Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe Murdaca | -- | 2434 | 2023-08-01 08:13:43 | | | |
| 2 | Peter Tang | Meta information modification | 2434 | 2023-08-01 08:26:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Effects of AntagomiRs on Different Lung Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/47471 (accessed on 07 February 2026).
Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Effects of AntagomiRs on Different Lung Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/47471. Accessed February 07, 2026.
Murdaca, Giuseppe, Alessandro Tonacci, Simone Negrini, Monica Greco, Matteo Borro, Francesco Puppo, Sebastiano Gangemi. "Effects of AntagomiRs on Different Lung Diseases" Encyclopedia, https://encyclopedia.pub/entry/47471 (accessed February 07, 2026).
Murdaca, G., Tonacci, A., Negrini, S., Greco, M., Borro, M., Puppo, F., & Gangemi, S. (2023, August 01). Effects of AntagomiRs on Different Lung Diseases. In Encyclopedia. https://encyclopedia.pub/entry/47471
Murdaca, Giuseppe, et al. "Effects of AntagomiRs on Different Lung Diseases." Encyclopedia. Web. 01 August, 2023.
Copy Citation
MiRNAs have been shown to play a crucial role among lung cancer, pulmonary fibrosis, tuberculosis (TBC) infection, and bronchial hypersensitivity, thus including chronic obstructive pulmonary disease (COPD) and asthma. The oncogenic effect of several miRNAs has been ruled out. In order to act on miRNAs turnover, antagomiRs have been developed.
antagomiR
miRNAs
lung diseases
human models
cellular models
animal models
1. Introduction
MicroRNAs (miRNAs) are small molecules made of 21 nucleotides, which modulate several biological processes through post-transcriptional gene expression regulation [1]. In addition, many miRNA knockout strains have differential responses to models of several disorders such as neuronal, cardiac, pulmonary, vascular, renal, immunological, while some of them have altered susceptibility to fungal or bacterial infections, or altered propensity to develop tumors in cancer models [2]. In fact, miRNAs may control tumor development, both acting as tumor-promoting miRNAs (oncomiRNAs and metastamiRNAs) or as tumor suppressor miRNAs [3][4]. Furthermore, it was demonstrated that several human miRNAs are located in specific genomic sites which are involved in cancer [5]. During the last decade, researchers investigated miRNAs functioning and regulation. More recently, they focused on miRNA stability and turnover. Indeed, it was established that miRNAs are very dynamic molecules, presenting with a rapid turnover, which depends on their activation. On these bases, researchers investigated miRNA-induced silencing complex (miRISC) and Argonaute (AGO) proteins, which directly interact with miRNAs and are key factors in the assembly and function of miRISCs [1]. In order to act on miRNAs turnover, antagomiRs have been developed. They are a novel class of chemically engineered oligonucleotides, which are specific silencers of endogenous miRNAs. Specifically, two major molecular changings have been developed in order to increase chemical stability. Thus, including switching of the phosphodiester support with a phosphorothioate linkage between nucleotides or including a 2′O-methyl group. Additionally, antagomiRs with a cholesterol moiety are thought to promote cellular uptake [6]. Considering the complex role of miRNAs, these new molecules can be powerful tools to silence specific miRNAs in vivo and may represent a therapeutic strategy for silencing miRNAs in disease. Indeed, Krützfeldt et al. [7] conducted a study on mice in order to study the biological significance of miR-122, which is abundant in the liver. Analysis of gene expression of messenger RNA from antagomiR-treated animals disclosed that the 3′ untranslated areas of upregulated genes were extremely enriched with miR-122 recognition motives, while down-regulated genes were poor of these motives. Moreover, researchers found that cholesterol biosynthesis genes would have been modulated by miR-122; in fact, plasma cholesterol levels were reduced in antagomiR-122-treated mice. So far, evidence on antagomiR function has been collected on cellular, animal, and human models. Moreover, as described below, data in these three groups are often coherent. This in support of the hypothesis that miRNAs are molecular factors capable of influencing the expression of several disorders. To date, new studies regarding the use of miRNAs as therapeutic targets are ongoing, especially in the treatment of HCV infection, atherosclerosis, and oncologic diseases. As Gambari et al. [3] highlighted in their review, until now, several antagomiRs have been studied among oncologic diseases as therapeutic agents, both alone or in combination with standard drugs. The promising results explain the reason why these agents will improve the therapy of several tumors, such as gastric cancer, gliomas, and breast cancer [8][9][10]. Indeed, the role of miRNAs upon lung disorders has been extensively studied. MiRNAs have proven to play a crucial role among lung cancer, pulmonary fibrosis, tuberculosis (TBC) infection, and bronchial hypersensitivity, thus including chronic obstructive pulmonary disease (COPD) and asthma. The oncogenic effect of several miRNAs has been recently ruled out. COPD is a complex disease with a high rate of morbidity and mortality, especially in Western countries. Disease exacerbations and the associated hospitalizations often represent a considerable expense at a socio-economic level. Reduced lung function predicts mortality and is key to the diagnosis of COPD. Shrine et al. [11] conducted a genome-wide associated study in order to highlight new genetic mechanisms in order to improve future preventive and therapeutic strategies for COPD. More recently, many authors investigated miRNAs expression in COPD, noticing that wide networks composed of miRNA and messenger RNA (mRNAs) cooperate in COPD pathogenesis [12]. Moreover, Faiz et al. [13] investigated whether miRNA expression was modulated by inhaled corticosteroid (ICS) treatment and identified miR-320d as a novel mediator of ICS, regulating the pro-inflammatory response of the airway epithelium. Nowadays, among chronic inflammatory lung diseases, asthma is one of the most prevalent. Pathological mechanisms rely on activation of mast cells and eosinophils and dysregulation of Th 2 response. Asthmatic patients often have a strong genetic background, however recent studies demonstrated the role of epigenetic factors such as miRNAs [14]. Gold standard therapies for asthma control include inhaled β-agonists, both short and long acting, and steroids. On this background, Yu at al. [15] recently demonstrated that a specific miRNA, miR-16, may be used as a predictive biomarker of therapeutic response in asthma, thus, suggesting the role of miRNAs not only upon disease physiopathology but also upon drug response. MiRNAs have shown to have an effect also upon pulmonary arterial hypertension (PAH). PAH is a complex disease with different clinical manifestations and high genetic variability. ESC/ERS Guidelines outlined some of the most frequently involved genetic factors such as nuclear factor of activated T cells (NFAT), hypoxia-inducible factor 1α (HIF-1α), and signal transducer and activator of transcription 3 (STAT3) [16]. New research showed that miRNAs may play a crucial role in vascular remodeling, thus inhibiting or promoting pulmonary vascular resistance. Novel genetic studies have not only focused on chronic lung diseases. Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) are well defined clinical disorders caused by many clinical insults to the lung or because of predispositions to lung injury [17]. These conditions are characterized by massive lung inflammatory response and alveolar barrier damage, and supportive care combined with anti-inflammatory drugs and fluid replacement is fundamental. Currently, there is significant evidence endorsing the crucial role of miRNAs as a new class of gene regulators in ALI [18]. Although several goals have been reached among oncologic disorders, lung cancer still represents a high mortality disease. New discoveries on genetic factors and molecular pathways involved in the disease pathogenesis have been ruled out, however further steps are needed. Indeed, during the last years, miRNAs were found to be useful screening tools. Moreover, they can help clinicians to discriminate between primary lung tumors and lung metastases [19]. Finally, some studies demonstrated that specific miRNAs expression may predict lung tumor prognosis [20]. Most importantly, anti-miRNAs molecules, as antagomiRs, are now emerging as new therapeutic tools. Therefore, the goal of the actual research is to use miRNAs as therapeutic agents. Single miRNAs modulate various mRNA target expressions and may have wide impacts on various cellular processes. Therapies that target individual miRNAs can therefore have wider effects than traditional single-molecule/single-target methods. Indeed, changing numerous downstream objectives, miRNAs may improve the probability of adverse effects occurrence, especially when systemic drug delivery is used. Considering all these, biopharmaceutical companies are actually trying to use miRNAs as novel therapeutics. Indeed, two clinical studies have been launched for hepatitis C virus infection and advanced hepatocellular carcinoma [21][22] treatment.
2. Effects of AntagomiRs on Different Lung Diseases in Human, Cellular, and Animal Models
It is well established that miRNAs are pleiotropic molecules involved in almost all major biological processes. This concept was particularly studied in the lung, where specific miRNAs demonstrated to cooperate with organ development and pulmonary diseases. During the last years, much data has been collected on this topic, with special regards to obstructive and restrictive lung diseases [23][24][25]. In fact, as Sessa and Hata [26] reported, a typical miRNAs expression profile was noticed and different miRNAs play an active role among different processes including hemostasis, viral infection, and inflammation. Lung-specific miRNAs can be used as novel biomarkers in lung disorders. To date, several pieces of research focused on specific lung disease miRNAs expression patterns. However, specific miRNAs expression profiles may be noticed also among different organs. Previously, Wang et al. [19] conducted a study on rats, demonstrating that two specific miRNAs (miR-195 and miR-200c) were peculiarly expressed in the lung, while eight miRNAs were co-expressed in the lung and heart and one miRNA was co-expressed in the lung and kidney. As interest increased on this topic, accessible databases as, for example, MiRmine were created in order to allow researchers to retrieve expression profiles of single or multiple miRNAs for a specific tissue or cell line, either normal or with disease information [27]. According to the researchers' results, miR-21 seems to be the most represented miRNA among lung conditions. MiR-21 is often up-regulated in lung carcinoma. This fact is believed to be a result of the capacity of miR-21 to inhibit tumor suppressor phosphatase and tensin-homolog [28]. Collison et al. [29] characterized miRNAs expression among house dust mite allergic mice. A group was treated with antagomiRs that inhibited the function of specific miRNAs in the lung, and the other group received standard steroid therapy with dexamethasone. Finally, inflammatory lesions and airway hyper-responsiveness were measured. Researchers found that, although miR-21 and let-7b were highly expressed during allergic inflammation, blockade of their function was ineffective at modulating the expression of disease. On the other hand, Kim et al. [30] conducted a study on BALB/c mice noticing that antagomiR-21 increased phosphatase and tensin homolog (PTEN) levels (p < 0.05). Treatment with Ant-21 reduced phosphoinositide 3-kinase (PI3K) activity and restored histone deacetylase (HDAC2) levels (p < 0.05), leading to suppression of airway hyper-responsiveness and to restore of steroid sensitivity to allergic airway disease. Lee et al. [31] also investigated allergic inflammation among mouse models, reporting that MiR-21 expression was down-regulated in mice lungs treated with anti-miR-21. In fact, specific antagomiR reduced both eosinophil count (p < 0.01) and Th2 cytokines levels, including IL-5 and IL-13 in mice BAL fluid (p < 0.05). MiRNA21 demonstrated positive effects also upon lung ischemia. Ischemia/reperfusion injury (IRI) is the primary cause of acute lung injury (ALI) and primary graft dysfunction (PGD) after lung transplantation [32]. Li et al. [33] conducted a study on murine lung ischemia/reperfusion (I/R) and in vitro hypoxia/reoxygenation (H/R) models demonstrating that pre-treatment of mesenchymal stromal cells with miR-21-5p antagomiR ameliorated IRI in the lung. Regarding PAH, Pullamsetti et al. [34] conducted a study both on animal models and cell lines demonstrating that Ant-17 and Ant-21 reduced right ventricular systolic pressure and pulmonary arterial muscularization. Moreover, Ant-17 decreased hypoxia-induced right ventricular hypertrophy and improved pulmonary artery acceleration time. In mice, Ant-17 therapy reduced right ventricular systolic pressure and total pulmonary vascular resistance index, stabilized cardiac output and reduced pulmonary vascular remodeling. In human pulmonary artery smooth muscle cells, Ant-17 increased the cyclin-dependent kinase inhibitor 1A (p21). MiRNA 21 demonstrated to have a role also upon lung fibrosis. In fact, Shentu et al. [35] demonstrated that human mesenchymal stem cell-derived extracellular vesicles (mEVs) do contain several specific miRNAs including 21-5p and 630. MEVs suppress TGFβ1-induced myofibroblastic differentiation of normal and idiopathic pulmonary fibrosis (IPF) lung fibroblasts, thus mitigating tissue fibrotic response. Investigating the role of miRNA regarding the pathogenesis and progression of lung fibrosis, Liu et al. [36] found that miR-21 was up-regulated both in the lungs of mice presenting with bleomycin-induced lung fibrosis and IPF patients. In this setting, miR-21 was highly expressed by myofibroblasts in the fibrotic lungs. Furthermore, researchers noticed that miR-21 reduced bleomycin-induced lung fibrosis in rats’ lungs.
Overall, a simple explanation of the mechanisms involving antagomiR-21 in lung conditions is provided in Figure 1.
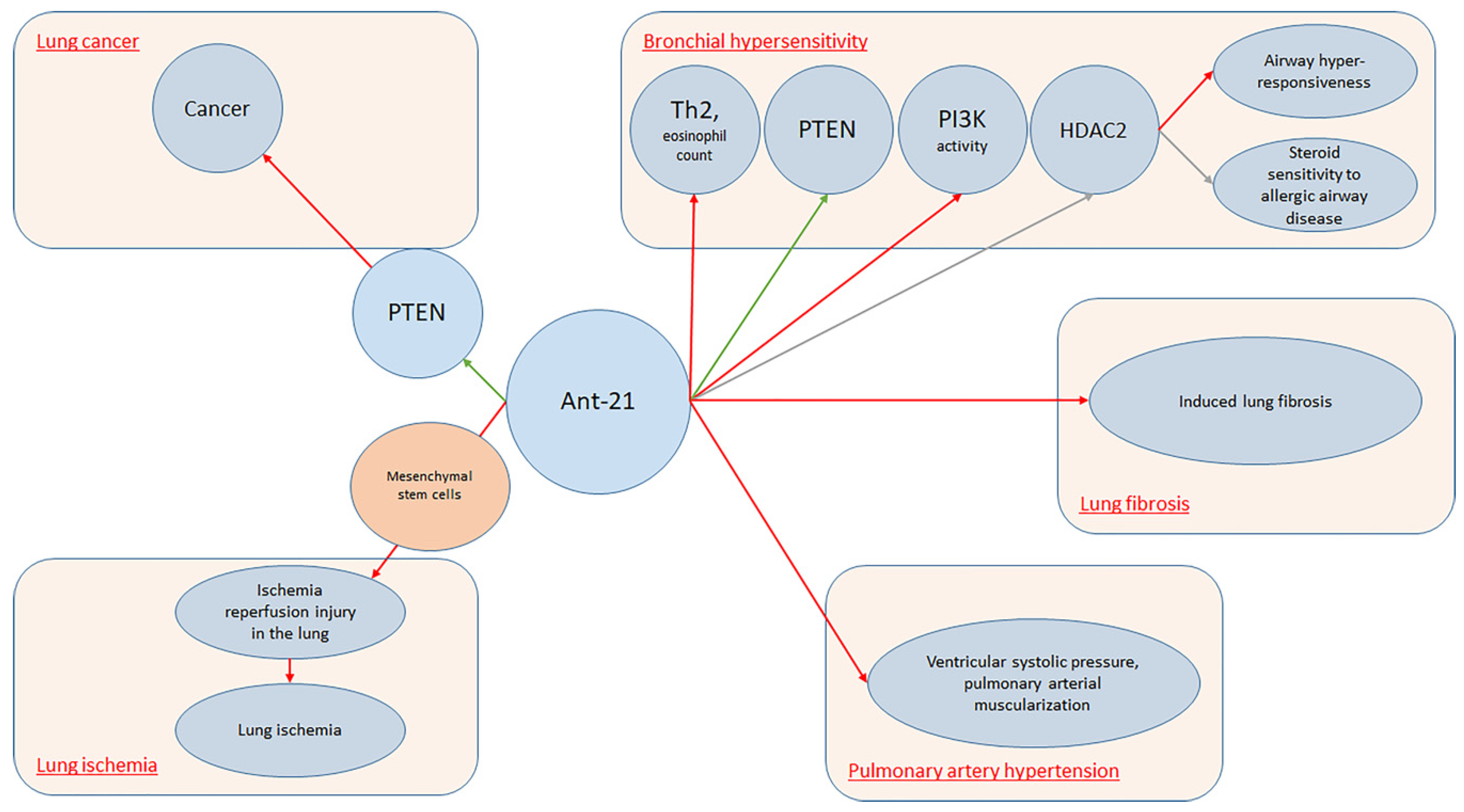
Figure 1. AntagomiR-21 in lung conditions.
Another miRNA molecule, which is widely expressed in several lung conditions, is miR-155. Several studies demonstrated that this molecule is upregulated in activated immune cells, such as T and B lymphocytes, macrophages, and dendritic cells (DCs). Indeed, miR-155 levels increase in response to inflammatory mediators. Moreover, miRNA-155 can regulate B-cell proliferation, malignancy, antibody production, and the differentiation and function of IL-17-producing helper T cells. Furthermore, miR-155 is induced by LPS, as well as other TLR ligands and proinflammatory cytokines [37][38][39]. As miR-155 is involved in several processes, it is feasible to understand its role upon many lung disorders. Basing on the hypothesis that miR-155 upregulation could inhibit IL-17 expression and therefore increase susceptibility to secondary bacterial pneumonia, Podsiad et [40] al. conducted a study on wild-type C57BL/6 mice and human lung macrophages in order to investigate the role of miR-155 and the respective antagoMiR upon viral and bacterial pneumonia. They concluded that miR-155 antagomiR ameliorated lung bacterial clearance compared with controls. MiR-155 plays a crucial role also upon ARDS. Triggering receptors expressed on myeloid cells (TREM) proteins are a family of immunoglobulin cell surface receptors expressed on myeloid cells and they are considered as amplifiers of Toll-like receptor (TLR)-induced inflammation. Experiments with antagomiR-155 confirmed that TREM-1-mediated changes were dependent on miR-155. Yuan et al. [41] conducted a study on wild-type C57BL/6J mice and bone marrow-derived macrophages demonstrating that TREM-1 boosted inflammatory response by inducing the expression of miR-155 in macrophages. Therefore, researchers inhibited TREM-1 using a nanomicellar strategy. Neutrophilic inflammation was reduced, thus suggesting that TREM-1 inhibition is a potential therapeutic target for neutrophilic lung inflammation and ARDS. Systemic lupus erythematosus (SLE) is a complex auto-immune disease which can involve several systems, including lungs. Diffuse alveolar hemorrhage (DAH) is a rare but severe complication of SLE and miR-155 showed to have a relevant role. In fact, Zhou et al. [42] found that miR-155 expression was up-regulated during the development of DAH, noticing that this molecule targets several pro-inflammatory mediators. The extent of lung inflammation was markedly reduced in miR-155–knockout (miR-1552/2) mice. Moreover, in vivo silencing of miR-155 using miR-155 antagomiR reduced the incidence of iatrogenic-induced DAH. MiR-155 cooperates with Th2 responses too. In fact, it is extensively expressed in the Th cell, DCs, and macrophages in the lung. MiR-155 was also found to be up-regulated in the nasal mucosa and airway smooth muscle cells of allergic asthmatic patients [30][38][39]. Recently, Plank et al. [43] conducted a study on murine asthmatic models noticing that MiR-155-5p is highly upregulated in mice. However, while targeting of miR-155-5p with a specific antagomiR resulted in specific inhibition in vivo, it was not able to alter the disease phenotype. Authors hypothesized that this could be due to the variation in antagomiR uptake, which demonstrated to be effective in myeloid cells and weak in lymphocytes. Cystic fibrosis (CF) is an autosomal recessive disease, due to the occurrence of cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations and it is characterized by a variable cytokines pro-inflammatory milieu. Bhattacharyya et al. [44] demonstrated that antagomiR-155 down-regulates miR-155 expression suppressing IL-8 and other proinflammatory genes in CF cells.
The mechanisms involving antagomiR-155 in lung conditions are displayed in Figure 2.
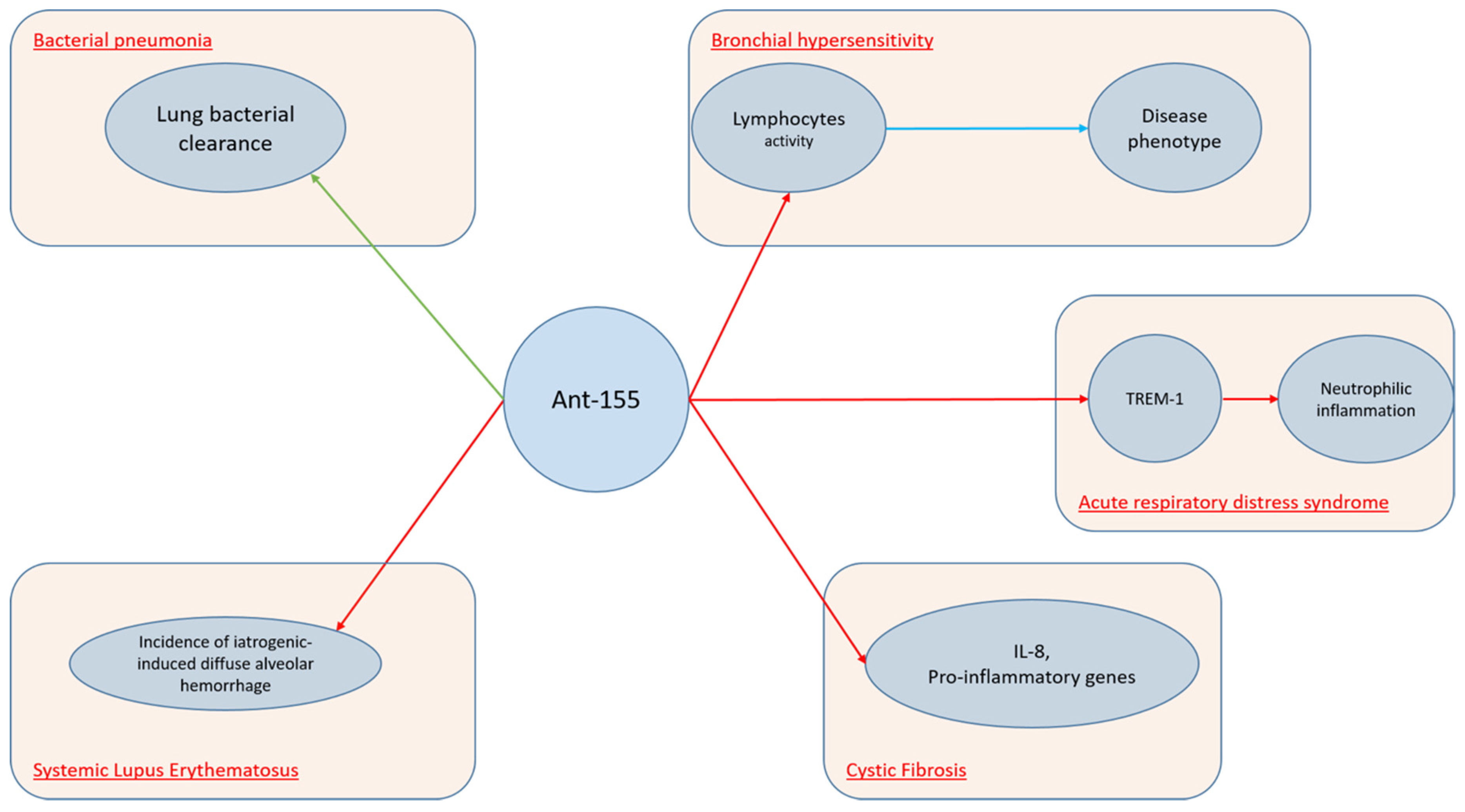
Figure 2. AntagomiR-155 in lung conditions.
References
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microrna biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610.
- Bartel, D.P. Metazoan micrornas. Cell 2018, 173, 20–51.
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomirnas and mimicking tumor suppressor mirnas: Nuew trends in the development of mirna therapeutic strategies in oncology (review). Int. J. Oncol. 2016, 49, 5–32.
- Nguyen, D.D.; Chang, S. Development of novel therapeutic agents by inhibition of oncogenic micrornas. Int. J. Mol. Sci. 2017, 19.
- Jadideslam, G.; Ansarin, K.; Sakhinia, E.; Babaloo, Z.; Abhari, A.; Ghahremanzadeh, K.; Khalili, M.; Radmehr, R.; Kabbazi, A. Diagnostic biomarker and therapeutic target applications of mir-326 in cancers: A systematic review. J. Cell Physiol. 2019.
- Yoo, B.H.; Bochkareva, E.; Bochkarev, A.; Mou, T.C.; Gray, D.M. 2’-o-methyl-modified phosphorothioate antisense oligonucleotides have reduced non-specific effects in vitro. Nucleic Acids Res. 2004, 32, 2008–2016.
- Krutzfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of micrornas in vivo with ’antagomirs’. Nature 2005, 438, 685–689.
- Lee, S.H.; Jung, Y.D.; Choi, Y.S.; Lee, Y.M. Targeting of runx3 by mir-130a and mir-495 cooperatively increases cell proliferation and tumor angiogenesis in gastric cancer cells. Oncotarget 2015, 6, 33269–33278.
- Brognara, E.; Fabbri, E.; Montagner, G.; Gasparello, J.; Manicardi, A.; Corradini, R.; Bianchi, N.; Finotti, A.; Breveglieri, G.; Borgatti, M.; et al. High levels of apoptosis are induced in human glioma cell lines by co-administration of peptide nucleic acids targeting mir-221 and mir-222. Int. J. Oncol. 2016, 48, 1029–1038.
- Liang, Z.; Ahn, J.; Guo, D.; Votaw, J.R.; Shim, H. Microrna-302 replacement therapy sensitizes breast cancer cells to ionizing radiation. Pharm. Res. 2013, 30, 1008–1016.
- Shrine, N.; Guyatt, A.L.; Erzurumluoglu, A.M.; Jackson, V.E.; Hobbs, B.D.; Melbourne, C.A.; Batini, C.; Fawcett, K.A.; Song, K.; Sakornsakolpat, P.; et al. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat. Genet. 2019, 51, 481–493.
- Hobbs, B.D.; Tantisira, K.G. Micrornas in copd: Small molecules with big potential. Eur. Respir. J. 2019, 53.
- Faiz, A.; Steiling, K.; Roffel, M.P.; Postma, D.S.; Spira, A.; Lenburg, M.E.; Borggrewe, M.; Eijgenraam, T.R.; Jonker, M.R.; Koppelman, G.H.; et al. Effect of long-term corticosteroid treatment on microrna and gene-expression profiles in copd. Eur. Respir. J. 2019, 53.
- Heffler, E.; Allegra, A.; Pioggia, G.; Picardi, G.; Musolino, C.; Gangemi, S. Microrna profiling in asthma: Potential biomarkers and therapeutic targets. Am. J. Respir. Cell Mol. Biol. 2017, 57, 642–650.
- Yu, B.; Yao, L.; Liu, C.; Tang, L.; Xing, T. Upregulation of microrna16 alters the response to inhaled betaagonists in patients with asthma though modulating expression of adrb2. Mol. Med. Rep. 2019, 19, 4027–4034.
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 esc/ers guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (esc) and the european respiratory society (ers): Endorsed by: Association for european paediatric and congenital cardiology (aepc), international society for heart and lung transplantation (ishlt). Eur. Heart J. 2016, 37, 67–119.
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564.
- Zhou, T.; Garcia, J.G.; Zhang, W. Integrating micrornas into a system biology approach to acute lung injury. Transl. Res. 2011, 157, 180–190.
- Wang, Q.Z.; Xu, W.; Habib, N.; Xu, R. Potential uses of microrna in lung cancer diagnosis, prognosis, and therapy. Curr. Cancer Drug Targets 2009, 9, 572–594.
- Yu, S.L.; Chen, H.Y.; Chang, G.C.; Chen, C.Y.; Chen, H.W.; Singh, S.; Cheng, C.L.; Yu, C.J.; Lee, Y.C.; Chen, H.S.; et al. Microrna signature predicts survival and relapse in lung cancer. Cancer Cell 2008, 13, 48–57.
- Janssen, H.L.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of hcv infection by targeting microrna. N. Engl. J. Med. 2013, 368, 1685–1694.
- Bouchie, A. First microrna mimic enters clinic. Nat. Biotechnol. 2013, 31, 577.
- Liao, W.; Dong, J.; Peh, H.Y.; Tan, L.H.; Lim, K.S.; Li, L.; Wong, W.F. Oligonucleotide therapy for obstructive and restrictive respiratory diseases. Molecules 2017, 22.
- Mei, D.; Tan, W.S.D.; Wong, W.S.F. Pharmacological strategies to regain steroid sensitivity in severe asthma and copd. Curr. Opin. Pharmacol. 2019, 46, 73–81.
- Testa, U.; Pelosi, E.; Castelli, G.; Labbaye, C. Mir-146 and mir-155: Two key modulators of immune response and tumor development. Noncoding RNA 2017, 3.
- Sessa, R.; Hata, A. Role of micrornas in lung development and pulmonary diseases. Pulm. Circ. 2013, 3, 315–328.
- Panwar, B.; Omenn, G.S.; Guan, Y. Mirmine: A database of human mirna expression profiles. Bioinformatics 2017, 33, 1554–1560.
- Zhang, J.G.; Wang, J.J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G.H. Microrna-21 (mir-21) represses tumor suppressor pten and promotes growth and invasion in non-small cell lung cancer (nsclc). Clin. Chim. Acta 2010, 411, 846–852.
- Collison, A.; Mattes, J.; Plank, M.; Foster, P.S. Inhibition of house dust mite-induced allergic airways disease by antagonism of microrna-145 is comparable to glucocorticoid treatment. J. Allergy Clin. Immunol. 2011, 128, 160–167.e4.
- Kim, R.Y.; Horvat, J.C.; Pinkerton, J.W.; Starkey, M.R.; Essilfie, A.T.; Mayall, J.R.; Nair, P.M.; Hansbro, N.G.; Jones, B.; Haw, T.J.; et al. Microrna-21 drives severe, steroid-insensitive experimental asthma by amplifying phosphoinositide 3-kinase-mediated suppression of histone deacetylase 2. J. Allergy Clin. Immunol. 2017, 139, 519–532.
- Silveyra, P.; DiAngelo, S.L.; Floros, J. An 11-nt sequence polymorphism at the 3’utr of human sftpa1 and sftpa2 gene variants differentially affect gene expression levels and mirna regulation in cell culture. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L106–L119.
- Porteous, M.K.; Lee, J.C. Primary graft dysfunction after lung transplantation. Clin. Chest Med. 2017, 38, 641–654.
- Li, J.W.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic mir-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76.
- Pullamsetti, S.S.; Doebele, C.; Fischer, A.; Savai, R.; Kojonazarov, B.; Dahal, B.K.; Ghofrani, H.A.; Weissmann, N.; Grimminger, F.; Bonauer, A.; et al. Inhibition of microrna-17 improves lung and heart function in experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2012, 185, 409–419.
- Shentu, T.P.; Huang, T.S.; Cernelc-Kohan, M.; Chan, J.; Wong, S.S.; Espinoza, C.R.; Tan, C.; Gramaglia, I.; van der Heyde, H.; Chien, S.; et al. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Sci. Rep. 2017, 7, 18052.
- Liu, G.; Friggeri, A.; Yang, Y.; Milosevic, J.; Ding, Q.; Thannickal, V.J.; Kaminski, N.; Abraham, E. Mir-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 2010, 207, 1589–1597.
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. Microrna-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609.
- Comer, B.S.; Camoretti-Mercado, B.; Kogut, P.C.; Halayko, A.J.; Solway, J.; Gerthoffer, W.T. Cyclooxygenase-2 and microrna-155 expression are elevated in asthmatic airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2015, 52, 438–447.
- Suojalehto, H.; Toskala, E.; Kilpelainen, M.; Majuri, M.L.; Mitts, C.; Lindstrom, I.; Puustinen, A.; Plosila, T.; Sipila, J.; Wolff, H.; et al. Microrna profiles in nasal mucosa of patients with allergic and nonallergic rhinitis and asthma. Int. Forum Allergy Rhinol. 2013, 3, 612–620.
- Podsiad, A.; Standiford, T.J.; Ballinger, M.N.; Eakin, R.; Park, P.; Kunkel, S.L.; Moore, B.B.; Bhan, U. Microrna-155 regulates host immune response to postviral bacterial pneumonia via il-23/il-17 pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L465–L475.
- Yuan, Z.; Syed, M.; Panchal, D.; Joo, M.; Bedi, C.; Lim, S.; Onyuksel, H.; Rubinstein, I.; Colonna, M.; Sadikot, R.T. Trem-1-accentuated lung injury via mir-155 is inhibited by lp17 nanomedicine. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L426–L438.
- Zhou, S.; Wang, Y.; Meng, Y.; Xiao, C.; Liu, Z.; Brohawn, P.; Higgs, B.W.; Jallal, B.; Jia, Q.; Qu, B.; et al. In vivo therapeutic success of microrna-155 antagomir in a mouse model of lupus alveolar hemorrhage. Arthritis Rheumatol. 2016, 68, 953–964.
- Plank, M.W.; Maltby, S.; Tay, H.L.; Stewart, J.; Eyers, F.; Hansbro, P.M.; Foster, P.S. Microrna expression is altered in an ovalbumin-induced asthma model and targeting mir-155 with antagomirs reveals cellular specificity. PLoS ONE 2015, 10, e0144810.
- Bhattacharyya, S.; Balakathiresan, N.S.; Dalgard, C.; Gutti, U.; Armistead, D.; Jozwik, C.; Srivastava, M.; Pollard, H.B.; Biswas, R. Elevated mir-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J. Biol. Chem. 2011, 286, 11604–11615.
More
Information
Subjects:
Medicine, General & Internal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
531
Revisions:
2 times
(View History)
Update Date:
01 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




