Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianliang Cao | -- | 1843 | 2023-08-01 10:51:36 | | | |
| 2 | Sirius Huang | Meta information modification | 1843 | 2023-08-02 02:39:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, R.; Qin, C.; Bala, H.; Wang, Y.; Cao, J. Spinel Ferrite (MFe2O4) Chemiresistive Based Gas Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/47482 (accessed on 06 March 2026).
Zhang R, Qin C, Bala H, Wang Y, Cao J. Spinel Ferrite (MFe2O4) Chemiresistive Based Gas Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/47482. Accessed March 06, 2026.
Zhang, Run, Cong Qin, Hari Bala, Yan Wang, Jianliang Cao. "Spinel Ferrite (MFe2O4) Chemiresistive Based Gas Sensors" Encyclopedia, https://encyclopedia.pub/entry/47482 (accessed March 06, 2026).
Zhang, R., Qin, C., Bala, H., Wang, Y., & Cao, J. (2023, August 01). Spinel Ferrite (MFe2O4) Chemiresistive Based Gas Sensors. In Encyclopedia. https://encyclopedia.pub/entry/47482
Zhang, Run, et al. "Spinel Ferrite (MFe2O4) Chemiresistive Based Gas Sensors." Encyclopedia. Web. 01 August, 2023.
Copy Citation
Gas-sensing technology has gained significant attention in recent years due to the increasing concern for environmental safety and human health caused by reactive gases. In particular, spinel ferrite (MFe2O4), a metal oxide semiconductor with a spinel structure, has emerged as a promising material for gas-sensing applications.
spinel ferrite
metal oxide semiconductor
chemiresistive gas sensor
nanostructure
doping
heterostructure
1. Introduction
Metal oxide semiconductor (MOS) gas sensors operate by detecting alterations in the electrical conductivity of a semiconducting metal oxide when exposed to a gas [1]. When the MOS sensor comes into contact with the target gas, the gas molecules adhere to the sensor material’s surface, resulting in a modification of the sensor’s electrical resistance [2]. The extent and direction of the resistance alteration correlate with the gas concentration and its chemical properties. Numerous metal oxide semiconducting materials, such as tin oxide (SnO2) [3], zinc oxide (ZnO) [4], titanium dioxide (TiO2) [5], and tungsten oxide (WO3) [6], have been widely utilized in the production of MOS sensors. These materials exhibit diverse sensing characteristics towards various gases, and their sensitivity, selectivity, and stability can be adjusted via material doping, surface modification, and operating conditions. To enhance the performance of MOS gas sensors, novel sensing structures such as nanowires [7], nanotubes [8], and nanostructured thin films [9] have been developed, offering larger surface-to-volume ratios and improved gas adsorption capabilities. Additionally, advanced fabrication techniques such as atomic layer deposition (ALD) [10], chemical vapor deposition (CVD) [11], and spray pyrolysis [12] have been employed to achieve precise control over the sensor’s morphology, composition, and functionality. In conclusion, MOS gas sensors have become indispensable tools for monitoring environmental pollution, ensuring industrial safety, and safeguarding public health [13][14]. The ongoing progress in sensor technology and its integration with information and communication systems will create novel opportunities for real-time, reliable, and intelligent gas-sensing solutions.
MOS-gas-sensitive materials can be classified into two categories based on the number of metal ions present in the single-phase metal oxide material: single metal oxides and composite metal oxides. The gas sensors based on single metal oxides exhibit excellent attributes, including easy integration, good repeatability, and effective detection of various gases [15][16][17]. Nonetheless, there is still room for improvement in terms of the selectivity and recovery performance of single-phase gas-sensitive materials. Researchers have explored strategies to enhance the sensing performance by incorporating precious metal catalysts or combining them with other materials to modify the morphology of single metal oxides, aiming to provide activation energy for reactions or form p–n heterojunctions.
In recent times, the distinctive magnetic properties [18], electrical properties [19], microwave absorption [20], and photocatalytic properties [21] of composite metal oxides, specifically spinel ferrites, have garnered significant attention. The primary preparation techniques for MFe2O4-based gas-sensitive materials include the co-precipitation method [22][23][24][25], sol–gel method [26][27][28], and template synthesis method [29]. These methods enable the production of spinel ferrite nanomaterials with diverse morphologies such as nanorods, nanotubes, nanofilms, and core–shell microspheres. The combination of novel synthesis approaches and the integration of new functional materials has led to the development of spinel ferrite and spinel ferrite composite materials with controllable structures and morphologies, thereby expanding their application potential. For instance, the controlled synthesis of spinel ferrite nanoparticles has exhibited promising outcomes in biomedical applications such as drug delivery and cancer therapy [30]. Furthermore, the combination of spinel ferrite with graphene oxide enhances its magnetic and electrical properties, positioning it as a potential candidate for spintronics [31] and electromagnetic shielding applications [32]. Additionally, the incorporation of metal ions or other functional materials into spinel ferrite has shown improved catalytic and photocatalytic properties, thereby finding application in areas such as wastewater treatment [33] and hydrogen production [34]. Overall, the advancement of novel synthesis methods and the integration of functional materials have broadened the scope of zinc ferrite materials and opened up avenues for future research.
As a semiconducting, magnetic oxide material, spinel ferrite has excellent chemical stability, enabling the effective adsorption of various gases [35]. Its inherent catalytic properties stimulate chemisorption processes that result in changes in its electrical resistance when exposed to different gases [36]. This allows for accurate gas detection and measurement. Additionally, spinel ferrite can operate at lower temperatures compared with other gas sensors, which leads to increased energy efficiency [37]. Its high sensitivity [38] and selectivity [39] towards particular gases, coupled with its capacity for miniaturization, make spinel ferrite an optimal material for building reliable, efficient, and compact gas sensors.
2. Gas-Sensing Mechanism
With its spinel crystal structure, spinel ferrite emerges as a promising sensing material possessing exceptional properties. Figure 1 illustrates the crystal structure of zinc ferrite, where the face-centered cube of O2− accumulates within its crystal lattice, while the metal ions M2+ and Fe3+ are embedded in the tetrahedral and octahedral gaps formed by O2−. This structure readily facilitates the formation of defects, including oxygen vacancies, both internally and on the surface, making it highly advantageous for gas-sensitive materials. The unique crystal structure, specifically the insertion of the transition metal cation Zn2+ into the Fe2+Fe3+O4 structure, plays a crucial role in the effective detection of reducing gases.
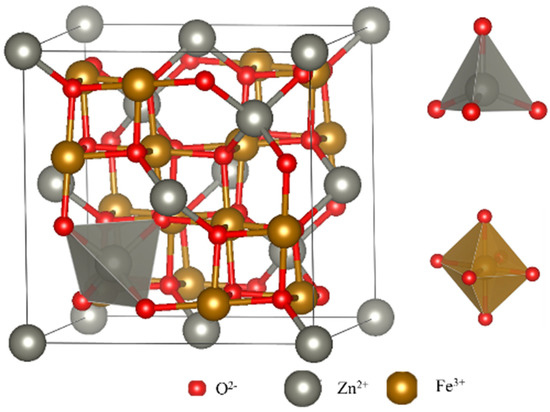
Figure 1. The crystal structure of ZnFe2O4, with Zn2+ in the tetrahedron gap and Fe3+ in the octahedron gap.
The gas-sensing response of spinel ferrite is determined by the complex interaction that occurs at the interface between the gas and solid material. However, a unified definition of gas sensor mechanisms is lacking. A commonly proposed sensing mechanism for spinel ferrite sensors is as follows: when a spinel-ferrite-based sensor is exposed to air, oxygen molecules adsorb onto its surface, capturing free electrons from the conduction band and forming oxygen anions. The specific form of these oxygen anions depends on the operating temperature. The loss of electrons generates an electron depletion layer (n-type) on the semiconductor surface, resulting in an increase in resistance. In a reducing gas atmosphere, Equation (6) occurs, leading to a reduction in the resistance of the electron depletion region and sensor. It is worth noting that the reaction described in Equation (6) may vary depending on the operating temperature or target gas.
The unique microstructure and high specific surface area of pure MFe2O4 nanomaterials offer numerous adsorption sites, leading to an enhancement in gas-sensing performance. The addition of metal ions through doping reduces the barrier height of grain boundaries, facilitating improved carrier diffusion and transfer rates; heterostructures [40], on the other hand, allow for the modulation of the electron depletion region and potential barrier at the interface by leveraging the interaction between Fermi energy levels and energy bands [41]. These mechanisms collectively contribute to the enhancement of gas sensitivity in the respective materials. More detailed explanations of the gas-sensitive mechanisms specific to these new materials can be found in later sections.
3. Nanostructure
The gas-sensing application has significantly benefited from the use of nanostructured materials, primarily due to their high surface-to-volume ratio, which allows for better interaction with the gas molecules. In particular, zinc ferrite, a type of spinel ferrite, has been widely used due to its specific surface area, contact area, porosity, grain size, and grain stacking order. These factors all contribute to its gas-sensing properties. The operating temperature, humidity, and gas concentration are several external factors that can influence the performance of zinc ferrite-based gas sensors. For instance, at higher operating temperatures, the sensor’s sensitivity can increase due to the enhanced surface reaction rates [42]. On the other hand, excessive humidity may cause the surface of the sensor to become water-saturated, which could inhibit its response to target gases [35]. Apart from these external factors, the morphology-related characteristics of spinel ferrite also play a significant role in its gas-sensing properties. The development of unique morphologies and structures in spinel ferrite is considered a promising approach to enhance its gas-sensing performance. For example, porous spinel ferrite with large specific surface areas can provide more active sites for gas molecule adsorption, facilitating improved surface effects, electronic transfer efficiency, and ultimately a better gas-sensing performance. Various synthesis methods can be employed to create spinel ferrite materials with different morphologies. These include sol–gel, hydrothermal, and co-precipitation methods, among others. Each method offers unique advantages in terms of controlling the size, shape, and distribution of the nanoparticles, thereby allowing for the optimization of the sensor’s performance.
4. Doping
Element doping is indeed a powerful strategy to enhance the structure and performance of spinel ferrite materials, and there has been a growing interest in this research area recently. While earlier studies on spinel ferrite doping mostly concentrated on applications such as electrodes and magnetism, recent advancements have shed light on the importance of doping for optimizing gas-sensing properties. However, not all metallic elements are suitable for doping in spinel ferrite materials. Preferably, elements with donor characteristics (high valence elements that can donate electrons) or acceptor characteristics (low valence elements that can accept electrons) are used for modification. Doping in spinel ferrite materials can occur in two forms. The first form of doping involves displacement, where the M2+ (A site) and Fe3+ (B site) ions in the spinel ferrite are replaced by the doping elements. This changes the composition of the spinel ferrite and can affect its properties, such as A-site doping [43], B-site doping [44], and AB-site doping [45]. The second involves the incorporation of doping elements into the tetrahedral and octahedral interstices of MFe2O4 crystals. This results in a solid solution structure, where the doping elements are homogeneously dispersed within the host material [46]. Doping can significantly alter the composition and microstructure of spinel ferrite materials, influencing characteristics such as crystallinity [47]. These changes can, in turn, affect the reference resistance [48] and gas-sensing performance [49] of the ferrite-based gas sensors. For instance, doping can enhance the sensitivity [50], selectivity [51], response speed [28], and stability [52] of the sensors.
5. Heterostructure
It has been discussed how the gas-sensitive performance of spinel ferrite sensors can be enhanced through the manipulation of their morphology or the introduction of doping elements. However, to achieve the desired properties, researchers have explored the development of spinel ferrite composites, which find more extensive applications in the fields of photocatalysis and sensing. The development of spinel ferrite composites has gained significant attention due to their potential to synergistically enhance the gas-sensitive performance. These composites often involve combining spinel ferrite with other materials such as metal oxides, carbon-based materials, or polymers. The unique properties of these composite materials can be leveraged to improve the gas-sensing properties of spinel ferrite sensors. For example, metal-oxide-based spinel ferrite composites have demonstrated an improved gas-sensing performance due to the enhanced specific surface area and increased active sites provided by the metal oxide component. The combination of spinel ferrite with carbon-based materials, such as graphene or carbon nanotubes, can enhance the electrical conductivity and provide additional adsorption sites, leading to enhanced gas-sensing capabilities. In summary, the development of spinel ferrite composites has opened up new avenues for enhancing the gas-sensitive properties of spinel ferrite sensors. These composites, whether metal-oxide-based, carbon-based, or incorporating polymers, offer unique advantages that can be leveraged to achieve an improved gas-sensing performance.
References
- Nadargi, D.Y.; Umar, A.; Nadargi, J.D.; Lokare, S.A.; Akbar, S.; Mulla, I.S.; Suryavanshi, S.S.; Bhandari, N.L.; Chaskar, M.G. Gas sensors and factors influencing sensing mechanism with a special focus on MOS sensors. J. Mater. Sci. 2023, 58, 559–582.
- Yang, X.; Deng, Y.; Yang, H.; Liao, Y.; Cheng, X.; Zou, Y.; Wu, L.; Deng, Y. Functionalization of Mesoporous Semiconductor Metal Oxides for Gas Sensing: Recent Advances and Emerging Challenges. Adv. Sci. 2023, 10, 2204810.
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255.
- Kurugundla, G.K.; Godavarti, U.; Saidireddy, P.; Pothukanuri, N. Zinc oxide based gas sensors and their derivatives: A critical review. J. Mater. Chem. C 2023, 11, 3906–3925.
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403.
- Kukkola, J.; Mäklin, J.; Halonen, N.; Kyllönen, T.; Tóth, G.; Szabó, M.; Shchukarev, A.; Mikkola, J.-P.; Jantunen, H.; Kordás, K. Gas sensors based on anodic tungsten oxide. Sens. Actuators B 2011, 153, 293–300.
- Cho, S.-Y.; Yoo, H.-W.; Kim, J.Y.; Jung, W.-B.; Jin, M.L.; Kim, J.-S.; Jeon, H.-J.; Jung, H.-T. High-resolution p-type metal oxide semiconductor nanowire array as an ultrasensitive sensor for volatile organic compounds. Nano Lett. 2016, 16, 4508–4515.
- Kauffman, D.R.; Star, A. Carbon nanotube gas and vapor sensors. Angew. Chem. Int. Ed. 2008, 47, 6550–6570.
- Beckers, N.; Taschuk, M.; Brett, M. Selective room temperature nanostructured thin film alcohol sensor as a virtual sensor array. Sens. Actuators B 2013, 176, 1096–1102.
- Pan, H.; Zhou, L.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Atomic layer deposition to heterostructures for application in gas sensors. Int. J. Extreme Manuf. 2023, 5, 22008.
- Srivastava, S.; Pal, P.; Sharma, D.K.; Kumar, S.; Senguttuvan, T.; Gupta, B.K. Ultrasensitive Boron–Nitrogen-Codoped CVD Graphene-Derived NO2 Gas Sensor. ACS Mater. Au 2022, 2, 356–366.
- Sriram, S.R.; Parne, S.R.; Pothukanuri, N.; Edla, D.R. Prospects of spray pyrolysis technique for gas sensor applications-A comprehensive review. J. Anal. Appl. Pyrolysis 2022, 164, 105527.
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform metal oxide-based hybrid nanostructures for gas sensing with versatile prospects. Coord. Chem. Rev. 2020, 413, 213272.
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369.
- Selvakumar, D.; Sonu, K.; Ramadoss, G.; Sivaramakrishnan, R.; Jayavel, R.; Eswaramoorthy, M.; Rao, K.V.; Pugazhendhi, A. Heterostructures of polyaniline and Ce-ZnO nanomaterial coated flexible PET thin films for LPG gas sensing at standard environment. Chemosphere 2023, 314, 137492.
- Souri, M.; Yamini, Y.; Amoli, H.S. The synergistic effect of Ce dopant/Cotton bio-template on the performance of the SnO2 gas sensor for the detection of Ethanol. Mater. Sci. Eng. 2023, 294, 116501.
- Qu, Z.; Li, Y.; Xu, R.; Li, C.; Wang, H.; Wang, H.; Zhang, Y.; Wei, Q. Candy-like heterojunction nanocomposite of WO3/Fe2O3-based semiconductor gas sensor for the detection of triethylamine. Microchim. Acta 2023, 190, 139.
- Zhou, J.; Lin, Y.; Yang, H.; Ren, Y.; Xu, F. Structural, morphological and magnetic properties of low temperature sintered LiZnTiBi ferrites. J. Alloys Compd. 2023, 932, 167616.
- Vinod, G.; Rajashekhar, K.; Naik, J.L. Dysprosium doped Cu0.8Cd0.2DyxFe2-xO4 nano ferrites: A combined impact of Dy3+ on enhanced physical, optical, magnetic, and DC-electrical properties. Ceram. Int. 2023, 49, 2829–2851.
- Wang, X.; Lv, X.; Liu, Z.; Zhang, H.; Liu, M.; Xu, C.; Zhou, X.; Yuan, M.; Yang, L.; You, W. Multi-interfacial 1D magnetic C fibers for Broadband microwave absorption. Mater. Today Phys. 2023, 35, 101140.
- Kumari, S.; Dhanda, N.; Thakur, A.; Gupta, V.; Singh, S.; Kumar, R.; Hameed, S.; Thakur, P. Nano Ca-Mg-Zn ferrites as tuneable photocatalyst for UV light-induced degradation of rhodamine B dye and antimicrobial behavior for water purification. Ceram. Int. 2023, 49, 12469–12480.
- Singh, S.; Singh, A.; Yadav, B.C.; Tandon, P. Synthesis, characterization, magnetic measurements and liquefied petroleum gas sensing properties of nanostructured cobalt ferrite and ferric oxide. Mater. Sci. Semicond. Process. 2014, 23, 122–135.
- Srivastava, R.; Yadav, B.C.; Singh, M.; Yadav, T.P. Synthesis, Characterization of Nickel Ferrite and Its Uses as Humidity and LPG Sensors. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1404–1412.
- Rathore, D.; Kurchania, R.; Pandey, R.K. Fabrication of Ni1xZnxFe2O4 (x = 0, 0.5 and 1) nanoparticles gas sensor for some reducing gases. Sens. Actuators A 2013, 199, 236–240.
- Abu-Hani, A.F.S.; Mahmoud, S.T.; Awwad, F.; Ayesh, A.I. Design, fabrication, and characterization of portable gas sensors based on spinel ferrite nanoparticles embedded in organic membranes. Sens. Actuators B 2017, 241, 1179–1187.
- Maharajan, M.; Mursalin, M.D.; Narjinary, M.; Rana, P.; Sen, S.; Sen, A. Synthesis, Characterization and Vapour Sensing Properties of Nanosized ZnFe2O4. Trans. Indian Ceram. Soc. 2014, 73, 102–104.
- De Oliveira, R.C.; Pontes Ribeiro, R.A.; Cruvinel, G.H.; Ciola Amoresi, R.A.; Carvalho, M.H.; Aparecido de Oliveira, A.J.; de Oliveira, M.C.; de Lazaro, S.R.; da Silva, L.F.; Catto, A.C.; et al. Role of Surfaces in the Magnetic and Ozone Gas-Sensing Properties of ZnFe2O4 Nanoparticles: Theoretical and Experimental Insights. ACS Appl. Mater. Interfaces 2021, 13, 4605–4617.
- Chethan, B.; Ravikiran, Y.T.; Vijayakumari, S.C.; Rajprakash, H.G.; Thomas, S. Nickel substituted cadmium ferrite as room temperature operable humidity sensor. Sens. Actuators A 2018, 280, 466–474.
- Li, X.; Lu, D.; Shao, C.; Lu, G.; Li, X.; Liu, Y. Hollow CuFe2O4/α-Fe2O3 composite with ultrathin porous shell for acetone detection at ppb levels. Sens. Actuators B 2018, 258, 436–446.
- Gavilán, H.; Rizzo, G.M.; Silvestri, N.; Mai, B.T.; Pellegrino, T. Scale-up approach for the preparation of magnetic ferrite nanocubes and other shapes with benchmark performance for magnetic hyperthermia applications. Nat. Protoc. 2023, 18, 783–809.
- Shen, L.; Lan, G.; Lu, L.; Ma, C.; Cao, C.; Jiang, C.; Fu, H.; You, C.; Lu, X.; Yang, Y. A strategy to modulate the bending coupled microwave magnetism in nanoscale epitaxial lithium ferrite for flexible spintronic devices. Adv. Sci. 2018, 5, 1800855.
- Wang, W.; Gumfekar, S.P.; Jiao, Q.; Zhao, B. Ferrite-grafted polyaniline nanofibers as electromagnetic shielding materials. J. Mater. Chem. C 2013, 1, 2851–2859.
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A. Application of spinel ferrite nanoparticles in water and wastewater treatment: A review. Sep. Purif. Technol. 2017, 188, 399–422.
- Scheffe, J.R.; Allendorf, M.D.; Coker, E.N.; Jacobs, B.W.; McDaniel, A.H.; Weimer, A.W. Hydrogen production via chemical looping redox cycles using atomic layer deposition-synthesized iron oxide and cobalt ferrites. Chem. Mater. 2011, 23, 2030–2038.
- Wei, K.; Huai, H.-X.; Zhao, B.; Zheng, J.; Gao, G.-Q.; Zheng, X.-Y.; Wang, C.-C. Facile synthesis of CoFe2O4 nanoparticles and their gas sensing properties. Sens. Actuators B 2022, 369, 132279.
- Nemufulwi, M.I.; Swart, H.C.; Shingange, K.; Mhlongo, G.H. ZnO/ZnFe2O4 heterostructure for conductometric acetone gas sensors. Sens. Actuators B 2023, 377, 133027.
- Rathore, D.; Mitra, S.; Kurchania, R.; Pandey, R.K. Physicochemical properties of CuFe2O4 nanoparticles as a gas sensor. J. Mater. Sci. Mater. Electron. 2018, 29, 1925–1932.
- Zhang, J.; Chen, D.; Chen, L. Preparation of ultrafine ZnFe2O4 and its gas-sensing properties for Cl2. Sens. Mater. 2006, 18, 277–282.
- Zhang, Y.; Zhou, Y.; Li, Z.; Chen, G.; Mao, Y.; Guan, H.; Dong, C. MOFs-derived NiFe2O4 fusiformis with highly selective response to xylene. J. Alloys Compd. 2019, 784, 102–110.
- Wu, K.; Lu, Y.; Liu, Y.; Liu, Y.; Shen, M.; Debliquy, M.; Zhang, C. Synthesis and acetone sensing properties of copper (Cu2+) substituted zinc ferrite hollow micro-nanospheres. Ceram. Int. 2020, 46, 28835–28843.
- Yang, T.; Yang, X.; Zhu, M.; Zhao, H.; Zhang, M. Coral-like ZnFe2O4-ZnO mesoporous heterojunction architectures: Synthesis and enhanced sensing properties for triethylamine. Inorg. Chem. Front. 2020, 7, 1918–1926.
- Afzal, A.; Mujahid, A.; Iqbal, N.; Javaid, R.; Qazi, U.Y. Enhanced High-Temperature (600 degrees C) NO2 Response of ZnFe2O4 Nanoparticle-Based Exhaust Gas Sensors. Nanomaterials 2020, 10, 2133.
- Mukherjee, K.; Majumder, S.B. Synthesis of embedded and isolated Mg0.5Zn0.5Fe2O4 nano-tubes and investigation on their anomalous gas sensing characteristics. Sens. Actuators B 2013, 177, 55–63.
- Patil, J.Y.; Nadargi, D.Y.; Mulla, I.S.; Suryavanshi, S.S. Cerium doped MgFe2O4 nanocomposites: Highly sensitive and fast response-recoverable acetone gas sensor. Heliyon 2019, 5, e01489.
- Mugutkar, A.B.; Gore, S.K.; Mane, R.S.; Patange, S.M.; Jadhav, S.S.; Shaikh, S.F.; Al-Enizi, A.M.; Nafady, A.; Thamer, B.M.; Ubaidullah, M. Structural modifications in Co-Zn nanoferrites by Gd substitution triggering to dielectric and gas sensing applications. J. Alloys Compd. 2020, 844, 11.
- Rao, P.; Godbole, R.V.; Bhagwat, S. Nanocrystalline Pd:NiFe2O4 thin films: A selective ethanol gas sensor. J. Magn. Magn. Mater. 2016, 416, 292–298.
- George, J.; Abraham, K.E.; Thomas, K.J. Influence of zinc on the multifunctional properties of ferrites M1-xZnxFe2O4 (M = Cu, Mg, Ni, x = 0, 0.35). J. Magn. Magn. Mater. 2022, 546, 168904.
- Sutka, A.; Mezinskis, G.; Lusis, A.; Stingaciu, M. Gas sensing properties of Zn-doped p-type nickel ferrite. Sens. Actuators B 2012, 171, 354–360.
- Singh, A.; Singh, S.; Joshi, B.D.; Shukla, A.; Yadav, B.C.; Tandon, P. Synthesis, characterization, magnetic properties and gas sensing applications of ZnxCu1-xFe2O4 (0.0 <= x <= 0.8) nanocomposites. Mater. Sci. Semicond. Process. 2014, 27, 934–949.
- Bodade, A.B.; Bodade, A.B.; Wankhade, H.G.; Chaudhari, G.N.; Kothari, D.C. Conduction mechanism and gas sensing properties of CoFe2O4 nanocomposite thick films for H2S gas. Talanta 2012, 89, 183–188.
- Srinivas, C.; Ranjith Kumar, E.; Tirupanyam, B.V.; Singh Meena, S.; Bhatt, P.; Prajapat, C.L.; Chandrasekhar Rao, T.V.; Sastry, D.L. Study of magnetic behavior in co-precipitated Ni–Zn ferrite nanoparticles and their potential use for gas sensor applications. J. Magn. Magn. Mater. 2020, 502, 166534.
- Wu, S.; Li, X.; Xu, Y.; Wu, J.; Wang, Z.; Han, Y.; Zhang, X. Hierarchical spinel NixCo1-xFe2O4 microcubes derived from Fe-based MOF for high-sensitive acetone sensor. Ceram. Int. 2018, 44, 19390–19396.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
02 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





