Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jin-ping Shu | -- | 1378 | 2023-08-01 03:01:31 | | | |
| 2 | Jessie Wu | Meta information modification | 1378 | 2023-08-01 07:23:28 | | | | |
| 3 | Jessie Wu | Meta information modification | 1378 | 2023-08-01 10:14:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Geng, X.; Liu, Y.; Li, J.; Li, Z.; Shu, J.; Wu, G. Nectria pseudotrichia Associated with Camellia Canker Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/47459 (accessed on 07 February 2026).
Geng X, Liu Y, Li J, Li Z, Shu J, Wu G. Nectria pseudotrichia Associated with Camellia Canker Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/47459. Accessed February 07, 2026.
Geng, Xiansheng, Ying Liu, Jiyuan Li, Zhihong Li, Jinping Shu, Guiyang Wu. "Nectria pseudotrichia Associated with Camellia Canker Disease" Encyclopedia, https://encyclopedia.pub/entry/47459 (accessed February 07, 2026).
Geng, X., Liu, Y., Li, J., Li, Z., Shu, J., & Wu, G. (2023, August 01). Nectria pseudotrichia Associated with Camellia Canker Disease. In Encyclopedia. https://encyclopedia.pub/entry/47459
Geng, Xiansheng, et al. "Nectria pseudotrichia Associated with Camellia Canker Disease." Encyclopedia. Web. 01 August, 2023.
Copy Citation
Camellia japonica is a native tree species with high economic value that is widely cultivated in southern China. Canker disease was a serious threat to the growth of camellia trees. However, the pathogen causing this disease in China has not yet been reported.
Camellia japonica
canker disease
1. Background
Camellia japonica L. is an evergreen shrub or tree in the family Theaceae that is native to China, South Korea, and Japan [1][2]. Over the last 200 years, C. japonica has been gradually introduced into Europe, the Americas, and Oceania, and has become a popular garden plant in most warm-temperate regions of the world [2][3]. C. japonica is a multipurpose tree species with high ornamental, economic, and medicinal value. C. japonica is popular due to its bright color and beautiful flowers, which are responsible for its high ornamental value. The content of unsaturated fatty acids, such as oleic acid, in the seeds of C. japonica accounts for more than 90% of the total fatty acid content, giving this species high economic value [4]. C. japonica plants have been found to contain triterpenes, saponins, flavonoids, tannins, fatty acids, and phenolic compounds, which exhibit various biological activities, including antioxidant, antiaging, antiviral, anti-inflammatory, antihyperuricemic, antiosteoporotic, and antiallergic activities [1][5][6].
Canker disease was first described in C. japonica in the United States in 1924 [7] and was subsequently observed in the United Kingdom [8], Spain [9] and China. The causal agent of this disease was reported to be Glomerella cingulate (Stonem) Spauld & Schrenk in the United States [10] and in the United Kingdom [8] and to be Neofusicoccum luteum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips, and N. parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips in Spain [9]. However, the pathogen causing this disease in China has not yet been reported. In recent years, canker disease in C. japonica has been found in many areas of Zhejiang Province, China. Among these infections, C. japonica plantations in Ningbo and Jinhua are the most seriously impacted, with more than 20% of camellia plants being infected. In Zhejiang Province, canker disease has only been observed on the trunk and branches of C. japonica. Mildly infected plants exhibit large necrotic lesions on the bark, while severe infection can cause the death of the entire branch or even the entire tree. Canker disease has become the most serious disease affecting C. japonica.
2. Field Surveys of Canker Disease in C. japonica
Field surveys indicated that canker disease occurred on both camellia plantations, and that the infections only occurred in C. japonica cultivar ‘Hongluzhen’. The disease incidence rates of C. japonica ‘Hongluzhen’ in Ningbo and Jinhua were 46.2% ± 9.4% and 20.0% ± 0.0%, respectively.
The pathogenic fungus can infect the trunk and branches of C. japonica ‘Hongluzhen’. When the pathogen infects the trunk, it often causes the necrosis of inner bark and xylem surface tissues, revealing the xylem (Figure 1a). In natural conditions, it is difficult for a callus to form around the lesion, meaning that it is possible for the lesion to continuously expand, forming longer and larger lesions. When the branches are infected, the bark of the infected regions becomes dark and slightly sunken (Figure 1b), and light red synnematal structures are present on some of the portions of diseased bark. The synnema were erumpent through the epidermis, solitary, slightly spherical at the top, 284.0–414.0 μm in diameter, and had a long (1323.0–1584.0 μm) stipe at the base (Figure 1c).

Figure 1. Disease symptoms and synnematal morphology of the pathogenic fungus. (a,b) Trunk canker and branch canker observed on C. japonica ‘Hongluzhen’; (c) synnemata on diseased bark.
3. Isolation, Morphological Structures and Cultural Characteristics of the Pathogenic Fungus of Camellia Canker
In this research, a total of 15 samples were collected, and Nectriaceae-like fungi were grown on PDA. The isolates grew quickly on PDA, with colony diameters of 8.1 ± 1.6 cm after 6 days of incubation at 25 °C. The upper side of the colonies on PDA was red at the center, and the color gradually lightened from the center to the margin of each colony (Figure 2a). The reverse side of each colony was red at the center and was creamy at the margin (Figure 2b). The isolates formed colorless colonies on SNA (Figure 2c). Vegetative hyphae were observed on SNA, but no aerial hyphae were present (Figure 2c). When the PDA plate was observed using the VHX-5000 microscope, the conidiophores, conidial mass, and conidia were observed in the center of each colony (Figure 2d). The conidia varied in size and morphology on PDA, with young conidia that were ellipsoidal to cylindrical in shape and that were generally 4.5–5.6 μm × 2.6–3.3 μm in size, and mature conidia that were ellipsoidal, cylindrical to allantoid in shape, and approximately 9.1–12.2 μm × 2.9–3.9 μm in size. When the isolate was cultured on PDA for 20 days, a large number of synnemata were produced near the side wall of the Petri dish (Figure 2e). However, only a small number of synnemata were produced on SNA after 60 days (Figure 2f).
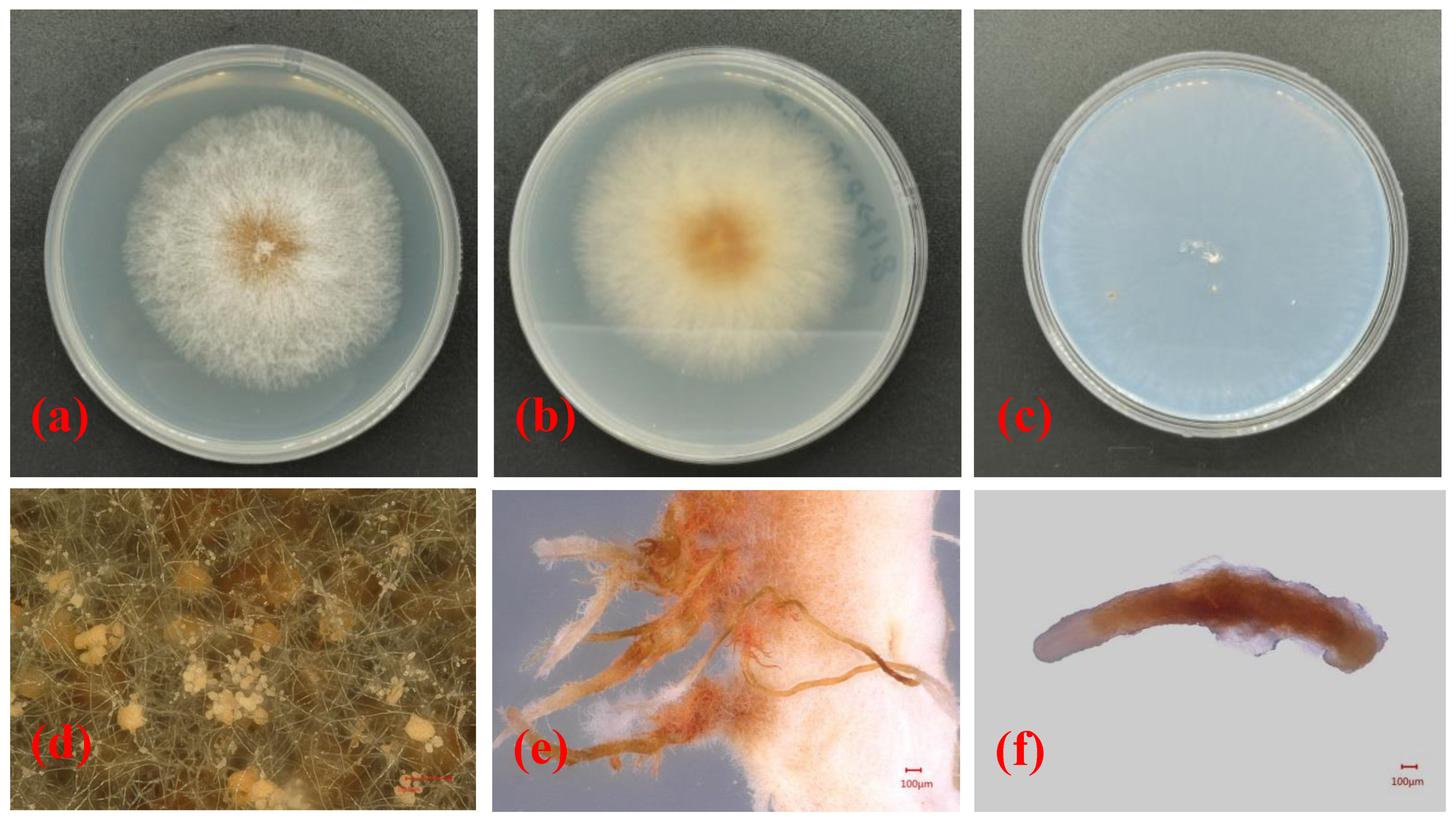
Figure 2. Morphological structures and cultural characteristics of Nectria pseudotrichia. (a,b) Upper and reverse sides of colonies on PDA, respectively; (c) upper side of colony on SNA; (d) conidial masses produced on PDA; (e,f) synnemata produced on PDA and SNA, respectively.
Based on the morphological characteristics of the mycelia, synnema, conidiophores, conidial mass, and conidia of the isolated pathogenic fungi as well as their cultural characteristics on PDA and SNA, the pathogen affecting camellia C. japonica in Zhejiang Province was preliminarily identified as Nectria pseudotrichia.
4. Phylogenetic Analysis
Phylogenetic analyses of 29 strains of Nectria were performed using the concatenated datasets for three loci. A total of 1561 characters (LSU: 1–799, ITS: 800–1308, tef1-α: 1309–1561) were included in the datasets. For Bayesian inference (BI) analysis, GTR + I + G, GTR + I + G, and SYM + G were selected as the best nucleotide substitution models for LSU rDNA, ITS rDNA, and tef1-α, respectively. The phylogenetic tree that was generated from BI analysis showed that the isolate LPNP clustered with 10 strains of N. pseudotrichia from Genbank and had a high posterior probability value (Figure 3). Multilocus phylogenetic analysis confirmed the assignment of the isolate LPNP as a strain of N. pseudotrichia. Multilocus phylogenetic analysis also revealed that within the N. pseudotrichia Clade, the LPNP strain and the two strains ICMP2245 and MAFF241394 from Japan clustered into a subclade, indicating that the genetic distance between these three strains is the closest (Figure 3).
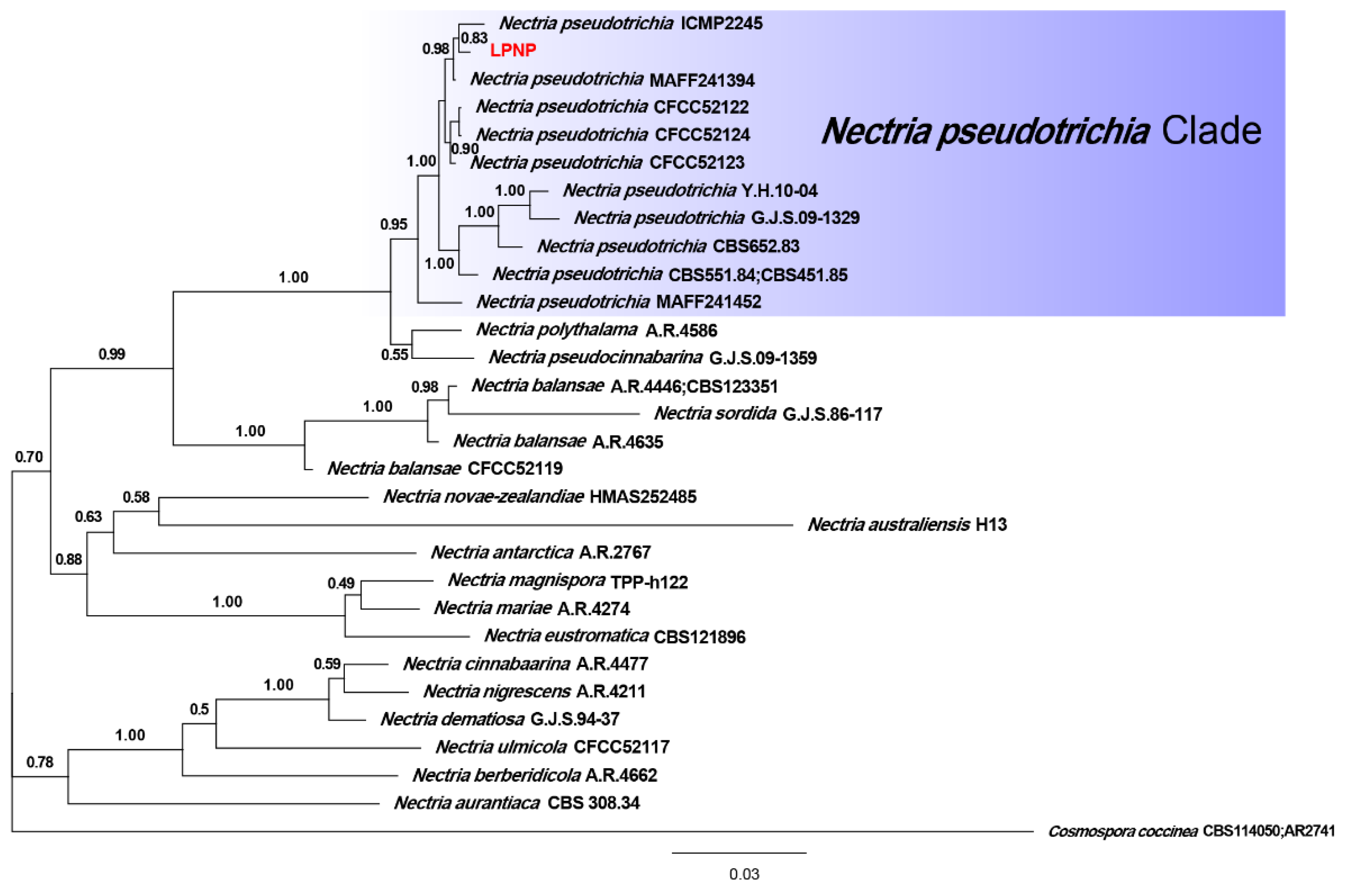
Figure 3. Phylogenetic tree inferred from BI analysis based on concatenated alignments of LSU rDNA, ITS rDNA, and tef1-α sequences. Cosmospora coccinea was used as an outgroup. Numbers on the nodes represent Bayesian posterior probabilities. The isolate obtained in the current study is highlighted in red.
5. Pathogenicity Tests
At 28 days after inoculation, symptoms were observed at the inoculation site on the stem of C. japonica ‘Hongluzhen’ (Figure 4a,b). The stems of the seedlings that had been inoculated with sterile PDA plugs produced a large amount of callus at the inoculated site, and the bark surface was flat with no necrotic lesions beneath the bark (Figure 4c,d). However, the stems of seedlings that had been inoculated with N. pseudotrichia produced a negligible callus at the inoculation site; the bark was slightly sunken, and necrotic lesions had formed beneath it (Figure 4a,b). In addition, at 14 days after inoculation, the detached leaves of the seedlings that had been inoculated with N. pseudotrichia showed no lesions (Figure 4e), indicating that N. pseudotrichia had no pathogenicity to the leaves of C. japonica ‘Hongluzhen’. In the re-isolation experiment, a fungus could be isolated from the necrotic tissue of symptomatic seedlings, and the morphological characterization as well as the ITS rDNA sequence of the isolate were consistent with those of LPNP. This pathogen was not isolated from the bark around the inoculation sites of negative control seedlings.
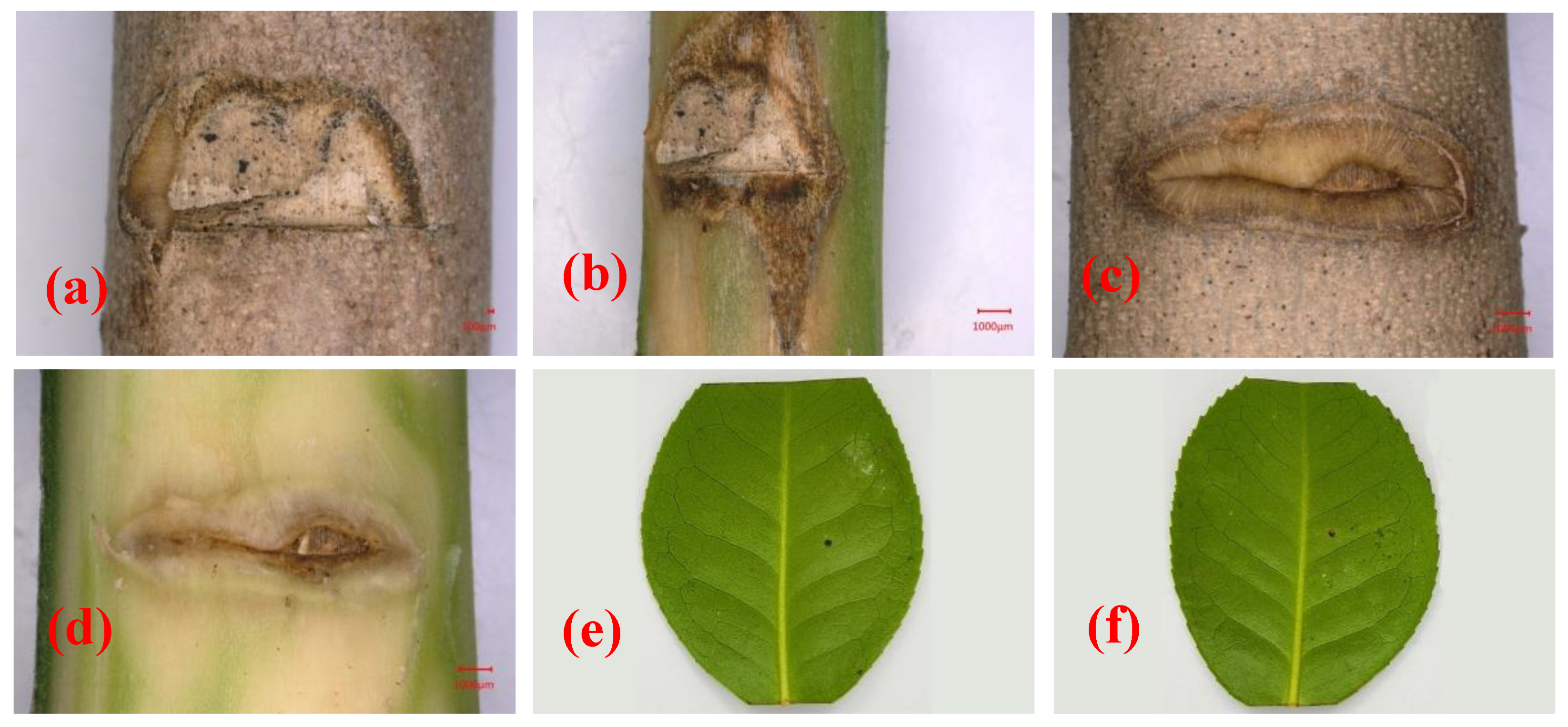
Figure 4. Pathogenicity analysis of Nectria pseudotrichia on the stems and detached leaves of Camellia japonica ‘Hongluzhen’. (a,b) Lesions on and beneath the bark of a camellia stem inoculated with N. pseudotrichia, respectively; (c,d) lesions on and beneath the bark of a camellia stem inoculated with a sterile PDA plug, respectively; (e,f) lesions on detached leaves inoculated with sterile PDA and N. pseudotrichia, respectively.
N. pseudotrichia can cause necrotic lesions on the stem of C. japonica and can be re-isolated from necrotic tissue, indicating that N. pseudotrichia is the causal agent of camellia canker disease in Zhejiang Province, China.
References
- Lim, T.K. (Ed.) Camellia japonica. In Edible Medicinal and Non Medicinal Plants; Springer: Dordrecht, The Netherlands, 2014; Volume 8, pp. 764–776.
- Gao, J.Y.; Parks, C.R.; Du, Y.Q. Collected Species of the Genus Camellia: An Illustrated Outline; Zhejiang Science and Technology Press: Hangzhou, China, 2005; pp. 43–44.
- Vela, P.; Salinero, C.; Sainz, M. Phenological growth stages of Camellia japonica. Ann. Appl. Biol. 2012, 162, 182–190.
- Garcia-Jares, C.; Sanchez-Nande, M.; Lamas, J.P.; Lores, M. Profiling the Fatty Acids Content of Ornamental Camellia Seeds Cultivated in Galicia by an Optimized Matrix Solid-Phase Dispersion Extraction. Bioengineering 2017, 4, 87.
- Zhang, Y.-L.; Yin, C.-P.; Kong, L.-C.; Jiang, D.-H. Extraction optimisation, purification and major antioxidant component of red pigments extracted from Camellia japonica. Food Chem. 2011, 129, 660–664.
- Yoon, I.-S.; Park, D.-H.; Kim, J.-E.; Yoo, J.-C.; Bae, M.-S.; Oh, D.-S.; Shim, J.-H.; Choi, C.-Y.; An, K.-W.; Kim, E.-I.; et al. Identification of the biologically active constituents of Camellia japonica leaf and anti-hyperuricemic effect in vitro and in vivo. Int. J. Mol. Med. 2017, 39, 1613–1620.
- Baxter, L.W. Studies on Control of Root Rot and Dieback of Camellias; Louisiana State University: Baton Rouge, FL, USA, 1955.
- Dickens, J.S.W.; Cook, R.T.A. Glomerella cingulata on camellia. Plant Pathol. 1989, 38, 75–85.
- Pintos, C.; Redondo, V.; Chaves, M.; Rial, C.; Mansilla, P. Camellia japonica dieback caused by Neofusicoccum luteum and N. parvum in Spain. In Proceedings of the International Camellia Congress, Chuxiong, China, 5–9 February 2012.
- Ngo, H.C.; Baxter, L.W.; Fagan, S.G. The status of our knowledge in 1978 of twig blight, canker and dieback of camellias caused by a strain of Glomerella cingulata. Am. Camellia Yearb. 1978, 33, 75–95.
More
Information
Subjects:
Pathology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
749
Revisions:
3 times
(View History)
Update Date:
01 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




