important for understanding its normal functions and mechanisms responsible for changing the mechanical properties of connective tissue.
2. Proteins Modifying Extracellular Matrix of Echinoderms
2.1. Collagen Formation
The synthesis of ECM and change in its properties depend, first, on the enzymes responsible for the assembly of various types of fibrils that constitute the basis of connective tissue. Transglutaminase-2 [22] and lysyl oxidase (Lox) are involved in the formation of collagen fibrils [23][24]. An analysis of the NCBI databases has shown that echinoderms possess one Lox and some transglutaminase genes.
2.1.1. Lysyl Oxidase
Lysyl oxidases (LOXs) are a family of copper-dependent amino oxidases capable of ECM remodeling by forming various inter- and intra-chain cross-links in collagens and elastins [25]. LOXs can oxidize lysine and hydroxylysine residues to reactive aldehyde species that eventually form associations with other oxidized groups or intact lysines [25]. Vertebrates have five LOXs genes, while echinoderms have only one (Figure 1). Apparently, LOXs of deuterostomes diverged from a single ancestral gene during the divergence of Ambulacraria and Chordata. The function of LOX in echinoderms most likely does not differ from that in vertebrates and consists of establishing cross-links between collagens.
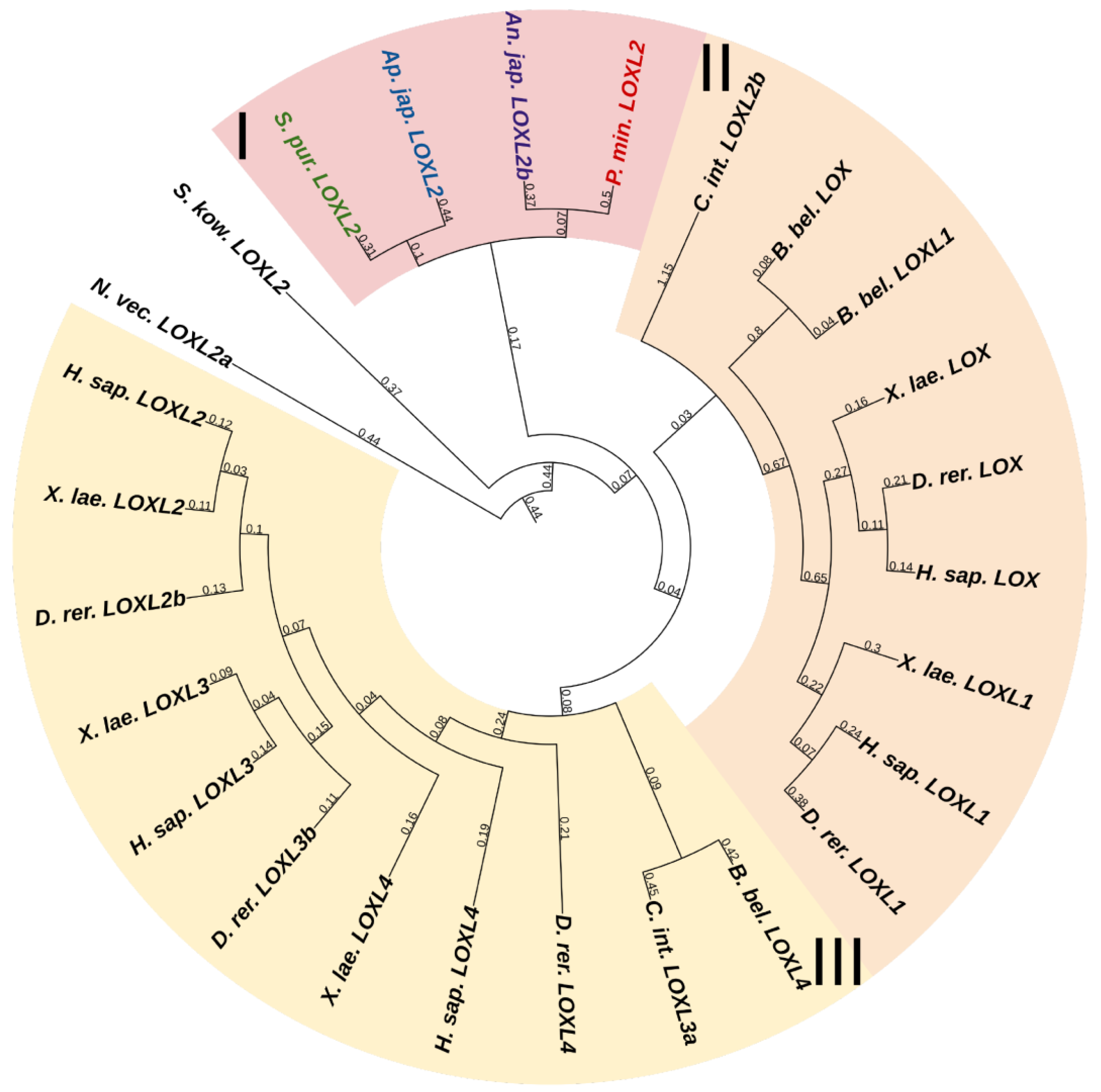
Figure 1. Phylogenetic trees showing the relationships of Lysyl oxidase of chordates, hemichordates, and echinoderms. Crinoids (Anneissia japonica)—purple color; asteroids (Patiria miniata)—red color; echinoids (Strongylocentrotus purpuratus)—green color; holothurians (Apostichopus japonicus)—blue color; hemichordates (Saccoglossus kowalevskii) and chordates (Ciona intestinalis, Branchiostoma belcheri and Homo sapiens)—black color. Groups of proteins (I–III) are marked with colored areas.
2.1.2. Transglutaminases
Transglutaminases (TGM) are a family of Ca2+-dependent enzymes that covalently bind amino groups of one protein to the γ-carboxamide groups of glutamines of another [26]. Vertebrates have eight to nine TGM genes that perform many functions in various tissues such as apoptosis, adhesion, ECM stabilization, signal transmission, coagulation of germ and blood cells, and formation of bone tissue and cell membrane of keratinocytes [27]. One protein from this family, EPB42, does not have catalytic activity but is, nevertheless, involved in signaling, structural scaffolding, and adhesive functions [27]. The domain structures of all vertebrate TGMs are similar and include the N-terminal, catalytic middle domains, and one or two domains located at the C-terminal site. Transglutaminases of echinoderms have a structure identical to those of vertebrates. Three TGM genes have been found in all classes, except for crinoids, which have four genes (Figure 2). In a phylogenetic tree, all the echinoderm TGMs are grouped separately from vertebrate TGMs, which indicates an earlier divergence from the ancestral gene and the lack of correspondence between them. It has been shown that transglutaminases in sea urchins are involved in embryonic development and affect cell proliferation [28]. Also, as evidenced by experiments with their inhibitors, transglutaminases probably cause stiffening of MCT [29].
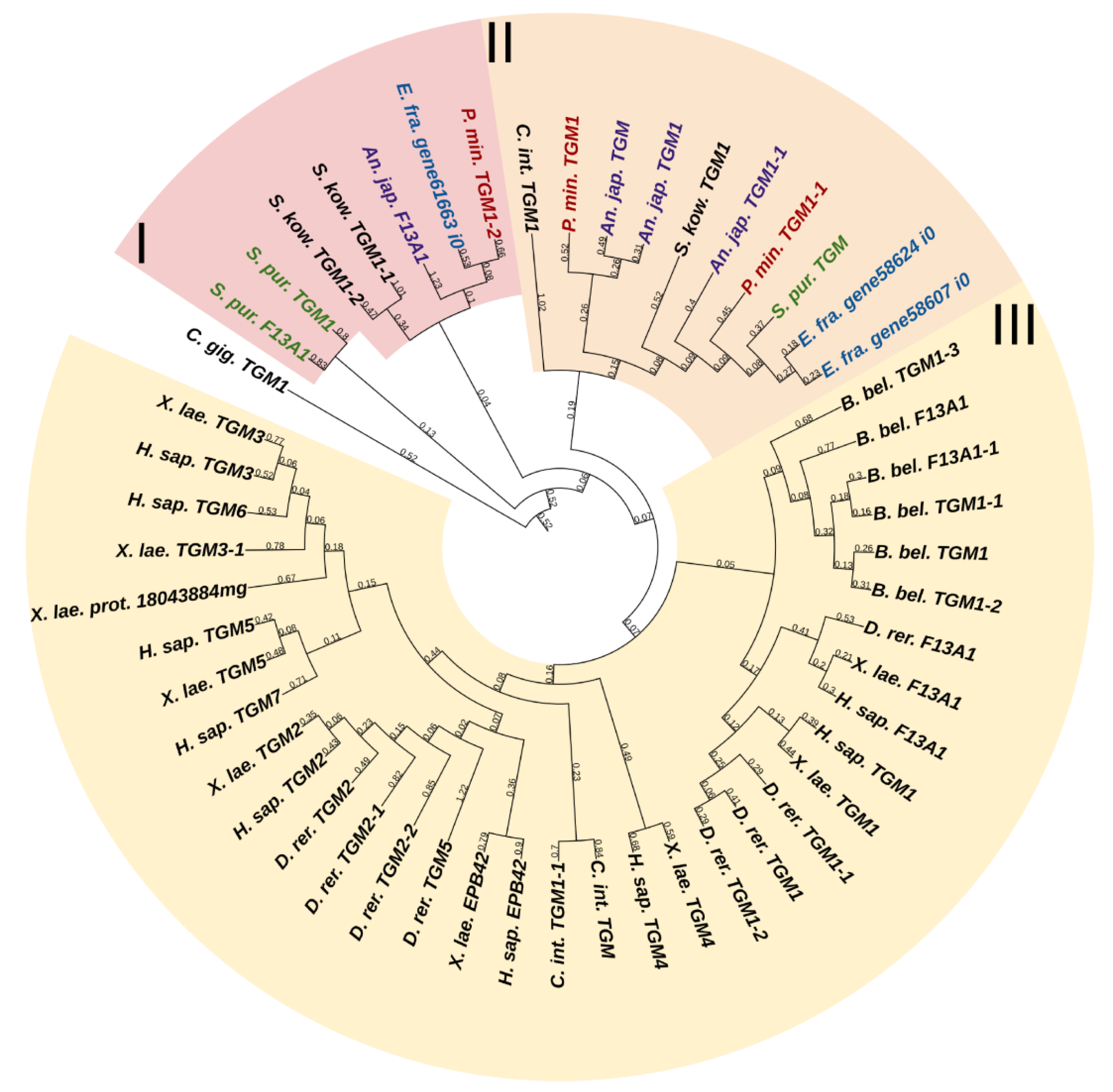
Figure 2. Phylogenetic trees showing the relationships of transglutaminases of chordates, hemichordates, and echinoderms. Crinoids (Anneissia japonica)—purple color; asteroids (Patiria miniata)—red color; echinoids (Strongylocentrotus purpuratus)—green color; holothurians (Eupentacta fraudatrix)—blue color; hemichordates (Saccoglossus kowalevskii) and chordates (Ciona intestinalis, Branchiostoma belcheri and Homo sapiens)—black color. Groups of proteins (I–III) are marked with colored areas.
2.2. Proteases
Animals possess a wide variety of proteases capable of degrading ECM proteins. These are serine, cysteine, aspartyl, and metal peptidases. The transformation of connective tissue during fission in echinoderms has been shown to be accompanied by variation in the expression of genes of numerous proteases and their inhibitors such as matrix metalloproteinases (MMPs), ADAMTSs, a tissue inhibitor of metalloproteinases (TIMPs), and Cathepsin D [20]. This emphasizes the importance of these proteins in morphogenetic processes accompanied by rearrangements in connective tissue.
2.2.1. Serine Proteases
The family of serine proteinases comprises a large number of proteolytic enzymes involved in many biological processes [30]. It is known that many serine proteases of various types can degrade connective tissue proteins. These include plasmin, cathepsin G, fibroblast activation protein α, kallikrein 12 (KLK12), neurotrypsin, furin, matriptase (ST14), hepsin, neutrophil elastase, activated protein C, KLK 4 and 14, etc. [31][32][33]. They can lyse ECM components directly or indirectly, by activating other proteinases such as MMP [34][35]. Of all the above proteins, only furin is reliably identified in echinoderms.
The domain organization of furins does not fundamentally differ between vertebrates and echinoderms. The N-terminal signal peptide and the subsequent propeptide are involved in successive posttranslational modifications such as proteolytic cleavage, glycosylation, and folding [36]. A catalytic, P domain stabilizing it, and a cysteine-rich domain, are located next [36]. These are followed by a cytoplasmic domain responsible for protein localization and a transmembrane domain. Removing the propeptide, furin activates extracellular proteases MMPs and a disintegrin and metalloproteinases (ADAMs) by cleaving the molecule in the region of furin-activated motif (R-X-R/K-R) [37]. In addition, it is involved in the maturation of some of ECM components: collagen type V, XIII, XXV [38][39][40] (see above), integrins, and various growth and differentiation factors [36][41].
Besides furin, proteases referred to in NCBI as serine proteases have been identified in echinoderms. Eight serine proteinases have been found in the holothurian C. schmeltzii, of which four are expressed only in fissioning individuals [20]. A study on the holothurian A. japonicus has shown that serine proteases are capable of effectively degrading collagen [42][43]. Thus, an assumption can be made that in echinoderms, serine proteinases are involved in the processes of connective tissue remodeling.
The use of BLAST on echinoderms has shown proteases Hepsin and KLK4, 12 and 14 to best match with the sequences designated as trypsins. However, a search for these “trypsins” among mammals provides ambiguous matches, which hampers identification of these proteins and assumption on their functions. The situation is similar to that with other serine proteases, e.g., plasmin. The systematics of echinoderm serine proteases requires dedicated studies, which may identify new candidates to the role of ECM remodeling among proteins of this family.
2.2.2. Cysteine Proteases
Cysteine proteases are a group of enzymes that play a major role in a variety of biological processes including digestion, apoptosis, and protein processing. These enzymes are characterized by the nucleophilic cysteine residue in the active center that catalyzes the hydrolysis of peptide bonds [44]. One of the subgroups of cysteine proteases is cathepsins (CTS). As a rule, these have intracellular localization, but some of them (cathepsins B, L, K and S) can be secreted into the intercellular space and degrade ECM proteins [45][46]. For example, STSK is secreted by osteoclasts and is involved in bone remodeling. Among ECM proteins, the substrates for the listed proteinases are collagens I, II, and IV, aggrecan, perlecan, nidogen, and laminin [45].
Cysteine cathepsins have a similar structure: a signal peptide is located at the N-terminus, followed by the propeptide inhibitor I29 required for post-translational modifications and activation, and then by the peptidase domain C1 containing a catalytic site [47]. Of cathepsins capable of degrading extracellular components, CTSB and CTSL were identified in echinoderms. In A. japonicus, the cathepsin L-like protein is found in the outer layer of dermis [36]. It is assumed to be involved in the autolysis of the body-wall connective tissue in holothurians. In C. schmeltzii, cathepsin L is expressed during asexual reproduction [20]. CTSB may be involved in regenerative processes in echinoderms, as it has been detected in spines of the sea urchin Echinometra lucunter capable of regeneration [48]. Thus, cathepsins should be taken into account when analyzing the mechanisms changing the MCT properties, since these proteases can be involved in ECM remodeling in echinoderms.
2.2.3. Aspartyl Protease
Aspartyl proteases represent a group of peptidases that cleave protein substrates using two aspartic acid residues located in their catalytic center [49]. One of proteases of this type is cathepsin D. It is a lysosomal enzyme, but it can also be localized in the extracellular space [50], where it can cleave aggrecan molecules [51]. The CTSD structures in echinoderms and vertebrates are similar and do not fundamentally differ from the structural organization of cysteine cathepsins. There is evidence of the involvement of CTSD in morphogenetic processes in holothurians. In A. japonicus, it is involved in autolysis of body wall, muscles, and gut [52]. In C. schmeltzii, CTSD begins to be expressed in the constriction area of fissioning individuals [20]. Thus, CTSD is likely to be involved in processes of MCT transformation.
2.2.4. Matrix Metalloproteinases
Among the enzymes involved in the ECM remodeling, proteases of the metzincin superfamily are of particular interest [53]. This group includes most of the well-known metalloendoproteinases: matrix metalloproteinases (MMPs), a disintegrin and metalloproteinases (ADAMs), a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs), pappalysins (pregnancy-associated plasma proteins), serralysins (bacterial enzymes), leishmanolysins (protozoan proteinases), and astacins [53][54]. All of them contain zinc in the active center. Many of these proteases are involved in ECM degradation, but the most significant group of enzymes involved in connective tissue remodeling is MMPs, also referred to as matrixins [55]. Depending on their specialization, MMPs can either degrade extracellular matrix components or perform site-specific proteolysis by activating or inactivating various proteins [56][57]. The number of MMPs varies between different echinoderm species. In the A. japonica genome, a total of 22 MMP genes have been identified; in P. miniata, 20 MMPs; in S. purpuratus, 21 MMPs; and in A. japonicus, 18 [58]. These are comparable to the number of MMPs genes in vertebrates (25–33). The structure and functions of echinoderm MMPs are described in detail in the review by Dolmatov et al. [58]. MMPs are assumed to play an important role in the mechanisms changing the mechanical properties of MCT [13][59]. Galardin (the synthetic MMP inhibitor) stiffens ligaments in sea urchins [59]. Furthermore, MMPs are involved in dermal liquefaction in holothurians [60][61].
2.2.5. ADAMs and ADAMTSs
Humans have 21 ADAMs and 19 ADAMTSs [62]. ADAMs and ADAMTs are anchored on the cell membrane. They differ from MMPs by the absence of hemopexin-like repeats and the presence of EGF-like and disintegrin domains. ADAMTSs also have thrombospondin repeats [62]. The number of ADAMTS and ADAM genes in echinoderms differs between members of different classes. The greatest number of ADAMTS genes (14) have been found in the crinoid A. japonica, the smallest number being found in the holothurian A. japonicus and the sea urchin S. purpuratus, with 11 in each. The sea star P. miniata has 12 ADAMTS genes. Five genes encoding ADAM have been identified in each of the echinoderm species studied.
Unlike ADAMs, ADAMTSs are mostly specialized in degradation of ECM components and are, thus, actively involved in the processes of cell migration, proliferation, and differentiation [63]. They cut N-propeptides of collagens I and II, thereby being involved in the assembly of collagen fibrils [64]. ADAMTSs also cut off the prodomains in some of proteoglycans (aggrecan, versican, brevican, and neurocan) and glycoprotein COMP [63]. Furthermore, fibulins, TGFbRIII, LOX, perlecan, and THBS-1 may be potential substrates for ADAMTSs [65]. ADAMTSs in echinoderms may be involved in the processes of degradation of ECM components in MCT. Seven transcripts of ADAMTs have been identified in C. schmeltzii [20]. ADAMTS7 and ADAMTS9 are positively regulated in fissioning individuals, while ADAMTS13 and ADAMTS14, vice versa, are negatively regulated.
References
- Adams, J.C. Extracellular Matrix Evolution: An Overview. In Evolution of Extracellular Matrix; Keeley, F.W., Mecham, R.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–25.
- Wilkie, I.C. Variable Tensility in Echinoderm Collagenous Tissues: A Review. Mar. Behav. Physiol. 1984, 11, 1–34.
- Motokawa, T. Connective Tissue Catch in Echinoderms. Biol. Rev. 1984, 59, 255–270.
- Motokawa, T.; Shintani, O.; Birenheide, R. Contraction and Stiffness Changes in Collagenous Arm Ligaments of the Stalked Crinoid Metacrinus rotundus (Echinodermata). Biol. Bull. 2004, 206, 4–12.
- Takemae, N.; Nakaya, F.; Motokawa, T. Low Oxygen Consumption and High Body Content of Catch Connective Tissue Contribute to Low Metabolic Rate of Sea Cucumbers. Biol. Bull. 2009, 216, 45–54.
- Wilkie, I.C. Autotomy as a Prelude to Regeneration in Echinoderms. Microsc. Res. Tech. 2001, 55, 369–396.
- Wilkie, I.C.; Candia Carnevali, M.D. Morphological and Physiological Aspects of Mutable Collagenous Tissue at the Autotomy Plane of the Starfish Asterias rubens L. (Echinodermata, Asteroidea): An Echinoderm Paradigm. Mar. Drugs 2023, 21, 138.
- Motokawa, T.; Sato, E.; Umeyama, K. Energy Expenditure Associated with Softening and Stiffening of Echinoderm Connective Tissue. Biol. Bull. 2012, 222, 150–157.
- Motokawa, T.; Tsuchi, A. Dynamic Mechanical Properties of Body-Wall Dermis in Various Mechanical States and Their Implications for the Behavior of Sea Cucumbers. Biol. Bull. 2003, 205, 261–275.
- Uthicke, S. Influence of Asexual Reproduction on the Structure and Dynamics of Holothuria (Halodeima) atra and Stichopus chloronotus Populations of the Great Barrier Reef. Mar. Freshw. Res. 2001, 52, 205–215.
- Mladenov, P.V.; Burke, R.D. Echinodermata: Asexual Propagation. In Reproductive Biology of Invertebrates; Adiyodi, K.G., Adiyodi, R.G., Eds.; Oxford and IBH Publishing Co., PVT. Ltd.: New Delhi, India, 1994; Volume 6B, pp. 339–383.
- Tamori, M.; Yamada, A. Possible Mechanisms of Stiffness Changes Induced by Stiffeners and Softeners in Catch Connective Tissue of Echinoderms. Mar. Drugs 2023, 21, 140.
- Wilkie, I.C.; Sugni, M.; Candia Carnevali, M.D.; Elphick, M.R. The Mutable Collagenous Tissue of Echinoderms: From Biology to Biomedical Applications. In Soft Matter for Biomedical Applications; Azevedo, H.S., Mano, J.F., Borges, J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 3–33.
- Whittaker, C.A.; Bergeron, K.-F.; Whittle, J.; Brandhorst, B.P.; Burke, R.D.; Hynes, R.O. The Echinoderm Adhesome. Dev. Biol. 2006, 300, 252–266.
- Keeley, F.W. The Evolution of Elastin. In Evolution of Extracellular Matrix; Keeley, F.W., Mecham, R.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 73–119.
- Rodriguez-Pascual, F. The Evolutionary Origin of Elastin: Is Fibrillin the Lost Ancestor? In Extracellular Matrix—Developments and Therapeutics; Rama, S.M., Ed.; Intech Open: Rijeka, Croatia, 2021.
- Chiquet-Ehrismann, R.; Tucker, R.P. Tenascins and the Importance of Adhesion Modulation. Cold Spring Harb. Perspect. Biol. 2011, 3, a004960.
- Schwarzbauer, J.E.; DeSimone, D.W. Fibronectins, Their Fibrillogenesis, and in Vivo Functions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005041.
- Ba, H.; Yao, F.; Yang, L.; Qin, T.; Luan, H.; Li, Z.; Zou, X.; Hou, L. Identification and Expression Patterns of Extracellular Matrix-Associated Genes Fibropellin-Ia and Tenascin Involved in Regeneration of Sea Cucumber Apostichopus japonicus. Gene 2015, 565, 96–105.
- Dolmatov, I.Y.; Afanasyev, S.V.; Boyko, A.V. Molecular Mechanisms of Fission in Echinoderms: Transcriptome Analysis. PLoS ONE 2018, 13, e0195836.
- Hynes, R.O. The Evolution of Metazoan Extracellular Matrix. J. Cell Biol. 2012, 196, 671–679.
- Esposito, C.; Caputo, I. Mammalian Transglutaminases. Identification of Substrates as a Key to Physiological Function and Physiopathological Relevance. FEBS J. 2005, 272, 615–631.
- Kagan, H.M.; Li, W. Lysyl Oxidase: Properties, Specificity, and Biological Roles inside and Outside of the Cell. J. Cell. Biochem. 2003, 88, 660–672.
- Lucero, H.A.; Kagan, H.M. Lysyl Oxidase: An Oxidative Enzyme and Effector of Cell Function. Cell. Mol. Life Sci. 2006, 63, 2304–2316.
- Grau-Bové, X.; Ruiz-Trillo, I.; Rodriguez-Pascual, F. Origin and Evolution of Lysyl Oxidases. Sci. Rep. 2015, 5, 10568.
- Greenberg, C.S.; Birckbichler, P.J.; Rice, R.H. Transglutaminases: Multifunctional Cross-Linking Enzymes That Stabilize Tissues. FASEB J. 1991, 5, 3071–3077.
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.W.; Mehta, K. Transglutaminase Regulation of Cell Function. Physiol. Rev. 2014, 94, 383–417.
- Cariello, L.; Zanetti, L.; Lorand, L. Effects of Inhibiting Transglutaminase during Egg Fertilization and Development. Biochem. Biophys. Res. Commun. 1994, 205, 565–569.
- Diab, M.; Gilly, W.M.F. Mechanical Properties and Control of Nonmuscular Catch in Spine Ligaments of the Sea Urchin, Strongylocentrotus franciscanus. J. Exp. Biol. 1984, 111, 155–170.
- Antalis, T.M.; Buzza, M.S. Extracellular: Plasma Membrane Proteases—Serine Proteases. Encycl. Cell Biol. 2016, 1, 650–660.
- Wilkinson, D.J.; Arques, M.d.C.; Huesa, C.; Rowan, A.D. Serine Proteinases in the Turnover of the Cartilage Extracellular Matrix in the Joint: Implications for Therapeutics. Br. J. Pharmacol. 2019, 176, 38–51.
- Tagirasa, R.; Yoo, E. Role of Serine Proteases at the Tumor-Stroma Interface. Front. Immunol. 2022, 13, 832418.
- Ferrer-Ferrer, M.; Dityatev, A. Shaping Synapses by the Neural Extracellular Matrix. Front. Neuroanat. 2018, 12, 40.
- Hohenester, E. Structural Biology of Laminins. Essays Biochem. 2019, 63, 285–295.
- Kammerer, R.A.; Schulthess, T.; Landwehr, R.; Schumacher, B.; Lustig, A.; Yurchenco, P.D.; Ruegg, M.A.; Engel, J.; Denzer, A.J. Interaction of Agrin with Laminin Requires a Coiled-Coil Conformation of the Agrin-Binding Site within the Laminin Gamma1 Chain. EMBO J. 1999, 18, 6762–6770.
- Solovyeva, N.I.; Gureeva, T.A.; Timoshenko, O.S.; Moskvitina, T.A.; Kugaevskaya, E.V. Furin as proprotein convertase and its role in normaland pathological biological processes. Biomeditsinskaia Khimiia 2016, 62, 609–621.
- Wong, E.; Maretzky, T.; Peleg, Y.; Blobel, C.; Sagi, I. The Functional Maturation of A Disintegrin and Metalloproteinase (ADAM) 9, 10, and 17 Requires Processing at a Newly Identified Proprotein Convertase (PC) Cleavage Site. J. Biol. Chem. 2015, 290, 12135–12146.
- Imamura, Y.; Steiglitz, B.; Greenspan, D. Bone Morphogenetic Protein-1 Processes the NH2-Terminal Propeptide, and a Furin-like Proprotein Convertase Processes the COOH-Terminal Propeptide of pro- 1(V) Collagen. J. Biol. Chem. 1998, 273, 27511–27517.
- Hashimoto, T.; Wakabayashi, T.; Watanabe, A.; Kowa, H.; Hosoda, R.; Nakamura, A.; Kanazawa, I.; Arai, T.; Takio, K.; Mann, D.M.A.; et al. CLAC: A Novel Alzheimer Amyloid Plaque Component Derived from a Transmembrane Precursor, CLAC-P/Collagen Type XXV. EMBO J. 2002, 21, 1524–1534.
- Snellman, A.; Tu, H.; Väisänen, T.; Kvist, A.-P.; Huhtala, P.; Pihlajaniemi, T. A Short Sequence in the N-Terminal Region Is Required for the Trimerization of Type XIIIcollagen and Is Conserved in Other Collagenous Transmembrane Proteins. EMBO J. 2000, 19, 5051–5059.
- Lissitzky, J.C.; Luis, J.; Munzer, J.S.; Benjannet, S.; Parat, F.; Chrétien, M.; Marvaldi, J.; Seidah, N.G. Endoproteolytic Processing of Integrin Pro-Alpha Subunits Involves the Redundant Function of Furin and Proprotein Convertase (PC) 5A, but Not Paired Basic Amino Acid Converting Enzyme (PACE) 4, PC5B or PC7. Biochem. J. 2000, 346, 133–138.
- Yan, L.-J.; Zhan, C.-L.; Cai, Q.-F.; Weng, L.; Du, C.-H.; Liu, G.-M.; Su, W.-J.; Cao, M.-J. Purification, Characterization, CDNA Cloning and in Vitro Expression of a Serine Proteinase from the Intestinal Tract of Sea Cucumber (Stichopus japonicus) with Collagen Degradation Activity. J. Agric. Food Chem. 2014, 62, 4769–4777.
- Xu, S.-Q.; Zhang, Z.-Y.; Nie, B.; Du, Y.-N.; Tang, Y.; Wu, H.-T. Characteristics of the Intestine Extracts and Their Effect on the Crude Collagen Fibers of the Body Wall from Sea Cucumber Apostichopus japonicus. Biology 2023, 12, 705.
- Verma, S.; Dixit, R.; Pandey, K.C. Cysteine Proteases: Modes of Activation and Future Prospects as Pharmacological Targets. Front. Pharmacol. 2016, 7, 107.
- Fonović, M.; Turk, B. Cysteine Cathepsins and Extracellular Matrix Degradation. Biochim. Biophys. Acta 2014, 1840, 2560–2570.
- Brix, K.; Dunkhorst, A.; Mayer, K.; Jordans, S. Cysteine Cathepsins: Cellular Roadmap to Different Functions. Biochimie 2008, 90, 194–207.
- Gureeva, T.A.; Timoshenko, O.S.; Kugaevskaya, E.V.; Solovyova, N.I. Cysteine cathepsins: Structure, physiological functions and their role in carcinogenesis. Biomeditsinskaia Khimiia 2021, 67, 453–464.
- Sciani, J.M.; Antoniazzi, M.M.; Neves, A.d.C.; Pimenta, D.C. Cathepsin B/X Is Secreted by Echinometra lucunter Sea Urchin Spines, a Structure Rich in Granular Cells and Toxins. J. Venom. Anim. Toxins Trop. Dis. 2013, 19, 33.
- Battu, A.; Purushotham, R.; Dey, P.; Vamshi, S.S.; Kaur, R. An Aspartyl Protease-Mediated Cleavage Regulates Structure and Function of a Flavodoxin-like Protein and Aids Oxidative Stress Survival. PLoS Pathog. 2021, 17, e1009355.
- Sun, H.; Lou, X.; Shan, Q.; Zhang, J.; Zhu, X.; Zhang, J.; Wang, Y.; Xie, Y.; Xu, N.; Liu, S. Proteolytic Characteristics of Cathepsin D Related to the Recognition and Cleavage of Its Target Proteins. PLoS ONE 2013, 8, e65733.
- Handley, C.J.; Tuck Mok, M.; Ilic, M.Z.; Adcocks, C.; Buttle, D.J.; Robinson, H.C. Cathepsin D Cleaves Aggrecan at Unique Sites within the Interglobular Domain and Chondroitin Sulfate Attachment Regions That Are Also Cleaved When Cartilage Is Maintained at Acid PH. Matrix Biol. 2001, 20, 543–553.
- Yu, C.; Cha, Y.; Wu, F.; Xu, X.; Qin, L.; Du, M. Molecular Cloning and Functional Characterization of Cathepsin D from Sea Cucumber Apostichopus japonicus. Fish Shellfish. Immunol. 2017, 70, 553–559.
- Bond, J.S. Proteases: History, Discovery, and Roles in Health and Disease. J. Biol. Chem. 2019, 294, 1643–1651.
- Huxley-Jones, J.; Clarke, T.-K.; Beck, C.; Toubaris, G.; Robertson, D.L.; Boot-Handford, R.P. The Evolution of the Vertebrate Metzincins; Insights from Ciona intestinalis and Danio rerio. BMC Evol. Biol. 2007, 7, 63.
- Murphy, G.; Nagase, H. Progress in Matrix Metalloproteinase Research. Mol. Aspects Med. 2008, 29, 290–308.
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573.
- Marino-Puertas, L.; Goulas, T.; Gomis-Rüth, F.X. Matrix Metalloproteinases Outside Vertebrates. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2026–2035.
- Dolmatov, I.Y.; Nizhnichenko, V.A.; Dolmatova, L.S. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Echinoderms: Structure and Possible Functions. Cells 2021, 10, 2331.
- Ribeiro, A.R.; Barbaglio, A.; Oliveira, M.J.; Ribeiro, C.C.; Wilkie, I.C.; Candia Carnevali, M.D.; Barbosa, M.A. Matrix Metalloproteinases in a Sea Urchin Ligament with Adaptable Mechanical Properties. PLoS ONE 2012, 7, e49016.
- Sun, L.-M.; Wang, T.-T.; Zhu, B.-W.; Niu, H.-L.; Zhang, R.; Hou, H.-M.; Zhang, G.-L.; Murata, Y. Effect of Matrix Metalloproteinase on Autolysis of Sea Cucumber Stichopus japonicus. Food Sci. Biotechnol. 2013, 22, 1259–1261.
- Liu, Y.-X.; Liu, Z.-Q.; Song, L.; Ma, Q.-R.; Zhou, D.-Y.; Zhu, B.-W.; Shahidi, F. Effects of Collagenase Type I on the Structural Features of Collagen Fibres from Sea Cucumber (Stichopus japonicus) Body Wall. Food Chem. 2019, 301, 125302.
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313.
- McMahon, M.; Ye, S.; Pedrina, J.; Dlugolenski, D.; Stambas, J. Extracellular Matrix Enzymes and Immune Cell Biology. Front. Mol. Biosci. 2021, 8, 703868.
- Colige, A.; Vandenberghe, I.; Thiry, M.; Lambert, C.A.; Beeumen, J.V.; Li, S.-W.; Prockop, D.J.; Lapière, C.M.; Nusgens, B.V. Cloning and Characterization of ADAMTS-14, a Novel ADAMTS Displaying High Homology with ADAMTS-2 and ADAMTS-3. J. Biol. Chem. 2002, 277, 5756–5766.
- Bekhouche, M.; Leduc, C.; Dupont, L.; Janssen, L.; Delolme, F.; Goff, S.V.-L.; Smargiasso, N.; Baiwir, D.; Mazzucchelli, G.; Zanella-Cleon, I.; et al. Determination of the Substrate Repertoire of ADAMTS2, 3, and 14 Significantly Broadens Their Functions and Identifies Extracellular Matrix Organization and TGF-β Signaling as Primary Targets. FASEB J. 2016, 30, 1741–1756.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
515
Revisions:
2 times
(View History)
Update Date:
02 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




