You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Asghar Ali | -- | 2001 | 2023-08-01 11:25:31 | | | |
| 2 | Dean Liu | -19 word(s) | 1982 | 2023-08-02 02:26:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ali, A.; Zahra, A.; Kamthan, M.; Husain, F.M.; Albalawi, T.; Zubair, M.; Alatawy, R.; Abid, M.; Noorani, M.S. Microbial Biofilms. Encyclopedia. Available online: https://encyclopedia.pub/entry/47484 (accessed on 26 December 2025).
Ali A, Zahra A, Kamthan M, Husain FM, Albalawi T, Zubair M, et al. Microbial Biofilms. Encyclopedia. Available at: https://encyclopedia.pub/entry/47484. Accessed December 26, 2025.
Ali, Asghar, Andaleeb Zahra, Mohan Kamthan, Fohad Mabood Husain, Thamer Albalawi, Mohammad Zubair, Roba Alatawy, Mohammad Abid, Md Salik Noorani. "Microbial Biofilms" Encyclopedia, https://encyclopedia.pub/entry/47484 (accessed December 26, 2025).
Ali, A., Zahra, A., Kamthan, M., Husain, F.M., Albalawi, T., Zubair, M., Alatawy, R., Abid, M., & Noorani, M.S. (2023, August 01). Microbial Biofilms. In Encyclopedia. https://encyclopedia.pub/entry/47484
Ali, Asghar, et al. "Microbial Biofilms." Encyclopedia. Web. 01 August, 2023.
Copy Citation
Biofilms are complex communities of microorganisms that grow on surfaces and are embedded in a matrix of extracellular polymeric substances. These are prevalent in various natural and man-made environments, ranging from industrial settings to medical devices, where they can have both positive and negative impacts.
quorum sensing
quorum quenching
phage therapy
anti-virulence compounds
antimicrobial peptide
1. Introduction
Biofilms have gained popularity due to their extreme resistance to removal and treatment. Their resistant nature was brought to light by Characklis in 1973 [1]. They involve an immobile single- or sometimes multispecies colony that includes bacteria, fungi, diatoms, and protozoa that attach to living and non-living surfaces, i.e., are sessile [1][2]. William J. Costerton, in the year 1978, used the term biofilm for the first time [3]. Biofilms have gained importance in recent times because of their role in medical and pharmaceutical sectors as they cause several diseases in human and animal hosts, as well as contamination of medical implants and other equipment [4][5]. Biofilms are expensive in terms of both money and human life. It is estimated that out of all hospital infections, 65% are caused due to biofilms [6]. Apart from health, biofilms are detrimental to food-based industries, including, but not limited to, the dairy processing, brewing, seafood, meat, and poultry industries [7]. They also have a negative impact on the drinking and healthcare water distribution systems as they cause bio-corrosion of pipes, degradation of water quality, and hence, outbreaks of water-borne diseases [8][9]. The ability of microorganisms to form biofilms is known to have a beneficial effect in the agriculture sector, where biofilms offer plant protection and bioremediation of soil [8]. Biofilms are also known to aid in corrosion inhibition and wastewater treatment. As a result, they have a broad application spectrum in the field of biotechnology [8]. The NBIC Annual report (National Biofilms Innovation Centre’s annual Report 2022) provides data about the economic impact of the biofilms, which is represented in the form of a pie chart in Figure 1. An estimated USD 4 tn of value is associated with biofilms. It is seen that the highest cost is from corrosion, which accounts for about 69%, and then health, which has about a 10% contribution [10].
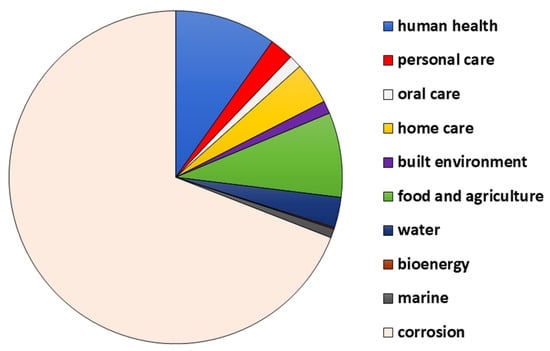
Figure 1. The distribution of cost (in percent) among various sectors.
Current estimates suggest that about 40–80% of bacterial cells on Earth can form biofilms [8]. The aggregation of bacteria precedes the formation of biofilms [11]. The aggregation of bacteria can either be surface-associated, as in the biofilms developed on medical implants, which is responsible for causing diseases such as prosthetic valve endocarditis, biofilms on orthopedic and dental implants, peritoneal dialysis catheters, and urinary catheters, or it could be non-surface-associated, as seen in biofilms developed in chronic infections such as cystic fibrosis (CF), and bacterial aggregates discovered from the ocean, freshwater, and water treatment systems [11][12][13]. If the collection involves genetically similar bacteria, it is called auto-aggregation, and if the bacteria are different, it is termed co-aggregation [11]. The biofilm development is determined by several conditions of the environment, such as pH, temperature, salinity, ionic strength, rate of flow of the medium, i.e., hydrodynamics, and nutrient availability [14]. It is also affected by the properties of the surface on which it is formed. Its formation is promoted by the hydrophobic nature of the carrier, increased roughness, and non-polarity of the surface [14][15]. Bacterial cell-surface characteristics such as the hydrophobicity, free energy, overall charge, topography, and geometrical features of the bacterial cell surface also affect its attachment and development [14]. The production of chemicals such as c-AMP (cyclic adenosine monophosphate), c-di-GMP (bis-(3′-5′)-cyclic-dimeric guanosine monophosphate), quorum sensing signal molecules, and EPS (extracellular polymeric substances) also determine cell attachment and growth [15][16]. The formation of biofilms provides a survival advantage as compared to the planktonic state by safeguarding the organism from changes in its environmental conditions. They are protected from the environment and are resistant to all kinds of stressors, such as desiccation, shear stress, toxins, and grazing by microfauna due to the presence of EPS, which constitutes 90% of the mass of dry biofilms and is the principal constituent of biofilms [11][17]. EPS is primarily composed of high-molecular-weight heteropolysaccharides arranged in linear or branched chains, along with extracellular DNA and glycoconjugates. This is collectively referred to as the matrixome, which forms a slippery surface by combining with water, and also forms a complex 3D architecture [3][17][18][19]. EPS varies with the bacterial composition and the stage of development. Biofilms are also known to retain certain special substances, such as particles of clay, blood, corroded fragments, and certain mineral crystals. This varies based on where the biofilms originated [15].
These colonies also promote immense cell-to-cell interactions as QS (quorum sensing) [1]. QS systems regulate bacterial behaviour in the population through signalling mediated by autoinducer molecules. The AI-2 and AI-3 (autoinducers 2 and 3) quorum sensing is used in both Gram-positive and Gram-negative species. In Gram-negative bacteria, QS signalling is mediated by AHLs (N-acyl homoserine lactones), also known as AI-1 (autoinducer 1). It is the best-studied signalling mechanism and is described in Figure 2a [20]. The AIP (autoinducing peptides) molecules are secreted by Gram-positive bacteria. AIP are small peptides that are processed post-translationally and are impermeable to the cell membrane [4]. The two-component pathway in Gram-positive bacteria is illustrated in detail in Figure 2b [20]. Autoinducers are released by bacterial cells in response to population density and accumulate in their environment. When the concentration of these QS molecules attains a threshold value, the AHL molecules, which are transcription factors, attach to the LuxR receptors and regulate the expression of certain genes that are responsible for synthesising virulence factors, such as exotoxins, enzymes, biofilms, and others [21]. The communication between microbes can alter gene expression in response to the concentration of QS molecules, and thereby regulate community behaviour [15][22]. The regulation of gene expression can, in turn, affect the virulence, pathogenicity, and ultimately, the survival of bacteria [23].
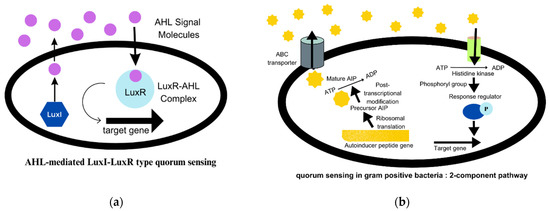
Figure 2. (a) AHL-mediated quorum sensing in Gram-negative bacteria. (b) Quorum signalling mechanism in Gram-positive bacteria.
To facilitate the research, a repository of all QSSM (quorum sensing signalling molecules), called SigMol, has been created. It contains information related to different families of signalling molecules, such as AHLs, HAQ (4-hydroxy-2-alkylquinolones), DPK (diketopiperazines), DSF (diffusible signal factors), AI-2, AI-3, QSPs (quorum sensing peptides), and many others [24].
It is now known that biofilm development occurs in a step-by-step pattern, known as the attached growth process, as follows: -reversible binding to the surface due to the presence of cations, adhesion that is irreversible, followed by the formation of a microcolony and the maturation of the colony, which disperses as free planktonic cells [15][16][19]. According to a survey, biofilm research has seen an upward trend in the last decade. The search with the keyword “biofilm” in the title yielded over 72,500 publications from 1975 to 2022 on PubMed, of which over 55,600 articles have been published in the last decade, from 2012 to 2022. On browsing the search engine VentureRadar with the keyword “biofilm”, a list of 227 companies was displayed. This indicates the growing commercial interest and also highlights the need for bench-side-to-bedside research in the field of biofilms and the development of anti-biofilm agents [25]. This growth in biofilm research has been triggered by the advancement and development of several techniques of molecular biology, such as microscopic, spectroscopic, bioinformatics, sequencing, and several other methods, which are summarised in Figure 3 [16][26][27][28][29]. Novel methods such as SAW (surface acoustic waves) and bioimpedance-based sensing are also used [30].
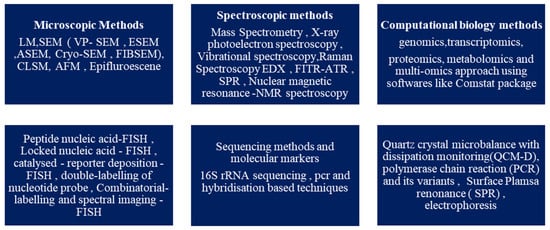
Figure 3. Characterisation techniques for biofilms.
2. Biofilm and Its Development
Biofilms are a predominant form of microbial growth. Over time, several perspectives have been put forward to explain the nature of biofilms. The most simplistic and commonly accepted model is to consider biofilms as aggregation of individual cells which are formed in a definite five-step process, as shown in Figure 4. The three major processes are attachment, colony formation, and dispersion [1].
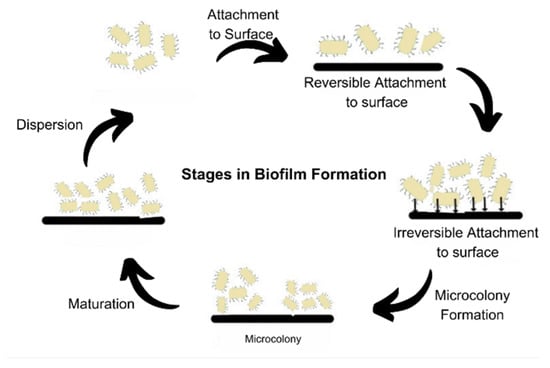
Figure 4. Stages of biofilm formation.
The prevailing model of biofilm development, consisting of five sequential steps, has been established using in vitro experimental conditions and is primarily focused on the growth of Pseudomonas aeruginosa. This model has also been extrapolated and widely accepted for the study of biofilm development in Staphylococcus aureus [11]. It delineates the lifecycle of biofilm-forming organisms into two distinct stages: the motile stage and the sessile stage [31]. The process begins by attachment of free-living microbes to the biotic or abiotic surface, reversibly, via non-specific interactions such as Vander Waals forces and ionic interactions. The attachment becomes irreversible once the adhesins and adhesive proteins are made and production of cyclic c-di-GMP begins. The levels of intracellular c-di-GMP govern the nature of bacterial existence in a planktonic or biofilm state. These cells then form micro-colonies that undergo maturation by EPS formation. The matured biofilms disperse some of the cells that serve as inoculum to spread the biofilm. This process is also called seeding dispersal [32]. Dispersion mainly occurs due to nutrient deficiency, mechanical stress, formation of flagella, degradation of EPS, or the formation of toxins [33].
Recent studies have pointed out disparities in the model and its limited applicability in vivo and in medicinal and industrial settings [11]. Thus, Sauer et al. have proposed an alternative, expanded, and open model of biofilm development that actually displays the bacteria as having the potential to switch forms in response to the factors such as the nature of the substrate, the colonising bacteria, and the conditions in the micro-environment [11]. Bacteria under in vivo conditions can be present in a planktonic or colonial form, as illustrated in Figure 5 [11].
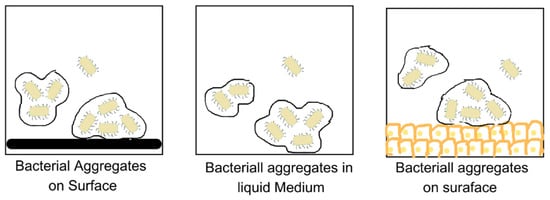
Figure 5. Bacterial aggregates on different kinds of surfaces.
Another approach is to consider biofilms as having characteristics similar to multi-cellular organisms. This is considered since cells in biofilm exhibit coordinated responses, and individual cells behave as differentiated ones in displaying the division of labour. In addition to this, different parts of biofilms have specific roles, such as the functioning of organ systems. They can also control the behaviour and maintain a stable internal environment via QS signalling. The QS system is responsible for interactions between cells, which can be positive as well as negative [31].
Penesyan et al. provided an in-depth insight into the above-mentioned approaches and proposed a novel vision that attempts to fill in the gaps and provide a holistic understanding of the unique nature of biofilms [31]. They stated that biofilms provide a protected environment for cells to accumulate mutations and incorporate genetic and phenotypic changes that enhance their fitness in response to environmental stress [31].
3. Biofilms and CRISPR-Cas System
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) are a family of DNA repeats widely distributed in prokaryotes. A CRISPR loci consists of a short sequence, 21–48 bp, repeated several times—about 250 times. The cas gene (CRISPR-associated gene) is located adjacent to the CRISPR loci [34]. The CRISPR-Cas system serves as a prokaryotic defence mechanism against plasmids and phages attacking the cell [35][36]. CRISPR/Cas adopts at least two basic mechanisms: (1) by acquiring proto-spacers from foreign DNA at the leader end of the CRISPR locus (adaptation stage), and (2) by targeting either invasive DNA or RNA (interference stage) [36]. There is growing evidence that biofilm formation is regulated by the CRISPR-Cas systems that exist in bacteria. The studies suggest an interrelation between the genes that are responsible for biofilm formation and the CRISPR-Cas system. It has been implicated in the regulation of bacterial physiology, virulence, pathogenicity, and the formation of EPS [37]. The CRISPR system can be used as a safe and targeted approach to treat microbial infections. It requires specific cleavage of the Cas9 complex component signalling system, which is a regulator of bacterial virulence [37]. The expressions of CRISPR-associated genes and proteins are seen in various Gram-positive and Gram-negative bacteria associated with humans. By controlling gene expressions of several virulence genes, several biofilm-related diseases can be cured. Researchers have been able to target this system for developing anti-biofilm strategies. Zuberi et al. introduced the concept of CRISPRi (CRISPR inhibition). This process can produce many levels of gene knockdown [38]. It targets the LuxS gene, which regulates quorum signalling. The LuxS gene that synthesises AI-2, a molecule which initiates biofilm formation, was made to hybridise with complementary “single-guide RNA” (sgRNA), and hence, its expression was silenced [39].
References
- Jamal, M.; Khan, A.W.; Andleeb, S. Bacterial Biofilm: Its Composition, Formation and Role in Human Infections. J. Microbiol. Biotechnol. 2015, 4, 3.
- Dobretsov, S.; Abed, R.M.M.; Teplitski, M. Mini-Review: Inhibition of Biofouling by Marine Microorganisms. Biofouling 2013, 29, 423–441.
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Artini, M.; Papa, R.; Selan, L. Microscopy Methods for Biofilm Imaging: Focus on Sem and VP-SEM Pros and Cons. Biology 2021, 10, 51.
- Bramhachari, P.V. (Ed.) Implication of Quorum Sensing and Biofilm Formation in Medicine, Agriculture and Food Industry; Springer: Singapore, 2019; ISBN 978-981-329-408-0.
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella Biofilm Formation, Chronic Infection, and Immunity within the Intestine and Hepatobiliary Tract. Front. Cell. Infect. Microbiol. 2021, 10, 1–17.
- Srivastava, S.; Bhargava, A. Biofilms and Human Health. Biotechnol. Lett. 2016, 38, 1–22.
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014.
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928.
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges. Front. Microbiol. 2016, 7, 45.
- National Board Inspection Code (NBIC). NBIC-Annual-Report-2022; National Board Inspection Code (NBIC): Taipei, Taiwan, 2022.
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle– Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620.
- Cai, Y.-M. Non-Surface Attached Bacterial Aggregates: A Ubiquitous Third Lifestyle. Front. Microbiol. 2020, 11.
- Kumar, S.; Chandra, N.; Singh, L.; Hashmi, M.Z.; Varma, A. Biofilms in Human Diseases: Treatment and Control; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-030-30757-8.
- Pal, M.K.; Lavanya, M. Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation. J. Bio Tribo Corros. 2022, 8, 76.
- Sonawane, J.M.; Rai, A.K.; Sharma, M.; Tripathi, M.; Prasad, R. Microbial Biofilms: Recent Advances and Progress in Environmental Bioremediation. Sci. Total Environ. 2022, 824, 153843.
- Huang, H.; Peng, C.; Peng, P.; Lin, Y.; Zhang, X.; Ren, H. Towards the Biofilm Characterization and Regulation in Biological Wastewater Treatment. Appl. Microbiol. Biotechnol. 2019, 103, 1115–1129.
- Miranda, A.F.; Ramkumar, N.; Andriotis, C.; Höltkemeier, T.; Yasmin, A.; Rochfort, S.; Wlodkowic, D.; Morrison, P.; Roddick, F.; Spangenberg, G.; et al. Applications of Microalgal Biofilms for Wastewater Treatment and Bioenergy Production. Biotechnol. Biofuels 2017, 10, 120.
- Tolker-Nielsen, T. Biofilm Development. Microbiol. Spectr. 2018, 4, 10.
- Bhatia, R.; Gulati, D.; Sethi, G. Biofilms and Nanoparticles: Applications in Agriculture. Folia Microbiol. 2021, 66, 159–170.
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory Mechanisms and Promising Applications of Quorum Sensing-Inhibiting Agents in Control of Bacterial Biofilm Formation. Front. Microbiol. 2020, 11, 589640.
- Coquant, G.; Grill, J.P.; Seksik, P. Impact of N-Acyl-Homoserine Lactones, Quorum Sensing Molecules, on Gut Immunity. Front. Immunol. 2020, 11.
- Abdul Hamid, N.W.; Nadarajah, K. Microbe Related Chemical Signalling and Its Application in Agriculture. Int. J. Mol. Sci. 2022, 23, 8998.
- Sakarikou, C.; Kostoglou, D.; Simões, M.; Giaouris, E. Exploitation of Plant Extracts and Phytochemicals against Resistant Salmonella Spp. in Biofilms. Food Res. Int. 2020, 128, 108806.
- Rajput, A.; Kaur, K.; Kumar, M. SigMol: Repertoire of Quorum Sensing Signaling Molecules in Prokaryotes. Nucleic Acids Res. 2016, 44, D634–D639.
- Rumbaugh, K.P. How Well Are We Translating Biofilm Research from Bench-Side to Bedside? Biofilm 2020, 2, 100028.
- Cai, P.; Sun, X.; Wu, Y.; Gao, C.; Mortimer, M.; Holden, P.A.; Redmile-Gordon, M.; Huang, Q. Soil Biofilms: Microbial Interactions, Challenges, and Advanced Techniques for Ex-Situ Characterization. Soil Ecol. Lett. 2019, 1, 85–93.
- Dufrêne, Y.F.; Boonaert, C.J.P.; Rouxhet, P.G. Surface Analysis by X-ray Photoelectron Spectroscopy in Study of Bioadhesion and Biofilms. Methods Enzymol. 1999, 310, 375–389.
- Sun, V.; Sc, B. Monitoring Early Stages of Bacterial Adhesion at Silica Surfaces through Image Analysis. Langmuir 2020, 36, 2120–2128.
- Nozhevnikova, A.N.; Botchkova, E.A.; Plakunov, V.K. Multi-Species Biofilms in Ecology, Medicine, and Biotechnology. Microbiology 2015, 84, 731–750.
- Xu, Y.; Dhaouadi, Y.; Stoodley, P.; Ren, D. Sensing the Unreachable: Challenges and Opportunities in Biofilm Detection. Curr. Opin. Biotechnol. 2020, 64, 79–84.
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three Faces of Biofilms: A Microbial Lifestyle, a Nascent Multicellular Organism, and an Incubator for Diversity. NPJ Biofilms Microbiomes 2021, 7, 80.
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial Biofilm Formation on Implantable Devices and Approaches to Its Treatment and Prevention. Heliyon 2018, 4, e01067.
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent Advances in Nanotechnology for Eradicating Bacterial Biofilm. Theranostics 2022, 12, 2383–2405.
- Horvath, P.; Romero, D.A.; Coûté-Monvoisin, A.-C.; Richards, M.; Deveau, H.; Moineau, S.; Boyaval, P.; Fremaux, C.; Barrangou, R. Diversity, Activity, and Evolution of CRISPR Loci in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1401–1412.
- Nie, M.; Dong, Y.; Cao, Q.; Zhao, D.; Ji, S.; Huang, H.; Jiang, M.; Liu, G.; Liu, Y. CRISPR Contributes to Adhesion, Invasion, and Biofilm Formation in Streptococcus agalactiae by Repressing Capsular Polysaccharide Production. Microbiol. Spectr. 2022, 10, e02113-21.
- Garneau, J.E.; Dupuis, M.-È.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas Bacterial Immune System Cleaves Bacteriophage and Plasmid DNA. Nature 2010, 468, 67–71.
- Wu, Q.; Cui, L.; Liu, Y.; Li, R.; Dai, M.; Xia, Z.; Wu, M. CRISPR-Cas Systems Target Endogenous Genes to Impact Bacterial Physiology and Alter Mammalian Immune Responses. Mol. Biomed. 2022, 3, 22.
- Mohamad, F.; Alzahrani, R.R.; Alsaadi, A.; Alrfaei, B.M.; Yassin, A.E.B.; Alkhulaifi, M.M.; Halwani, M. An Explorative Review on Advanced Approaches to Overcome Bacterial Resistance by Curbing Bacterial Biofilm Formation. Infect. Drug Resist. 2023, 16, 19–49.
- Zuberi, A.; Misba, L.; Khan, A.U. CRISPR Interference (CRISPRi) Inhibition of LuxS Gene Expression in E. Coli: An Approach to Inhibit Biofilm. Front. Cell. Infect. Microbiol. 2017, 7, 214.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
999
Revisions:
2 times
(View History)
Update Date:
02 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




