Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamin Ali | -- | 3394 | 2023-07-31 13:36:39 | | | |
| 2 | Lindsay Dong | Meta information modification | 3394 | 2023-08-01 02:44:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ali, J.; Bayram, A.; Mukarram, M.; Zhou, F.; Karim, M.F.; Hafez, M.M.A.; Mahamood, M.; Yusuf, A.A.; King, P.J.H.; Adil, M.F.; et al. Management Strategies of Peach–Potato Aphid Myzus persicae. Encyclopedia. Available online: https://encyclopedia.pub/entry/47435 (accessed on 14 January 2026).
Ali J, Bayram A, Mukarram M, Zhou F, Karim MF, Hafez MMA, et al. Management Strategies of Peach–Potato Aphid Myzus persicae. Encyclopedia. Available at: https://encyclopedia.pub/entry/47435. Accessed January 14, 2026.
Ali, Jamin, Ahmet Bayram, Mohammad Mukarram, Fanrui Zhou, Muhammad Fazal Karim, Mogeda Mohammed Abdel Hafez, Mohammad Mahamood, Abdullahi Ahmed Yusuf, Patricia Jie Hung King, Muhammad Faheem Adil, et al. "Management Strategies of Peach–Potato Aphid Myzus persicae" Encyclopedia, https://encyclopedia.pub/entry/47435 (accessed January 14, 2026).
Ali, J., Bayram, A., Mukarram, M., Zhou, F., Karim, M.F., Hafez, M.M.A., Mahamood, M., Yusuf, A.A., King, P.J.H., Adil, M.F., Ma, Z., & Shamsi, I.H. (2023, July 31). Management Strategies of Peach–Potato Aphid Myzus persicae. In Encyclopedia. https://encyclopedia.pub/entry/47435
Ali, Jamin, et al. "Management Strategies of Peach–Potato Aphid Myzus persicae." Encyclopedia. Web. 31 July, 2023.
Copy Citation
The peach–potato aphid, Myzus persicae (Sulzer), is one of the most important pests of economic crops. It damages the plant directly by consuming nutrients and water and indirectly by transmitting plant viruses. This pest has the unenviable title of having resistance to more insecticides than any other herbivorous insect pest. Due to the development of its resistance to chemical pesticides, it is necessary to find other control options. Consequently, increased efforts worldwide have been undertaken to develop new management approaches for M. persicae.
Myzus persicae
biocontrol agents
integrated pest management
parasitoid
insecticide resistance
crop protection
1. Introduction
Aphids are one of the most economically important hemipteran pests, with about 100 aphid species being reported to cause significant agricultural losses worldwide [1][2]. Aphids are silent feeders that cause less tissue damage than chewing insects and, during feeding, release effector proteins in their saliva that suppress host plant defence responses [3]. Damage caused by aphids reduces plant fitness due to their feeding on phloem sap, which makes the plant nutritionally weaker compared to uninfested ones [4][5]. They also serve as important vectors for the transmission of plant viruses and produce honeydew that serves as a breeding medium for pathogens, which affect plant photosynthetic activities [2][3]. The peach–potato aphid, M. persicae, also known as the green peach aphid, is a highly polyphagous and cosmopolitan pest that has a global distribution, predominantly in North America, Europe, and Asia [3][6]. Winged adults measure up to 2.1 mm and reproduce parthenogenetically by a single sexual generation with a life cycle of 15 days [7]. In terms of damage, M. persicae causes direct and indirect damage to a wide range of crop plants [6][8][9][10].
The peach–potato aphid is considered one of the most destructive agricultural pests that feeds on over 40 plant families including Apiaceae (carrot, Daucus carota (Hoffmann)); Asteraceae (lettuce, Lactuca sativa (Linnaeus); artichoke, Cynara cardunculus (L.)); Amaranthaceae (beet, Beta vulgaris (L.); spinach, Spinacia oleracea (L.)); Brassicaceae (broccoli, Brassica oleracea var. italica (L.); brussels sprouts, Brassica oleracea var. gemmifera; cabbage, Brassica oleracea var. capitata (L.); cauliflower, Brassica oleracea var. botrytis (L.); kale, Brassica oleracea var. acephala (L.); mustard, Brassica juncea (L.); radish, Raphanus raphanistrum (L.); and turnip, Brassica rapa (L.)); Cucurbitaceae (cucumber, Cucmis sativus (L.); squash, Cucurbita pepo L.)); Fabaceae (bean, Phaseolus vulgaris (L.); pea, Pisum sativum (L.)); Poaceae (maize, Zea mays (L.); wheat, Triticum aestivum (L.); barley, Hordeum vulgare (L.); and rice, Oryza sativa (L.)); and Solanaceae (potato, Solanum tuberosum (L.); pepper, Capsicum annuum (L.); and tomato, Solanum lycopersicum (L.) [9][10][11][12][13][14]. Additionally, the peach–potato aphid acts as an important vector and transmits over hundreds of plant viruses, including potato leafroll virus (PLV), potato virus Y (PVY), beet western yellows viruses, beet yellows viruses, lettuce mosaic virus, cauliflower mosaic virus, turnip mosaic virus, cucumber mosaic, and watermelon mosaic viruses, which indirectly affect the growth and development of the host plant [3][9][15][16][17][18].
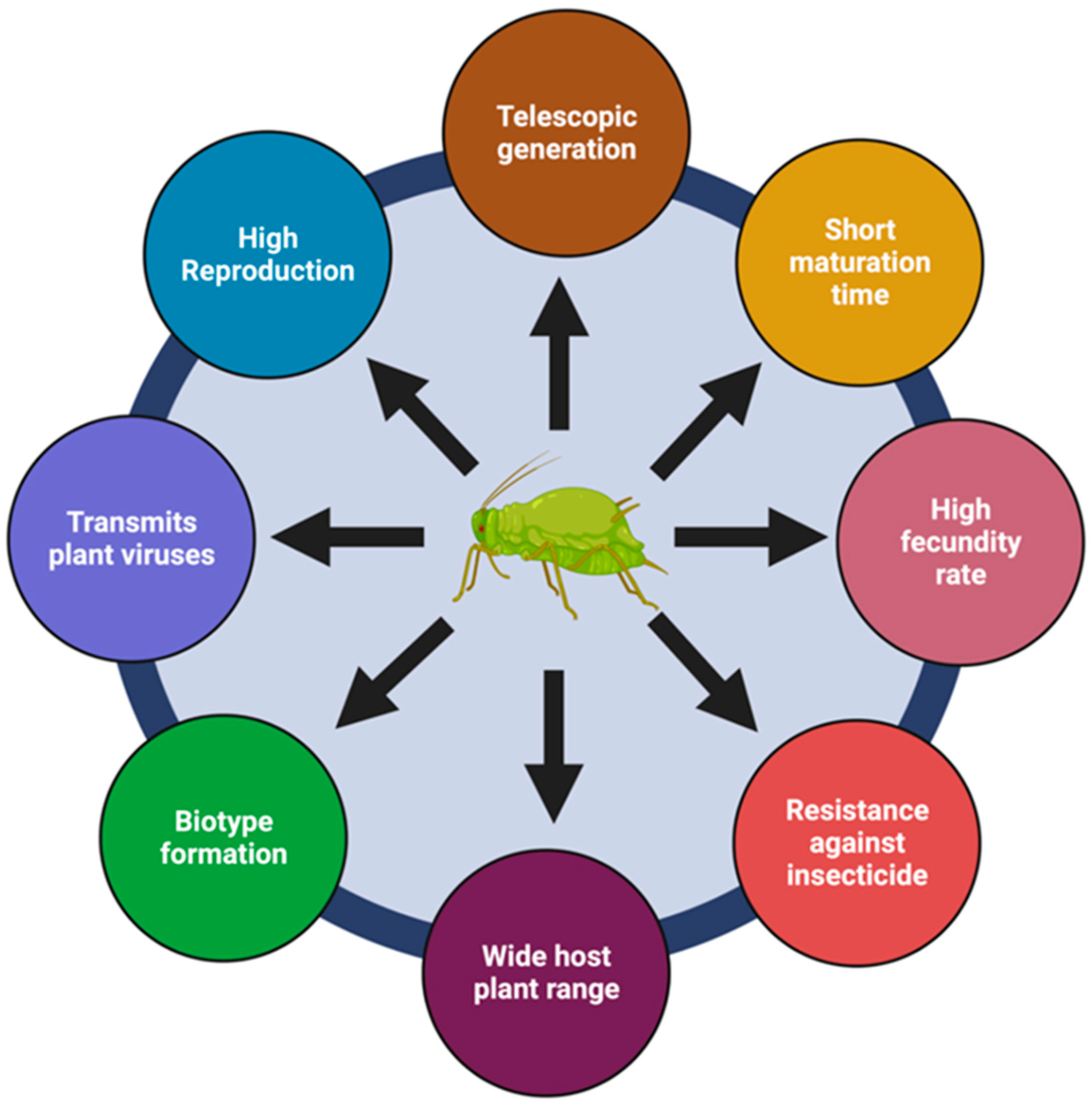
2. Pest Traits Influence Control Strategies
The ecological success of M. persicae as a polyphagous pest could be attributed to its short generation time, high fecundity, the presence of sexual and asexual populations, polyphenism (biotype), the ability to shift to new host plants, the transmission of viruses, and the development of resistance [6][11]. The transmission of plant viruses (persistent and non-persistent) by M. persicae is mostly facilitated by the winged forms due to their ability to fly, enabling the spreading of viruses between hosts [18][19]. This allows for both short-distance and long-distance transmission, with the latter playing a significant role in the spread of viral diseases across extensive geographic areas. The ability of aphids to transmit viruses over long distances is of great concern in an agricultural context, as it contributes to the rapid dissemination and establishment of viral infections in susceptible plant populations.
M. persicae also excretes honeydew (a sugary liquid), which affects the physiology of host plants and their interaction with other herbivores [20]. A list of challenges associated with M. persicae is presented in the chart given below in Figure 1.

Figure 1. Factors that ensure the ecological success of Myzus persicae as a polyphagous and cosmopolitan pest of economic importance.
3. Suppression of Plant Defence
M. persicae is known for its ability to subvert plant defences and disrupt defence-related pathways [21]. Unlike herbivores with chewing-type mouth parts, M. persicae is a silent feeder that causes less physical damage [22][23]. Previous studies have shown that plants can recognize the salivary proteins of M. persicae and modulate their defence strategies by producing deterrent compounds, including indole glucosinolates that covert indol-3-ylmethoxyglucosinolates (I3M) to 4-methoxyindol-3-ylmethoxygluosinolates (4MI3M)), callose formation, the plugging of sieve tubes, and the accumulation of reactive oxygen species (particularly hydrogen peroxide) [24][25][26][27]. However, M. persicae releases specific effector proteins, including Mp1, Mp55, MpC002, Mp59, Mp60, Mp61, Mp62, and Mp63, to counteract or reduce plant-induced defence responses, such as the synthesis of antifeedants and antibiotic compounds [28][29].
The expression of M. persicae salivary proteins in the host plant makes the plant more susceptible to it by increasing fecundity and reducing the synthesis of defensive compounds [27]. For example, the overexpression of the salivary proteins Mp55 and MpC003 is responsible for high aphid fecundity, reduced production of deterrent compounds, and making host plants more susceptible to M. persicae [30][31]. When Arabidopsis thaliana (L.) overexpressed Mp55 proteins, it resulted in high aphid fecundity; reduced levels of deterrent compounds, such as indole glucosinolates and 4MI3M; decreased callose deposition; and a lower abundance of defence signalling molecules, like hydrogen peroxide [27]. The expression of M. persicae-induced salivary proteins (Mp55) presumably avoids the activation of plant defence systems and promotes aphid feeding [32].
4. Economic Importance of Peach–Potato Aphid
Myzus persicae is responsible for both qualitative and quantitative losses in agricultural production by causing chlorosis, necrosis, wilting, defoliation, and fruit abortion. It directly affects plant nutritional status via feeding on plant sap [33]. According to previous studies, M. persicae is responsible for a considerable loss in crop yield, causing billions of dollars of losses globally [11][34]. Specifically, in the UK, aphid infestation causes up to a GBP 70 million loss per year [35]. M. persicae is considered one of the most common and devastating brassica (B. oleracea) pests in the UK, causing direct and indirect damage by spreading one of the most deleterious plant viruses, turnip yellow virus (TuYV), which can reduce final yield up to 26% [35]. Controlling this virus could result in an increase in total yield profit equivalent to GBP 60–90 million per year for oilseed rape growers in the UK [36]. In India, the peach–potato aphid is a key pest of sweet pepper that causes a 38 to 42% yield loss under a controlled environment [37]. In Brazil, the peach–potato aphid primarily serves as a vector for PYV and tomato yellow top virus (ToYTV), which causes a 20–70% loss of tomato (S. lycopersicum) yield and reduces fruit production by up to 85% [38]. In Australia, this pest is responsible for the transmission of TuYV in pea (P. sativum), faba bean (Vicia faba (L.)), lupin (Lupinus albus (L.)), chickpea (Cicer arietinum (L.)), and lentil (Lens orientalis (L.)) crop fields, and a loss of 46% in canola (Brassica napus (L.)) yield has been reported [39]. In New Zealand, M. persicae transmits potato virus Y (PVY) and leaflet curl virus (LCV), which are among the most damaging potato viruses and cause significant yield loss [40]. In China, M. persicae is also considered a primary pest for N. tabacum [41].
5. Current Management Practices
New pest management and control strategies are being developed in order to meet current and future challenges [42]. These pest management practices fall under the following categories “chemical, biological, and cultural” [43]. However, the use of chemical pesticides is one that is common due to their availability, efficacy, and ease of use [44]. Therefore, the majority of current management practices for M. persicae are based on chemical pesticides [45]. Synthetic pesticides containing active ingredients, such as pyrethroids, carbamate, organophosphates, and neonicotinoids, have a strong negative effect on a number of herbivores, including M. persicae [46][47][48].
Besides chemical methods, there are several other management approaches that show high potential in managing this pest, such as the use of natural defence elicitors and biocontrol agents, the application of entomopathogens, and cultural methods [3][49]. An accumulating body of evidence shows that the use of biocontrol agents, i.e., parasitoids and predators, can be an excellent managing tool to protect crop plants from direct damage caused by this pest [50][51]. Similarly, the use of entomopathogens against M. persicae have revealed a hidden potential for its management [52][53]. Cultural methods, such as intercropping companion plants [54], the application of neem leaf extract [55], and winter pruning, have also been found to be effective against the peach–potato aphid [56]. Altering plant defence mechanisms using natural compounds is another preventive measure, which presents some potential as a future pest management tool [3][57].
6. Challenges with Current Management Practices
6.1. Development of Insecticidal Resistance
The majority of current pest management approaches rely on the use of chemical insecticides, either solely or in combination [58]. However, the excessive use of these synthetic chemicals has led to an increased burden on insects to develop resistance against insecticides [45]; similarly, the control of M. persicae through chemical means remains difficult, owing to its ability to develop resistance [59]. The peach–potato aphid has developed resistance to various insecticides, with a total of 483 cases of insecticide resistance documented for this species on the APRD (Arthropod Pesticide Resistance Database) involving 84 active ingredients [60], making M. persicae the single most resistant aphid pest species and placing it in the overall top ten of the most resistant arthropod pests [61]. The exceptional ability of M. persicae to evolve a remarkable diversity of distinct mechanisms of resistance has made this species an emerging model of molecular evolution in insects.
6.2. Adverse Effect on Non-Target Organisms
The literature on the insecticidal side effects on arthropods reports that more than 400 agricultural chemicals have adverse effects on natural enemies, ranging from sublethal to lethal [62]. The excessive use of synthetic pesticides is responsible for deleterious effects on non-target organisms, such as pollinators (fields treated with spinosad residues showed the low survival of Apis mellifera (L.)), predators (spinosad derivatives caused the mortality of Hibana futilis (Banks) and Araneus prantensis (Emerton) upon treatment), and parasitoids (severe adverse effects of the dried residue of spinosad have been found on several parasitoids, including A. colemani, Aphytis melinus (DeBach), Leptomastix dactylopii (Howard), and Encarsia formosa (Gahan)) [63][64][65]. Parasitoids, which are of great economic significance in pest management and play a vital role in controlling pest populations, are badly affected by the excessive use of these synthetic chemicals [66].
6.3. Elicitation of Plant Defence: An Underutilized Tool
Despite excellent results, the use of natural defence compounds as plant defence elicitors under field conditions is still in its early stage [3][67]. The majority of these studies are limited to the laboratory and are still waiting for application in the field. For instance, CJ is a natural plant-derived compound that has been tested on several economically important crops (S. tuberosum, B. oleracea, T. aestivum, and S. lycopersicum) against various sucking pests, including M. persicae [3][68][69][70]. Many laboratory studies have investigated the role of CJ in inducing resistance in plants against pests, and almost all have validated that CJ induces resistance to pests [3][68][69][70][71].
6.4. Underutilisation of Cultural Practices
Cultural methods are commonly used to create unfavourable conditions for pests, which is one of the main goals of pest management approaches [72]. Cultural practices, such as crop rotation and sanitation, intercropping, the destruction of plant debris, and the avoidance of adjacent planting of crops, and trap crops, play a critical role in pest management [73][74]. For instance, winter peach pruning is practised, particularly in fruit orchards, to adjust plant load, which indirectly affects the performance of pests, i.e., M. persicae [75]. Reportedly, M. persicae is primarily found on mature leaves; therefore, winter pruning has been found to be an effective way to reduce pest infestations [56][75]. By removing some buds, a potential habitat for egg-laying is reduced, leading to a decrease in the overall population of M. persicae.
6.5. Lack of Resistant Cultivars
The use of aphid-resistant cultivars in combination with integrated pest management approaches represents an excellent option for managing M. persicae [76]. One of the significant differences between aphid-resistant and aphid-susceptible lines of the same crop is the length of time required by the aphid to reach the phloem during probing [77]. Aphids take a longer time to reach the phloem on resistant lines (Medicago truncatula (Gaertn.) cv. Jester) and cannot feed successively on it [78][79]. However, it has been observed that plant resistance is often overcome after a few years due to the emergence of new biotypes [80][81]. M. persicae has the ability to form biotypes, which differ in behaviour or physiology and allow this pest to expand its host range and rapidly occupy a new ecological niche [82].
7. Potential Biological Control Agents
7.1. Predators and Parasitoids
Extensive research has been conducted to evaluate the efficacy of certain biological control agents against M. persicae in laboratory and field settings [50]; a diverse range of 200 biocontrol agents from various families, including Coccinellidae, Cantharidae, Syrphidae, Anthocoridae, Pentatomidae, Aphelinidae, Braconidae, and Phytoseiidae, have been identified as potential insect agents for managing M. persicae populations [83][84]. The host plant plays a crucial role in implementing the biocontrol approach of naturally occurring biological control agents, and the effectiveness of these agents depends on the species and physiological status of the host plants. Plants release volatile compounds that serve as a cue for pollinators and other natural enemies of pests to locate the host plants [85][86]. Infestation with M. persicae has been observed to increase the release of volatile compounds known as herbivore-induced plant volatiles (HIPVs), which recruit parasitoids and predators [87].
7.2. Entomopathogenic Bacteria
Previous studies have shown that environmental microbes have the potential to kill herbivorous pests, including aphids, when applied in pest management programmes [88][89][90]. Several bacterial species (Pseudomonas fluorescens (Flügge) PpR24; Bacillus amyloliquefaciens [91] strains, CBMDDrag3, PGPBacCA2, and CBMDLO3; Saccharopolyspora spinosa [92]; and Bacillus thuringiensis (Berliner) [93]) and bacterial-derived insecticides (Bosal 10EC and Spinosad 240SC) have been tested on several important crops, including B. oleracea, A. thaliana, B. vulgaris, and C. annuum. On application of bacterial insecticides, a high reduction in the population of M. persicae has been recorded; for instance, a diet containing bacterial suspension showed 100% mortality of adults and nymphs [88] and a 57% population reduction in M. persicae on cauliflower [90]. C. annum, A. thaliana, and B. vulgaris showed a population reduction of 68%, 57%, and 69%, respectively, after application of P. fluorescens PpR24 [89].
7.3. Entomopathogenic Fungi
More than 750 species of entomopathogenic fungi (EPF) belonging to 85 genera are functionally known for their ability to infect arthropods [52][53][94][95][96]. However, most of them have not been used commercially to manage plant pests yet. These fungi are naturally present in agricultural soil, but their efficacy in nature is not high because of the low spore numbers. Their effectiveness can be improved through the inundative release of EPF. The most studied species of EPF belong to the genera Metarhizium, Beauveria, Hirsutella, Isaria, and Lecancillium [53].
7.4. Entomopathogenic Viruses (EPVs)
Plants come in contact with numerous viruses due to their interactions with herbivores. This plant–virus interaction serves as a reservoir for viruses and affects the performance of the associated herbivorous community. Plants take advantage of entomopathogenic viruses and utilise them as a defence tool against a number of plant pests [97]. For example, M. persicae spread Parvovirus (M. persicae densovirus MpDNV) during feeding on plants. The infected host plants use this EPV as a part of their defence strategies by spreading the infection to non-infected subsequent future visiting aphids [98].
7.5. Entomopathogenic Nematodes
Below-ground plant and root–herbivore interactions can affect above-ground plant–herbivore interactions [99][100]. Some nematodes are important entomopathogens that can impact the performance of attacking herbivores (below- and above-ground) on host plants by inducing plant defence mechanisms [101].
8. Sustainable Strategies
8.1. Augmentative Release of Biocontrol Agents
The biological control of insect pests is one of the most efficient and eco-friendly ways to manage them. It involves three basic approaches: the conservation, importation, and augmentation of natural enemies [102]. In Yunnan province, China, the augmentative release of the parasitoid, Aphidius gifuensis (Ashmead), significantly reduced the pest population in tobacco fields [103]. Similarly, in Himachal Pradesh, India, three species of parasitoids (Aphelinus asychis (Walker), Aphidius matricariae (Haliday), and A. ervi) showed high parasitism rates when tested against M. persicae in greenhouse experiments [104].
8.2. Resistant Plant Varieties
Previous studies have shown that breeding programmes with a primary goal of achieving high yield can have a negative impact on host plant resistance, making crops more susceptible to pests due to the manipulation of their genetics to produce high yields [105][106][107][108]. This hypothesis was investigated in an earlier study, where the performance of M. persicae on three wild potato, Solanum stoloniferum (Schltdl.) (23072, 22718 and 18333) and one cultivated potato, S. tubersoum (L.) cv., Desiree lines was observed [98]. All wild potato lines were highly resistant against M. persicae and caused high adult mortality in cage bioassays.
8.3. Natural Compounds as Plant Defence Elicitor
Extensive literature is available wherein natural compounds have been found effective in inducing plant defence [109]. Compounds, such as cis-Jasmone (CJ), β-ocimene, benzothiadiazole, and methyl jasmonate chitosan, have shown an elevated level of defence in plants, like B. napus, S. tuberosum, and L. esculentum, respectively [3][110]. In particular, the exogenous application of MeJA induces the formation of defence enzymes and reduces pupal/larval weights, performance, population densities, and feeding behaviour [109][111][112][113][114]. CJ is another well-known example of a plant defence elicitor, which has been tested on several important crops, including brassica, tomato, wheat, maize, sweet paper, and cotton, against sucking pests [3][71][115][116][117][118][119].
8.4. Intercropping Companion Plants
Another important aspect of management strategies for M. persicae is intercropping. There are various studies against different herbivores (Thrips tabaci (Lindeman), Aphis gossypii (Glover), and M. persicae) that support the idea of planting different crops in the same field [120][121]. A study that was conducted to investigate the effect of companion plants on M. persicae showed that T. patula and Basil release VOCs that directly and indirectly affect the performance and behaviour of M. persicae when used as companion plants with C. annuum in the field [49]. Similarly, intercropping garlic with tobacco, and celery, maize, and sunflower with potato reduces M. persicae populations significantly [122][123]. A volatile analysis of the blend released by companion plants showed the presence of several compounds, such as (E)-β-farnesene, β -linalool, caryophyllene, and pinene, that have a repellent effect on several herbivores, including M. persicae [49][117][124].
9. Proposed Integrated Pest Management for Myzus persicae
An accumulating body of evidence has reported the economic loss caused by M. persicae across a wide range of crop plants, and several studies have been performed to develop control strategies to manage this pest. However, the majority of these studies have focused on a single control measure, either a biological or chemical control, such as the effect of host plants [108][125]; predators [126][127]; EPFs [128][129][130]; EPNs (157); EPVs [131][132]; and push–pull strategies (intercropping and trap crops) [133]. A few pioneering studies used combined biocontrol agents, such as three species of EPF along with 1% azadirachtin [129][130], or biocontrol recruitment via host-plant-induced defence [3].
An IPM pyramid provides detailed information about the actions required to control pests, starting from the bottom (prevention) and progressing towards the top (chemical control), if the prevention and biocontrol strategies fail to suppress the population below the economic threshold [134][135]. Agronomic practices have been extensively reviewed [136], and incorporating these practices would be helpful in reducing the use of chemical pesticides and biological controls [137][138][139]. To synergise biological controls, the use of conventional aphicides in IPM is also valid while adhering to IPM guidelines regarding chemical pesticides [44]. To boost IPM strategies, the use of biocontrol agents in combination with priming agents benefits overall management strategies [43][134].
10. Conclusions
In conclusion, there are several obstacles that hinder the utilization of non-chemical pest control methods, including a lack of awareness, financial instability, a shortage of trained staff, and poor technical advancements [140]. In developing countries, people are either unaware of integrated pest management approaches or there is a lack of trained staff for the successful implementation of control strategies [140]. For example, a survey conducted in Chitwan, Nepal, revealed that only 17% of farmers received one short-term training session on integrated pest management. Financial instability and poor technical advancements are also significant factors that impede the adoption of such potential approaches [141]. In contrast, farmers show a high preference for chemical pesticides due to factors such as falling prices of generic pesticides, their easy availability and readiness for application, and quick response (less time consuming) [142][143][144].
The formation of biotypes means that developing insect-resistant crop varieties is a more complex and challenging process for this species, as it can easily adapt to new resistant varieties as well [145]. Therefore, resistant-cultivar-based agriculture should be investigated further to make crops more resistant and less palatable to M. persicae [11]. The application of natural plant defence elicitors on crops needs to be explored in the field [3][146].
References
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests; Cabi: New York, NY, USA, 2017.
- Dedryver, C.A.; Le Ralec, A.; Fabre, F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. Comptes Rendus Biol. 2010, 333, 539–553.
- Ali, J.; Covaci, A.D.; Roberts, J.M.; Sobhy, I.S.; Kirk, W.D.J.; Bruce, T.J.A. Effects of cis-Jasmone Treatment of Brassicas on Interactions with Myzus persicae Aphids and Their Parasitoid Diaeretiella rapae. Front. Plant Sci. 2021, 12, 711896.
- Goggin, F.L. Plant–aphid interactions: Molecular and ecological perspectives. Curr. Opin. Plant Biol. 2007, 10, 399–408.
- Rodriguez-Saona, C.R.; Musser, R.O.; Vogel, H.; Hum-Musser, S.M.; Thaler, J.S. Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. J. Chem. Ecol. 2010, 36, 1043–1057.
- Van Emden, H.F.; Eastop, V.F.; Hughes, R.D.; Way, M.J. The ecology of Myzus persicae. Annu. Rev. Entomol. 1969, 14, 197–270.
- Vorburger, C.; Lancaster, M.; Sunnucks, P. Environmentally related patterns of reproductive modes in the aphid Myzus persicae and the predominance of two ‘superclones’ in Victoria, Australia. Mol. Ecol. 2003, 12, 3493–3504.
- Capinera, J. Handbook of Vegetable Pests; Academic Press: Cambridge, MA, USA, 2020.
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; John Wiley & Sons Ltd.: New York, NY, USA, 2000.
- Holman, J. Host Plant Catalog of Aphids: Palaearctic Region; Springer: Berlin/Heidelberg, Germany, 2009.
- Ali, J. The Chemical Ecology of a Model Aphid Pest, Myzus persicae, and Its Natural Enemies; Keele University: Edinburgh, UK, 2022.
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51.
- Edde, P.A. Field Crop Arthropod Pests of Economic Importance; Academic Press: Cambridge, MA, USA, 2021.
- Blackman, R.L.; Eastop, V.F. Aphids on the World’ s Herbaceous; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 1–134.
- Naga, K.C.; Buckseth, T.; Subhash, S.; Bairwa, A.; Verma, G.; Kumar, R.; Malik, K.; Sharma, S.; Chakrabarti, S.K. Transmission efficiency of Potato leaf roll virus (PLRV) by potato aphid Aulacorthum solani and green peach aphid Myzus persicae. Indian J. Entomol. 2020, 82, 68–71.
- Capinera, J.L. Green Peach Aphid, Myzus persicae (Sulzer) (Insecta: Hemiptera: Aphididae); University of Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, EDIS: Panama City, FL, USA, 2001.
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. B Biol. Sci. 2002, 269, 455–460.
- Qi, Y.-H.; He, Y.-J.; Wang, X.; Zhang, C.-X.; Chen, J.-P.; Lu, G.; Li, J.-M. Physical contact transmission of Cucumber green mottle mosaic virus by Myzus persicae. PLoS ONE 2021, 16, e0252856.
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host–vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175.
- Hoffmann, K.H. Aphid honeydew: Rubbish or signaler. In Biology and Ecology of Aphids; CRC Press: Boca Raton, FL, USA, 2016; pp. 199–220.
- Chen, C.-Y.; Liu, Y.-Q.; Song, W.-M.; Chen, D.-Y.; Chen, F.-Y.; Chen, X.-Y.; Chen, Z.-W.; Ge, S.-X.; Wang, C.-Z.; Zhan, S. An effector from cotton bollworm oral secretion impairs host plant defense signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 14331–14338.
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests, 1st ed.; van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2007.
- Kallure, G.S.; Kumari, A.; Shinde, B.A.; Giri, A.P. Characterized constituents of insect herbivore oral secretions and their influence on the regulation of plant defenses. Phytochemistry 2022, 193, 113008.
- Kim, J.H.; Lee, B.W.; Schroeder, F.C.; Jander, G. Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J. 2008, 54, 1015–1026.
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 1–9.
- Will, T.; van Bel, A.J.E. Physical and chemical interactions between aphids and plants. J. Exp. Bot. 2006, 57, 729–737.
- Elzinga, D.A.; De Vos, M.; Jander, G. Suppression of Plant Defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol. Plant-Microbe Interact. 2014, 27, 747–756.
- Bhattacharya, S. Brassica-aphid interaction: Challenges and prospects of genetic engineering for integrated aphid management. Physiol. Mol. Plant Pathol. 2019, 108, 101442.
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428.
- Jaouannet, M.; Rodriguez, P.A.; Thorpe, P.; Lenoir, C.J.G.; MacLeod, R.; Escudero-Martinez, C.; Bos, J.I.B. Plant immunity in plant–aphid interactions. Front. Plant Sci. 2014, 5, 663.
- Bos, J.I.B.; Prince, D.; Pitino, M.; Maffei, M.E.; Win, J.; Hogenhout, S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010, 6, e1001216.
- Nalam, V.; Louis, J.; Shah, J. Plant defense against aphids, the pest extraordinaire. Plant Sci. 2019, 279, 96–107.
- Hewer, A.; Becker, A.; van Bel, A.J.E. An aphid’s Odyssey–the cortical quest for the vascular bundle. J. Exp. Biol. 2011, 214, 3868–3879.
- Ali, J. The Peach Potato Aphid (Myzus persicae): Ecology and Management; CRC Press: Boca Raton, FL, USA, 2023.
- Hemming, D.; Bell, J.; Collier, R.; Dunbar, T.; Dunstone, N.; Everatt, M.; Eyre, D.; Kaye, N.; Korycinska, A.; Pickup, J. Likelihood of extreme early flight of Myzus persicae (Hemiptera: Aphididae) across the UK. J. Econ. Entomol. 2022, 115, 1342–1349.
- Stevens, M.; McGrann, G.; Clark, B.; Authority, H. Turnip Yellows Virus (Syn Beet Western Yellows Virus): An Emerging Threat to European Oilseed Rape Production; HGCA: Cape Town, South Africa, 2008.
- Sharma, S.; Sood, A.K.; Ghongade, D.S. Assessment of losses inflicted by the aphid, Myzus persicae (Sulzer) to sweet pepper under protected environment in north western Indian Himalayan region. Phytoparasitica 2022, 50, 51–62.
- Pinheiro, P.F.; de Queiroz, V.T.; Rondelli, V.M.; Costa, A.V.; Marcelino, T.d.P.; Pratissoli, D. Insecticidal activity of citronella grass essential oil on Frankliniella schultzei and Myzus persicae. Ciência Agrotecnol. 2013, 37, 138–144.
- Nancarrow, N.; Aftab, M.; Hollaway, G.; Rodoni, B.; Trebicki, P. Symptomless turnip yellows virus infection causes grain yield loss in lentil and field pea: A three-year field study in south-eastern Australia. Front. Plant Sci. 2022, 13, 1049905.
- London, H.; Saville, D.J.; Merfield, C.N.; Olaniyan, O.; Wratten, S.D. The ability of the green peach aphid (Myzus persicae) to penetrate mesh crop covers used to protect potato crops against tomato potato psyllid (Bactericera cockerelli). PeerJ 2020, 8, e9317.
- Li, Y.; Xu, Z.; Shi, L.; Shen, G.; He, L. Insecticide resistance monitoring and metabolic mechanism study of the green peach aphid, Myzus persicae (Sulzer)(Hemiptera: Aphididae), in Chongqing, China. Pestic. Biochem. Physiol. 2016, 132, 21–28.
- Nauen, R.; Jeschke, P.; Velten, R.; Beck, M.E.; Ebbinghaus-Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Flupyradifurone: A brief profile of a new butenolide insecticide. Pest Manag. Sci. 2015, 71, 850–862.
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215.
- Deguine, J.-P.; Aubertot, J.-N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.G.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38.
- Wu, X.F.; Song, C.M. The resistance of Myzuss persicae (Sulzer) against Omethoate in Tobacco fields of Yunnan. J. Gansu Agric. Univ. 2007, 6, 102–105.
- Rawat, N.; Singh, R.; Sharma, P.L. Evaluation of some insecticides against the green peach aphid, Myzus persicae (sulzer)(hemiptera: Aphididae). Indian J. Entomol. 2013, 75, 113–117.
- Gibson, R.W.; Rice, A.D.; Sawicki, R.M. Effects of the pyrethroid deltamethrin on the acquisition and inoculation of viruses by Myzus persicae. Ann. Appl. Biol. 1982, 100, 49–54.
- Foster, S.P.; Denholm, I.; Devonshire, A.L. The ups and downs of insecticide resistance in peach-potato aphids (Myzus persicae) in the UK. Crop Prot. 2000, 19, 873–879.
- Dardouri, T.; Gautier, H.; Ben Issa, R.; Costagliola, G.; Gomez, L. Repellence of Myzus persicae (Sulzer): Evidence of two modes of action of volatiles from selected living aromatic plants. Pest Manag. Sci. 2019, 75, 1571–1584.
- Andorno, A.V.; López, S.N. Biological control of Myzus persicae (Hemiptera: Aphididae) through banker plant system in protected crops. Biol. Control 2014, 78, 9–14.
- Cabral, S.; Soares, A.O.; Garcia, P. Predation by Coccinella undecimpunctata L. (Coleoptera: Coccinellidae) on Myzus persicae Sulzer (Homoptera: Aphididae): Effect of prey density. Biol. Control 2009, 50, 25–29.
- Paschapur, A.; Subbanna, A.; Singh, A.K.; Jeevan, B.; Stanley, J.; Rajashekhar, H.; Mishra, K.K. Unraveling the Importance of Metabolites from Entomopathogenic Fungi in Insect Pest Management. In Microbes for Sustainable Lnsect Pest Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 89–120.
- Bamisile, B.S.; Akutse, K.S.; Siddiqui, J.A.; Xu, Y. Model application of entomopathogenic fungi as alternatives to chemical pesticides: Prospects, challenges, and insights for next-generation sustainable agriculture. Front. Plant Sci. 2021, 12, 741804.
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105.
- Déla, M.A.; Koffivi, K.G.; Komina, A.; Arnaud, A.; Philippe, G.; Adolé, G.I. Evaluation of neem leaves-based preparations as insecticidal agents against the green peach aphid, Myzus persicae (Sternorrhyncha: Aphididae). Afr. J. Agric. Res. 2014, 9, 1344–1352.
- Grechi, I.; Ould-Sidi, M.-M.; Hilgert, N.; Senoussi, R.; Sauphanor, B.; Lescourret, F. Designing integrated management scenarios using simulation-based and multi-objective optimization: Application to the peach tree–Myzus persicae aphid system. Ecol. Modell. 2012, 246, 47–59.
- Conboy, N.J.A.; McDaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, A.M.R.; Tosh, C.R. Volatile organic compounds as insect repellents and plant elicitors: An integrated pest management (IPM) strategy for glasshouse whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2020, 46, 1090–1104.
- Dent, D.; Binks, R.H. Insect Pest Management; Cabi: New York, NY, USA, 2020.
- Margaritopoulos, J.T.; Kati, A.N.; Voudouris, C.C.; Skouras, P.J.; Tsitsipis, J.A. Long-term studies on the evolution of resistance of Myzus persicae (Hemiptera: Aphididae) to insecticides in Greece. Bull. Entomol. Res. 2021, 111, 1–16.
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database; Michigan State University: East Lansing, MI, USA, 2021.
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128.
- Theiling, K.M.; Croft, B.A. Pesticide side-effects on arthropod natural enemies: A database summary. Agric. Ecosyst. Environ. 1988, 21, 191–218.
- Biondi, A.; Mommaerts, V.; Smagghe, G.; Vinuela, E.; Zappala, L.; Desneux, N. The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 2012, 68, 1523–1536.
- Stanley, J.; Preetha, G.; Stanley, J. Pesticide Toxicity to Non-Target Organisms; Springer: Berlin/Heidelberg, Germany, 2016; Volume 502.
- Serrão, J.E.; Plata-Rueda, A.; Martínez, L.C.; Zanuncio, J.C. Side-effects of pesticides on non-target insects in agriculture: A mini-review. Sci. Nat. 2022, 109, 17.
- Kampfraath, A.A.; Giesen, D.; Van Gestel, C.A.M.; Le Lann, C. Pesticide stress on plants negatively affects parasitoid fitness through a bypass of their phytophage hosts. Ecotoxicology 2017, 26, 383–395.
- Jamiołkowska, A. Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 2020, 10, 173.
- Birkett, M.A.; Campbell, C.A.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334.
- Sobhy, I.S.; Woodcock, C.M.; Powers, S.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. cis-Jasmone Elicits Aphid-Induced Stress Signalling in Potatoes. J. Chem. Ecol. 2017, 43, 39–52.
- Bruce, T.J.A.; Martin, J.L.; Pickett, J.A.; Pye, B.J.; Smart, L.E.; Wadhams, L.J. cis-Jasmone treatment induces resistance in wheat plants against the grain aphid, sitobion avenae (Fabricius) (Homoptera: Aphididae). Pest Manag. Sci. 2003, 59, 1031–1036.
- Bayram, A.; Tonğa, A. cis-Jasmone treatments affect pests and beneficial insects of wheat (Triticum aestivum L.): The influence of doses and plant growth stages. Crop Prot. 2018, 105, 70–79.
- Abate, T.; van Huis, A.; Ampofo, J.K.O. Pest management strategies in traditional agriculture: An African perspective. Annu. Rev. Entomol. 2000, 45, 631–659.
- Brader, L. Integrated pest control in the developing world. Annu. Rev. Entomol. 1979, 24, 225–254.
- Smit, N.; Matengo, L.O. Farmers’ cultural practices and their effects on pest control in sweet potato in South Nyanza, Kenya. Int. J. Pest Manag. 1995, 41, 2–7.
- Grechi, I.; Sauge, M.; Sauphanor, B.; Hilgert, N.; Senoussi, R.; Lescourret, F. How does winter pruning affect peach tree–Myzus persicae interactions? Entomol. Exp. Appl. 2008, 128, 369–379.
- Tetreault, H.M.; Grover, S.; Scully, E.D.; Gries, T.; Palmer, N.A.; Sarath, G.; Louis, J.; Sattler, S.E. Global responses of resistant and susceptible sorghum (Sorghum bicolor) to sugarcane aphid (Melanaphis sacchari). Front. Plant Sci. 2019, 10, 145.
- Dreyer, D.L.; Campbell, B.C. Chemical basis of host-plant resistance to aphids. Plant. Cell Environ. 1987, 10, 353–361.
- Klingler, J.; Creasy, R.; Gao, L.; Nair, R.M.; Calix, A.S.; Jacob, H.S.; Edwards, O.R.; Singh, K.B. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 2005, 137, 1445–1455.
- Greenslade, A.F.C.; Ward, J.L.; Martin, J.L.; Corol, D.I.; Clark, S.J.; Smart, L.E.; Aradottir, G.I. Triticum monococcum lines with distinct metabolic phenotypes and phloem-based partial resistance to the bird cherry–oat aphid Rhopalosiphum padi. Ann. Appl. Biol. 2016, 168, 435–449.
- Smith, C.M.; Chuang, W. Plant resistance to aphid feeding: Behavioral, physiological, genetic and molecular cues regulate aphid host selection and feeding. Pest Manag. Sci. 2014, 70, 528–540.
- Bosque-Perez, N.A.; Buddenhagen, I.W. The development of host-plant resistance to insect pests: Outlook for the tropics. In Proceedings of the 8th International Symposium on Insect-Plant Relationships; Springer: Berlin/Heidelberg, Germany, 1992; pp. 235–249.
- Tagu, D.; Klingler, J.P.; Moya, A.; Simon, J.-C. Early progress in aphid genomics and consequences for plant–aphid interactions studies. Mol. Plant-Microbe Interact. 2008, 21, 701–708.
- Acheampong, S.; Gillespie, D.R.; Quiring, D. Survey of parasitoids and hyperparasitoids (Hymenoptera) of the green peach aphid, Myzus persicae and the foxglove aphid, Aulacorthum solani (Hemiptera: Aphididae) in British Columbia. J. Entomol. Soc. Br. Columbia 2012, 109, 12–22.
- Mohammed, A.A.; Hatcher, P.E. Combining entomopathogenic fungi and parasitoids to control the green peach aphid Myzus persicae. Biol. Control 2017, 110, 44–55.
- Clavijo Mccormick, A.; Gershenzon, J.; Unsicker, S.B. Little peaks with big effects: Establishing the role of minor plant volatiles in plant–insect interactions. Plant. Cell Environ. 2014, 37, 1836–1844.
- Xu, H.; Turlings, T.C.J. Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 2018, 23, 100–111.
- Ahmed, Q.; Agarwal, M.; Alobaidi, R.; Zhang, H.; Ren, Y. Response of Aphid Parasitoids to Volatile Organic Compounds from Undamaged and Infested Brassica oleracea with Myzus persicae. Molecules 2022, 27, 1522.
- López-Isasmendi, G.; Alvarez, A.E.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C. Aphicidal activity of Bacillus amyloliquefaciens strains in the peach-potato aphid (Myzus persicae). Microbiol. Res. 2019, 226, 41–47.
- Paliwal, D.; Hamilton, A.J.; Barrett, G.A.; Alberti, F.; Van Emden, H.; Monteil, C.L.; Mauchline, T.H.; Nauen, R.; Wagstaff, C.; Bass, C. Identification of novel aphid-killing bacteria to protect plants. Microb. Biotechnol. 2022, 15, 1203–1220.
- Akbar, M.F.; Rana, H.U.; Perveen, F. Management of cauliflower aphid (Myzus persicae (Sulzer) Aphididae: Hemiptera) through environment friendly bioinsecticides. Pak. Entomol 2014, 36, 25–30.
- Priest, F.G.; Goodfellow, M.; Shute, L.A.; Berkeley, R.C.W. Bacillus amyloliquefaciens sp. nov., nom. rev. Int. J. Syst. Bacteriol. 1987, 37, 69–71.
- MERTZ, F.P.; Yao, R.C. Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still. Int. J. Syst. Evol. Microbiol. 1990, 40, 34–39.
- Torres-Quintero, M.C.; Arenas-Sosa, I.; Hernández-Velázquez, V.M.; Suárez-Rodríguez, R.; Peña-Chora, G. Characterization of Bacillus thuringiensis (Bacillaceae) strains pathogenic to Myzus persicae (Hemiptera: Aphididae). Florida Entomol. 2016, 99, 639–643.
- Mora, M.A.E.; Rouws, J.R.C.; Fraga, M.E. Occurrence of entomopathogenic fungi in Atlantic forest soils. Microbiol. Discov. 2016, 4, 1–7.
- Roberts, D.W.; Hajek, A.E. Entomopathogenic fungi as bioinsecticides. In Frontiers in Industrial Mycology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 144–159.
- Khachatourians, G.G.; Qazi, S.S. Entomopathogenic fungi: Biochemistry and molecular biology. In Human and Animal Relationships; Springer: Berlin/Heidelberg, Germany, 2008; pp. 33–61.
- Der Geest, V. Can plants use entomopathogens as bodyguards? Ecol. Lett. 2000, 3, 228–235.
- Van Munster, M.; Janssen, A.; Clérivet, A.; Van den Heuvel, J. Can plants use an entomopathogenic virus as a defense against herbivores? Oecologia 2005, 143, 396–401.
- Li, Y.; Zhen, S.; Shan, S.; Sun, B.; Li, J.; Hu, F.; Cui, Q.; Zhang, L.; Gu, X.; Cheng, W. Modulation of above-belowground plant-herbivore interactions by entomopathogenic nematodes. Appl. Soil Ecol. 2020, 148, 103479.
- Helms, A.M.; Ray, S.; Matulis, N.L.; Kuzemchak, M.C.; Grisales, W.; Tooker, J.F.; Ali, J.G. Chemical cues linked to risk: Cues from below-ground natural enemies enhance plant defences and influence herbivore behaviour and performance. Funct. Ecol. 2019, 33, 798–808.
- Kamali, S.; Javadmanesh, A.; Stelinski, L.L.; Kyndt, T.; Seifi, A.; Cheniany, M.; Zaki-Aghl, M.; Hosseini, M.; Heydarpour, M.; Asili, J. Beneficial worm allies warn plants of parasite attack below-ground and reduce above-ground herbivore preference and performance. Mol. Ecol. 2022, 31, 691–712.
- Shavanov, M.V.; Shigapov, I.I.; Niaz, A. Biological methods for pests and diseases control in agricultural plants. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2022; p. 30081.
- Yang, S.; Wei, J.; Yang, S.; Kuang, R. Current Status and Future Trends of Augmentative Release of Aphidius gifuensis for Control of Myzus persicae in Chinaâ€TM s Yunnan Province. J. Entomol. Res. Soc. 2011, 13, 87–99.
- Gavkare, O.; Kumar, S.; Japoshvili, G. Effectiveness of native parasitoids of Myzus persicae in greenhouse environments in India. Phytoparasitica 2014, 42, 141–144.
- Rodriguez-saona, C.; Vorsa, N.; Singh, A.P.; Johnson-cicalese, J.; Szendrei, Z.; Mescher, M.C.; Frost, C.J. Tracing the history of plant traits under domestication in cranberries: Potential consequences on anti-herbivore defences. J. Exp. Bot. 2011, 62, 2633–2644.
- Gepts, P. A comparison between crop domestication, classical plant breeding, and genetic engineering. Crop Sci. 2002, 42, 1780–1790.
- Mishra, M.; Lomate, P.R.; Joshi, R.S.; Punekar, S.A.; Gupta, V.S.; Giri, A.P. Ecological turmoil in evolutionary dynamics of plant–insect interactions: Defense to offence. Planta 2015, 242, 761–771.
- Ali, J.; Sobhy, I.S.; Bruce, T.J.A. Wild potato ancestors as potential sources of resistance to the aphid Myzus persicae. Pest Manag. Sci. 2022, 78, 3931–3938.
- Ali, J.; Wei, D.; Mahamood, M.; Zhou, F.; King, P.J.H.; Zhou, W.; Shamsi, I.H. Exogenous Application of Methyl Salicylate Induces Defence in Brassica against Peach Potato Aphid Myzus persicae. Plants 2023, 12, 1770.
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006, 120, 175–188.
- Erbilgin, N.; Krokene, P.; Christiansen, E.; Zeneli, G.; Gershenzon, J. Exogenous application of methyl jasmonate elicits defenses in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus. Oecologia 2006, 148, 426–436.
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013, 94, 272–277.
- Cao, H.H.; Wang, S.H.; Liu, T.X. Jasmonate- and salicylate-induced defenses in wheat affect host preference and probing behavior but not performance of the grain aphid, Sitobion avenae. Insect Sci. 2014, 21, 47–55.
- Xiao, L.; Carrillo, J.; Siemann, E.; Ding, J. Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. AoB Plants 2019, 11, plz003.
- Pavela, R. Essential oils from Foeniculum vulgare Miller as a safe environmental insecticide against the aphid Myzus persicae Sulzer. Environ. Sci. Pollut. Res. 2018, 25, 10904–10910.
- Wei, T.; Li, X.; Yashir, N.; Li, H.; Sun, Y.; Hua, L.; Ren, X.; Guo, J. Effect of exogenous silicon and methyl jasmonate on the alleviation of cadmium-induced phytotoxicity in tomato plants. Environ. Sci. Pollut. Res. 2021, 28, 51854–51864.
- Disi, J.O.; Zebelo, S.; Ngumbi, E.; Fadamiro, H.Y. cis-Jasmone primes defense pathways in tomato via emission of volatile organic compounds and regulation of genes with consequences for Spodoptera exigua oviposition. Arthropod. Plant. Interact. 2017, 11, 591–602.
- Oluwafemi, S.; Dewhirst, S.Y.; Veyrat, N.; Powers, S.; Bruce, T.J.A.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. Priming of production in maize of volatile organic defence compounds by the natural plant activator cis-jasmone. PLoS ONE 2013, 8, e62299.
- Matthes, M.; Bruce, T.; Chamberlain, K.; Pickett, J.; Napier, J. Emerging roles in plant defense for cis-jasmone-induced cytochrome P450 CYP81D11. Plant Signal. Behav. 2011, 6, 563–565.
- Tonğa, A.; Çakmak, S.; Şeker, K.; Temiz, M.G.; Bayram, A. cis-Jasmone treatments affect multiple sucking insect pests and associated predators in cotton. Entomol. Gen. 2020, 40, 49–61.
- Ben Issa, R.; Gautier, H.; Gomez, L. Influence of neighbouring companion plants on the performance of aphid populations on sweet pepper plants under greenhouse conditions. Agric. For. Entomol. 2017, 19, 181–191.
- Sidauruk, L.; Sipayung, P. Population of Myzus persicae (Sulzer) and insect diversity on intercropping potatoes with other plants which planting at different time. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 12018.
- Ameline, A.; Dorland, J.; Werrie, P.-Y.; Couty, A.; Fauconnier, M.-L.; Lateur, M.; Doury, G. Geranium macrorrhizum, a potential novel companion plant affecting preference and performance of Myzus persicae on sweet pepper. J. Pest Sci. 2022, 96, 671–682.
- Dardouri, T.; Gautier, H.; Costagliola, G.; Gomez, L. How French marigold (Tagetes patula L.) volatiles can affect the performance of green peach aphid. IOBC-WPRS Bull. 2017, 123, 71–78.
- Lai, R.; You, M.; Lotz, L.A.P.; Vasseur, L. Response of green peach aphids and other arthropods to garlic intercropped with tobacco. Agron. J. 2011, 103, 856–863.
- Ali, M.Y.; Naseem, T.; Arshad, M.; Ashraf, I.; Rizwan, M.; Tahir, M.; Rizwan, M.; Sayed, S.; Ullah, M.I.; Khan, R.R. Host-plant Variations affect the biotic potential, survival, and population projection of Myzus persicae (Hemiptera: Aphididae). Insects 2021, 12, 375.
- Foglar, H.; Malausa, J.C.; Wajnberg, E. The functional response and preference of Macrolophus caliginosus for two of its prey: Myzus persicae and Tetranychus urticae. Entomophaga 1990, 35, 465–474.
- Jaber, L.R.; Araj, S.-E. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol. Control 2018, 116, 53–61.
- Lee, W.W.; Shin, T.Y.; Bae, S.M.; Woo, S.D. Screening and evaluation of entomopathogenic fungi against the green peach aphid, Myzus persicae, using multiple tools. J. Asia. Pac. Entomol. 2015, 18, 607–615.
- Tiwari, S.; Sharma, S.; Wratten, S.D. Flowering alyssum (Lobularia maritima) promote arthropod diversity and biological control of Myzus persicae. J. Asia. Pac. Entomol. 2020, 23, 634–640.
- Ren, G.; Wang, X.; Chen, D.; Wang, X.; Fan, X.; Liu, X. Potato virus Y-infected tobacco affects the growth, reproduction, and feeding behavior of a vector aphid, Myzus persicae (Hemiptera: Aphididae). Appl. Entomol. Zool. 2015, 50, 239–243.
- Liu, J.; Liu, Y.; Donkersley, P.; Dong, Y.; Chen, X.; Zang, Y.; Xu, P.; Ren, G. Preference of the aphid Myzus persicae (Hemiptera: Aphididae) for tobacco plants at specific stages of potato virus Y infection. Arch. Virol. 2019, 164, 1567–1573.
- Singh, H.; Joshi, N. Management of the aphid, Myzus persicae (Sulzer) and the whitefly, Bemisia tabaci (Gennadius), using biorational on capsicum under protected cultivation in India. Egypt. J. Biol. Pest Control 2020, 30, 67.
- Naranjo, S.E. Conservation and evaluation of natural enemies in IPM systems for Bemisia tabaci. Crop Prot. 2001, 20, 835–852.
- Stenberg, J.A. A conceptual framework for integrated pest management. Trends Plant Sci. 2017, 22, 759–769.
- Lewis, W.J.; Van Lenteren, J.C.; Phatak, S.C.; Tumlinson Iii, J.H. A total system approach to sustainable pest management. Proc. Natl. Acad. Sci. USA 1997, 94, 12243–12248.
- Sunderland, K.; Samu, F. Effects of agricultural diversification on the abundance, distribution, and pest control potential of spiders: A review. Entomol. Exp. Appl. 2000, 95, 1–13.
- Whipps, J.M.; Lumsden, R.D. Commercial use of fungi as plant disease biological control agents: Status and prospects. In Fungi as Biocontrol Agents: Progress, Problems and Potential; Wiley: New York, NY, USA, 2001; pp. 9–22.
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents. Annu Rev Entomol. 2002, 47, 561–594.
- Rijal, J.P.; Regmi, R.; Ghimire, R.; Puri, K.D.; Gyawaly, S.; Poudel, S. Farmers’ knowledge on pesticide safety and pest management practices: A case study of vegetable growers in Chitwan, Nepal. Agriculture 2018, 8, 16.
- Bottrell, D.G.; Schoenly, K.G. Integrated pest management for resource-limited farmers: Challenges for achieving ecological, social and economic sustainability. J. Agric. Sci. 2018, 156, 408–426.
- Haggblade, S.; Diarra, A.; Traoré, A. Regulating agricultural intensification: Lessons from West Africa’s rapidly growing pesticide markets. Dev. Policy Rev. 2022, 40, e12545.
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, history, and classification. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–42.
- Denkyirah, E.K.; Okoffo, E.D.; Adu, D.T.; Aziz, A.A.; Ofori, A.; Denkyirah, E.K. Modeling Ghanaian cocoa farmers’ decision to use pesticide and frequency of application: The case of Brong Ahafo Region. Springerplus 2016, 5, 1113.
- Singh, B.; Jasrotia, P.; Crespo-Herreraa, L. Breeding for Aphid Resistance in Wheat: Status and Future Prospects. In New Horizons in Wheat and Barley Research; Springer: Berlin/Heidelberg, Germany, 2022; pp. 381–399.
- Tomilova, O.G.; Kryukova, N.A.; Efimova, M.V.; Kovtun, I.S.; Kolomeichuk, L.V.; Kryukov, V.Y.; Glupov, V.V. Early physiological response of potato plants to entomopathogenic fungi under hydroponic conditions. Horticulturae 2021, 7, 217.
More
Information
Subjects:
Agriculture, Dairy & Animal Science; Ecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
01 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




