
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe Murdaca | -- | 2666 | 2023-08-01 08:13:06 | | | |
| 2 | Peter Tang | Meta information modification | 2666 | 2023-08-01 08:24:05 | | |
Video Upload Options
Several allergic and immunologic diseases including asthma, food allergy (FA), chronic spontaneous urticaria (CSU), atopic dermatitis (AD), systemic lupus erythematosus (SLE), systemic sclerosis (SSc), rheumatoid arthritis (RA), and Behçet’s disease (BD) are characterized by the involvement of Th2 immunity. Several mediators lead to immunoglobulin (Ig)E production, thus including key cytokines such as interleukin (IL)-4, IL-5, and IL-13. Among them, IL-31 and IL-33 have been recently studied as novel biomarkers and future therapeutic targets for allergic and immunological disorders. IL-31 is a proinflammatory cytokine—it regulates cell proliferation and is involved in tissue remodeling. IL-33, acting through its receptor suppression of tumorigenity (ST2L), is an alarmin cytokine from the IL-1 family, whose expression is mediated by tissue damage. The latter has a pleiotropic effect, as it may modulate specific and innate immune cells functions.
1. Introduction
2. From IL-33 and IL-31 “Single Molecules” to the Idea of an IL-31/IL-33 Axis
3. Autoimmune Disorders
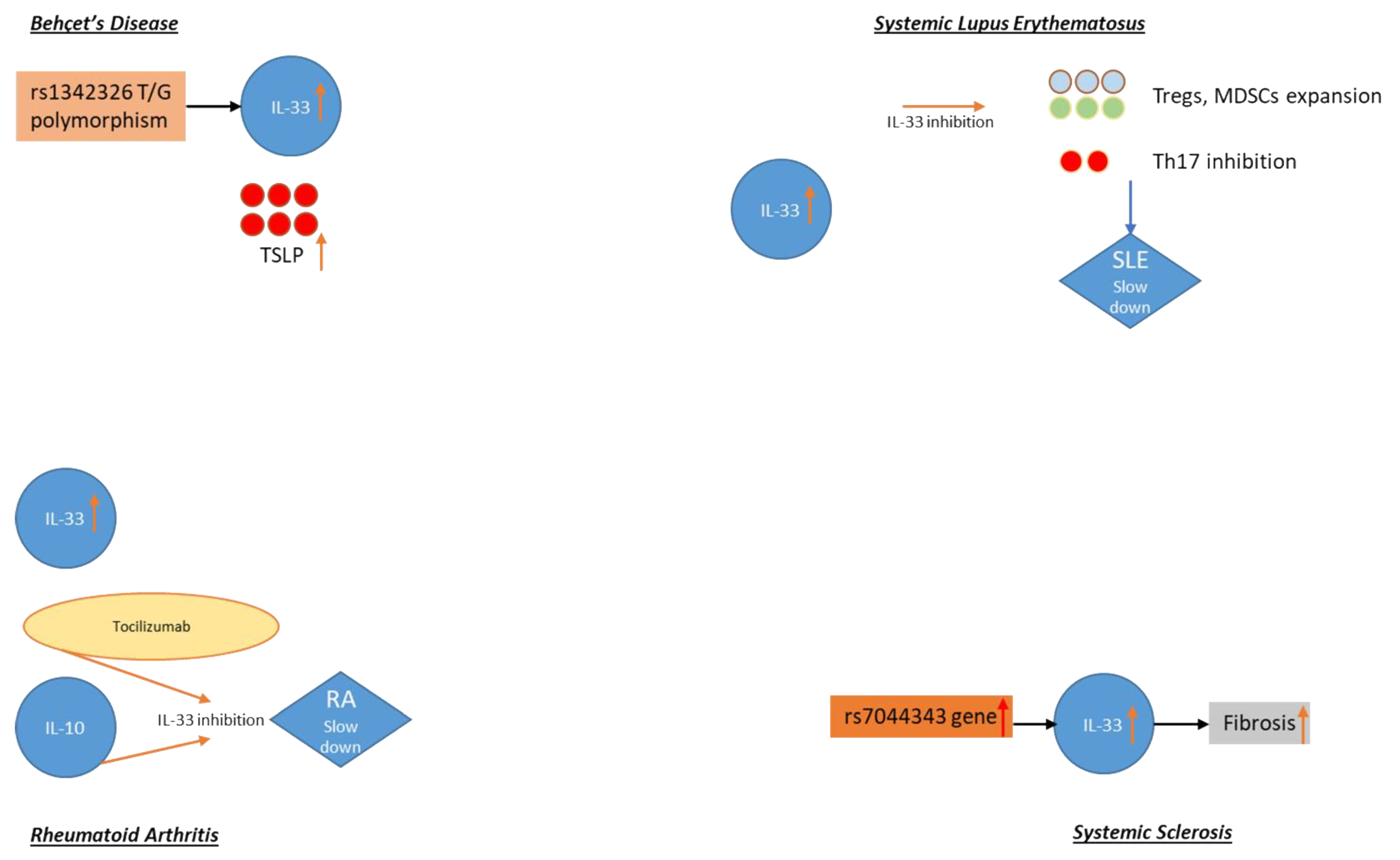
3.1. Behçet’s Disease
3.2. Systemic Lupus Erythematosus (SLE)
3.3. Rheumatoid Arthritis (RA)
3.4. Systemic Sclerosis (SSc)
References
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediat. Inflamm. 2018, 2018, 1–8.
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168.
- Ferretti, E.; Corcione, A.; Pistoia, V. The IL-31/IL-31 receptor axis: General features and role in tumor microenvironment. J. Leukoc. Biol. 2017, 102, 711–717.
- Ginaldi, L.; De Martinis, M.; Saitta, S.; Sirufo, M.M.; Mannucci, C.; Casciaro, M.; Ciccarelli, F.; Gangemi, S. Interleukin-33 serum levels in postmenopausal women with osteoporosis. Sci. Rep. 2019, 9, 3786.
- Ieni, A.; Casciaro, M.; Cardia, R.; Di Salvo, E.; Tuccari, G.; Ieni, A.; Gangemi, S. Interleukin-33 involvement in nonsmall cell lung carcinomas: An update. Biomolecules 2019, 9, 1–7.
- Yang, D.; Han, Z.; Oppenheim, J.J. Alarmins and immunity. Immunol. Rev. 2017, 280, 41–56.
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356.
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760.
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 29–36.
- Gibbs, B.F.; Patsinakidis, N.; Raap, U. Role of the Pruritic Cytokine IL-31 in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 1–6.
- Maier, E.; Werner, D.; Duschl, A.; Bohle, B.; Horejs, J. Europe PMC Funders Group Human Th2 but not Th9 cells release IL-31 in a STAT6/NF-κB- dependent way. J. Immunol. 2016, 193, 645–654.
- Stott, B.; Lavender, P.; Lehmann, S.; Pennino, D.; Durham, S.; Schmidt-Weber, C.B. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J. Allergy Clin. Immunol. 2013, 132, 446–454.
- Skef, W.; Hamilton, M.J.; Arayssi, T. Gastrointestinal behçet’s disease: A. review. World, J. Gastroenterol. 2015, 21, 3801–3812.
- Alpsoy, E. Behçet’s disease: A comprehensive review with a focus on epidemiology, etiology and clinical features, and management of mucocutaneous lesions. J. Dermatol. 2016, 43, 620–632.
- Tong, B.; Liu, X.; Xiao, J.; Su, G. Immunopathogenesis of Behcet’s disease. Front. Immunol. 2019, 10, 665.
- Talei, M.; Abdi, A.; Shanebandi, D.; Jadidi-Niaragh, F.; Khabazi, A.; Babaie, F.; Alipour, S.; Afkari, B.; Sakhinia, E.; Babaloo, Z. Interleukin-33 gene expression and rs1342326 polymorphism in Behçet’s disease. Immunol. Lett. 2019, 212, 120–124.
- Çerçi, P.; Altıner, S.; İnal, A.; Köse, K.; Keskin, G.; Ölmez, Ü. Investigating the role of IL-33 in the pathogenesis of Behçet’s Disease. Acta Clin. Belg. 2017, 72, 434–438.
- Kacem, O.; Kaabachi, W.; Dhifallah, I.; Ben Hamzaoui, A.; Hamzaoui, K. Elevated expression of TSLP and IL-33 in Behçet’s disease skin lesions: IL-37 alleviate inflammatory effect of TSLP. Clin. Immunol. 2018, 192, 14–19.
- Hamzaoui, K.; Borhani-Haghighi, A.; Kaabachi, W.; Hamzaoui, A. Increased interleukin 33 in patients with neuro-Behcet’s disease: Correlation with MCP-1 and IP-10 chemokines. Cell. Mol. Immunol. 2014, 11, 613–616.
- Takeuchi, M.; Karasawa, Y.; Harimoto, K.; Tanaka, A.; Shibata, M.; Sato, T.; Caspi, R.R.; Ito, M. Analysis of Th Cell-related Cytokine Production in Behçet Disease Patients with Uveitis Before and After Infliximab Treatment. Ocul. Immunol. Inflamm. 2017, 25, 52–61.
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019, 71, 1400–1412.
- D’Cruz, D.P.; Khamashta, M.A.; Hughes, G.R. Systemic lupus erythematosus. Lancet 2007, 369, 1208–1219.
- Ghodke-Puranik, Y.; Niewold, T.B. Immunogenetics of systemic lupus erythematosus: A comprehensive review. J. Autoimmun. 2015, 64, 125–136.
- Ippolito, A.; Petri, M. An update on mortality in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2008, 26, S72–79.
- Yang, Z.; Liang, Y.; Xi, W.; Li, C.; Zhong, R. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin. Exp. Med. 2011, 11, 75–80.
- Guo, J.; Xiang, Y.; Peng, Y.F.; Huang, H.T.; Lan, Y.; Wei, Y.S. The association of novel IL-33 polymorphisms with sIL-33 and risk of systemic lupus erythematosus. Mol. Immunol. 2016, 77, 1–7.
- Li, P.; Lin, W.; Zheng, X. IL-33 neutralization suppresses lupus disease in lupus-prone mice. Inflammation 2014, 37, 824–832.
- Zhu, X.; Xie, L.; Qin, H.; Liang, J.; Yang, Y.; Xu, J.; Zhang, T. Interaction between IL-33 gene polymorphisms and current smoking with susceptibility to systemic lupus erythematosus. J. Immunol. Res. 2019, 2019, 1547578.
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348.
- Huizinga, T.; Knevel, R. Rheumatoid arthritis: 2014 treat-To-Target RA recommendations-Strategy is key. Nat. Rev. Rheumatol. 2015, 11, 509–511.
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361.
- Chen, S.; Chen, B.; Wen, Z.; Huang, Z.; Ye, L. IL-33/ST2-mediated inflammation in macrophages is directly abrogated by IL-10 during rheumatoid arthritis. Oncotarget 2017, 8, 32407–32418.
- Macedo, R.B.V.; Kakehasi, A.M.; de Andrade, M.V.M. IL33 in rheumatoid arthritis: Potencial contribution to pathogenesis. Rev. Bras. Reumatol. 2016, 56, 451–457.
- Sellam, J.; Rivière, E.; Courties, A.; Rouzaire, P.O.; Tolusso, B.; Vital, E.V.; Emery, P.; Ferraccioli, G.; Soubrier, M.; Ly, B.; et al. Serum IL-33, a new marker predicting response to rituximab in rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 1–8.
- Choi, I.A.; Lee, S.J.; Park, W.; Park, S.H.; Shim, S.C.; Baek, H.J.; Yoo, D.H.; Kim, H.A.; Lee, S.K.; Lee, Y.J.; et al. Effects of tocilizumab therapy on serum interleukin-33 and interleukin-6 levels in patients with rheumatoid arthritis. Arch. Rheumatol. 2018, 33, 389–394.
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879.
- Rivière, E.; Sellam, J.; Pascaud, J.; Ravaud, P.; Gottenberg, J.E.; Mariette, X. Serum IL-33 level is associated with auto-antibodies but not with clinical response to biologic agents in rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 18–20.
- Sullivan, K.M.; Goldmuntz, E.A.; Keyes-Elstein, L.; McSweeney, P.A.; Pinckney, A.; Welch, B.; Mayes, M.D.; Nash, R.A.; Crofford, L.J.; Eggleston, B.; et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N. Engl. J. Med. 2018, 378, 35–47.
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699.
- Nagaraja, V.; Denton, C.P.; Khanna, D. Old medications and new targeted therapies in systemic sclerosis. Rheumatology 2015, 54, 1944–1953.
- Manetti, M.; Guiducci, S.; Ceccarelli, C.; Romano, E.; Bellando-Randone, S.; Conforti, M.L.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Increased circulating levels of interleukin 33 in systemic sclerosis correlate with early disease stage and microvascular involvement. Ann. Rheum. Dis. 2011, 70, 1876–1878.
- Vettori, S.; Cuomo, G.; Iudici, M.; D’Abrosca, V.; Giacco, V.; Barra, G.; De Palma, R.; Valentini, G. Early Systemic Sclerosis: Serum Profiling of Factors Involved in Endothelial, T-cell, and Fibroblast Interplay is Marked by Elevated Interleukin-33 Levels. J. Clin. Immunol. 2014, 34, 663–668.
- Terras, S.; Opitz, E.; Moritz, R.K.; Höxtermann, S.; Gambichler, T.; Kreuter, A. Increased serum IL-33 levels may indicate vascular involvement in systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 144–145.
- Zhang, Y.J.; Zhang, Q.; Yang, G.J.; Tao, J.H.; Wu, G.C.; Huang, X.L.; Duan, Y.; Li, X.P.; Ye, D.Q.; Wang, J. Elevated serum levels of interleukin-1β and interleukin-33 in patients with systemic sclerosis in Chinese population. Z. Rheumatol. 2018, 77, 151–159.
- Wagner, A.; Köhm, M.; Nordin, A.; Svenungsson, E.; Pfeilschifter, J.M.; Radeke, H.H. Increased Serum Levels of the IL-33 Neutralizing sST2 in Limited Cutaneous Systemic Sclerosis. Scand. J. Immunol. 2015, 82, 269–274.
- Yanaba, K.; Yoshizaki, A.; Asano, Y.; Kadono, T.; Sato, S. Serum IL-33 levels are raised in patients with systemic sclerosis: Association with extent of skin sclerosis and severity of pulmonary fibrosis. Clin. Rheumatol. 2011, 30, 825–830.




