
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Felix Antonio Acosta Arbelo | -- | 3231 | 2023-07-31 11:59:40 | | | |
| 2 | Fanny Huang | Meta information modification | 3231 | 2023-08-01 07:56:48 | | |
Video Upload Options
European sea bass production has increased. This increase is associated with an annually rising demand for sea bass, which encourages the aquaculture industries to increase their production to meet that demand. However, this intensification has repercussions on the animals, causing stress that is usually accompanied by dysbiosis, low feed-conversion rates, and immunodepression, among other factors. Therefore, the appearance of pathogenic diseases is common in these industries after immunodepression. Seeking to enhance animal welfare, researchers have focused on alternative approaches such as probiotic application. The use of probiotics in European sea bass production is presented as an ecological, safe, and viable alternative in addition to enhancing different host parameters such as growth performance, feed utilization, immunity, disease resistance, and fish survival against different pathogens through inclusion in fish diets through vectors and/or in water columns.
1. Introduction
2. Probiotic Modes of Action in European Sea Bass
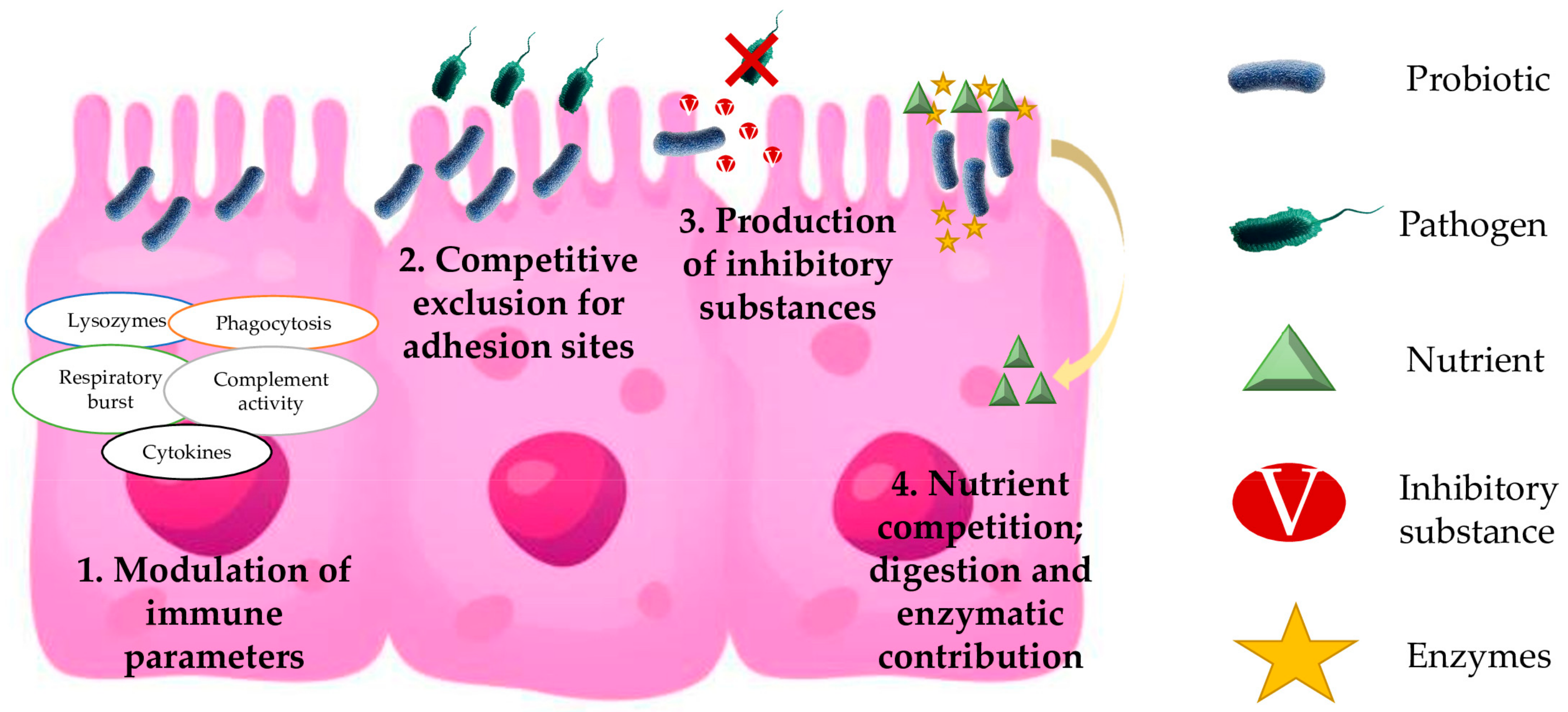
2.1. Modulation of Immune Parameters
2.2. Competitive Exclusion for Adhesion Sites
2.3. Production of Inhibitory Substances
2.4. Nutrient Competition: Digestion and Enzymatic Contribution
3. Probiotic Benefits in European Sea Bass Aquaculture
3.1. Increased Growth and Survival Rates
Probiotics in aquaculture promote fish growth by improving feed-conversion rates. The survival rate is another parameter that benefits after probiotic implementation [97]. As proof of these, many studies have reported that the application of different probiotics (single or combination) on European sea bass promote growth, growth performance, and survival [53–57,65].
3.2. Disease Resistance and Health Status
3.3. Elevation of Immune Parameters
3.4. Gut Morphology and Changes in Microbial Diversity
References
- Ulusoy, Ş.; Mol, S. Trace Elements in Seabass, Farmed by Turkey, and Health Risks to the Main Consumers: Turkish and Dutch Populations. Environ. Monit. Assess. 2022, 194, 224.
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Updating the Role of Probiotics, Prebiotics, and Synbiotics for Tilapia Aquaculture as Leading Candidates for Food Sustainability: A Review. Probiotics Antimicrob. Proteins 2021, 14, 130–157.
- Apromar. La Acuicultura en España 2022 (p. XX) Asociación Empresarial de Productores de Cultivos Marinos. 2022. Available online: https://apromar.es/apromar-publica-su-informe-anual-la-acuicultura-en-espana-2022/ (accessed on 12 April 2023).
- Henriksson, P.J.G.; Rico, A.; Troell, M.; Klinger, D.H.; Buschmann, A.H.; Saksida, S.; Chadag, M.V.; Zhang, W.; Se, P.H. Unpacking Factors Influencing Antimicrobial Use in Global Aquaculture and Their Implication for Management: A Review from a Systems Perspective. Sustain. Sci. 2018, 13, 1105–1120.
- De FreitasSouza, C.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential Oils as Stress-Reducing Agents for Fish Aquaculture: A Review. Front. Physiol. 2019, 10, 785.
- Delalay, G.; Berezowski, J.A.; Diserens, N.; Schmidt-Posthaus, H. An Understated Danger: Antimicrobial Resistance in Aquaculture and Pet Fish in Switzerland, a Retrospective Study from 2000 to 2017. J. Fish. Dis. 2020, 43, 1299–1315.
- Sánchez, J.L.F.; Le Breton, A.; Brun, E.; Vendramin, N.; Spiliopoulos, G.; Furones, D.; Basurco, B. NC-ND License Assessing the Economic Impact of Diseases in Mediterranean Grow-out Farms Culturing European Sea Bass. Aquaculture 2021, 547, 737530.
- Muniesa, A.; Basurco, B.; Aguilera, C.; Furones, D.; Reverté, C.; Sanjuan-Vilaplana, A.; Jansen, M.D.; Brun, E.; Tavornpanich, S. Mapping the Knowledge of the Main Diseases Affecting Sea Bass and Sea Bream in Mediterranean. Transbound Emerg. Dis. 2020, 67, 1089–1100.
- Suyamud, B.; Lohwacharin, J.; Yang, Y.; Sharma, V.K. Antibiotic Resistant Bacteria and Genes in Shrimp Aquaculture Water: Identification and Removal by Ferrate(VI). J. Hazard. Mater. 2021, 420, 126572.
- Wang, X.; Lin, Y.; Zheng, Y.; Meng, F. Antibiotics in Mariculture Systems: A Review of Occurrence, Environmental Behavior, and Ecological Effects. Environ. Pollut. 2022, 293, 118541.
- Pepi, M.; Focardi, S.; Area, M.; Int, J.E. Antibiotic-Resistant Bacteria in Aquaculture and Climate Change: A Challenge for Health in the Mediterranean Area. Public Health 2021, 18, 5723.
- Iwashita, M.K.P.; Addo, S.; Terhune, J.S. Use of Pre- and Probiotics in Finfish Aquaculture. In Feed and Feeding Practices in Aquaculture; Woodhead Publishing: Sawston, UK, 2015; pp. 235–249.
- Vivas, R.; Barbosa, A.A.T.; Dolabela, S.S.; Jain, S. Multidrug-Resistant Bacteria and Alternative Methods to Control Them: An Overview. Microb. Drug Resist. 2019, 25, 890–908.
- Cabello, F.C.; Godfrey, H.P. Even Therapeutic Antimicrobial Use in Animal Husbandry May Generate Environmental Hazards to Human Health. Environ. Microbiol. 2016, 18, 311–313.
- World Health Organization (WHO). Available online: https://www.who.int/ (accessed on 12 April 2023).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Chauhan, A.; Singh, R. Probiotics in Aquaculture: A Promising Emerging Alternative Approach. Symbiosis 2019, 77, 99–113.
- Archacka, M.; Celińska, E.; Białas, W. Techno-Economic Analysis for Probiotics Preparation Production Using Optimized Corn Flour Medium and Spray-Drying Protective Blends. Food Bioprod. Process. 2020, 123, 354–366.
- Ringø, E.; Harikrishnan, R.; Soltani, M.; Ghosh, K. The Effect of Gut Microbiota and Probiotics on Metabolism in Fish and Shrimp. Animals 2022, 12, 3016.
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the Latest Developments in the Role of Probiotics, Prebiotics and Synbiotics in Shrimp Aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281.
- Moriarty, D.J.W. Control of Luminous Vibrio Species in Penaeid Aquaculture Ponds. Aquaculture 1998, 164, 351–358.
- Van Hai, N.; Fotedar, R. Comparison of the Effects of the Prebiotics (Bio-Mos® and β-1,3-D-Glucan) and the Customised Probiotics (Pseudomonas synxantha and P. aeruginosa) on the Culture of Juvenile Western King Prawns (Penaeus latisulcatus Kishinouye, 1896). Aquaculture 2009, 289, 310–316.
- Merrifield, D.L.; Dimitroglou, A.; Foey, A.; Davies, S.J.; Baker, R.T.M.; Bøgwald, J.; Castex, M.; Ringø, E. The Current Status and Future Focus of Probiotic and Prebiotic Applications for Salmonids. Aquaculture 2010, 302, 1–18.
- Tinh, N.T.N.; Dierckens, K.; Sorgeloos, P.; Bossier, P. A Review of the Functionality of Probiotics in the Larviculture Food Chain. Mar. Biotechnol. 2008, 10, 1–12.
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174.
- Gómez-Llorente, C.; Muñoz, S.; Gil, A. 3rd International Immunonutrition Workshop Session 5: Early Programming of the Immune System and the Role of Nutrition Role of Toll-like Receptors in the Development of Immunotolerance Mediated by Probiotics. Proc. Nutr. Soc. 2010, 69, 373–380.
- Hasan, K.N.; Banerjee, G. Recent Studies on Probiotics as Beneficial Mediator in Aquaculture: A Review. J. Basic Appl. Zool. 2020, 81, 1–16.
- Vinderola, G.; Matar, C.; Perdigon, G. Role of Intestinal Epithelial Cells in Immune Effects Mediated by Gram-Positive Probiotic Bacteria: Involvement of Toll-like Receptors. Clin. Diagn. Lab. Immunol. 2005, 12, 1075–1084.
- Vizoso Pinto, M.G.; Rodriguez Gómez, M.; Seifert, S.; Watzl, B.; Holzapfel, W.H.; Franz, C.M.A.P. Lactobacilli Stimulate the Innate Immune Response and Modulate the TLR Expression of HT29 Intestinal Epithelial Cells In Vitro. Int. J. Food Microbiol. 2009, 133, 86–93.
- Ribeiro, C.M.S.; Hermsen, T.; Taverne-Thiele, A.J.; Savelkoul, H.F.J.; Wiegertjes, G.F. Evolution of Recognition of Ligands from Gram-Positive Bacteria: Similarities and Differences in the TLR2-Mediated Response between Mammalian Vertebrates and Teleost Fish. J. Immunol. 2010, 184, 2355–2368.
- Salinas, I.; Díaz-Rosales, P.; Cuesta, A.; Meseguer, J.; Chabrillón, M.; Moriñigo, M.Á.; Esteban, M.Á. Effect of Heat-Inactivated Fish and Non-Fish Derived Probiotics on the Innate Immune Parameters of a Teleost Fish (Sparus aurata L.). Vet. Immunol. Immunopathol. 2006, 111, 279–286.
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, Prebiotics and Synbiotics Improved the Functionality of Aquafeed: Upgrading Growth, Reproduction, Immunity and Disease Resistance in Fish. Fish Shellfish Immunol. 2022, 120, 569–589.
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y.; Hlordzi, V.; Sakyi, M.E.; Afriyie, G.; Wang, Z.; Li, Y.; Xie, C.X.; Kuebutornye, F.K.A.; et al. Mechanisms and the Role of Probiotic Bacillus in Mitigating Fish Pathogens in Aquaculture. Fish Physiol. Biochem. 2020, 46, 819–841.
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Gironés, O.; Múzquiz, J.L. In Vitro Competitive Adhesion and Production of Antagonistic Compounds by Lactic Acid Bacteria against Fish Pathogens. Vet. Microbiol. 2007, 122, 373–380.
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66.
- Dawood, M.A.O. Nutritional Immunity of Fish Intestines: Important Insights for Sustainable Aquaculture. Rev. Aquac. 2021, 13, 642–663.
- Cai, Y.; Benno, Y.; Nakase, T.; Oh, T.K. Specific Probiotic Characterization of Weissella hellenica DS-12 Isolated from Flounder Intestine. J. Gen. Appl. Microbiol. 1998, 44, 311–316.
- Lazado, C.C.; Caipang, C.M.A.; Brinchmann, M.F.; Kiron, V. In Vitro Adherence of Two Candidate Probiotics from Atlantic Cod and Their Interference with the Adhesion of Two Pathogenic Bacteria. Vet. Microbiol. 2011, 148, 252–259.
- Servin, A.L. Antagonistic Activities of Lactobacilli and Bifidobacteria against Microbial Pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440.
- Sorroza, L.; Padilla, D.; Acosta, F.; Román, L.; Grasso, V.; Vega, J.; Real, F. Characterization of the Probiotic Strain Vagococcus fluvialis in the Protection of European Sea Bass (Dicentrarchus labrax) against Vibriosis by Vibrio anguillarum. Vet. Microbiol. 2012, 155, 369–373.
- Monzón-Atienza, L.; Bravo, J.; Torrecillas, S.; Montero, D.; Canales, A.F.G.-D.; de la Banda, I.G.; Galindo-Villegas, J.; Ramos-Vivas, J.; Acosta, F. Isolation and Characterization of a Bacillus velezensis D-18 Strain, as a Potential Probiotic in European Seabass Aquaculture. Probiotics Antimicrob. Proteins 2021, 13, 1404–1412.
- Simón, R.; Docando, F.; Nuñez-Ortiz, N.; Tafalla, C.; Díaz-Rosales, P. Mechanisms Used by Probiotics to Confer Pathogen Resistance to Teleost Fish. Front. Immunol. 2021, 12, 1.
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The Functionality of Probiotics in Aquaculture: An Overview. Fish Shellfish Immunol. 2021, 117, 36–52.
- Makras, L.; Triantafyllou, V.; Fayol-Messaoudi, D.; Adriany, T.; Zoumpopoulou, G.; Tsakalidou, E.; Servin, A.; De Vuyst, L. Kinetic Analysis of the Antibacterial Activity of Probiotic Lactobacilli towards Salmonella enterica Serovar Typhimurium Reveals a Role for Lactic Acid and Other Inhibitory Compounds. Res. Microbiol. 2006, 157, 241–247.
- Hassan, M.; Kjos, M.; Nes, I.F.; Diep, D.B.; Lotfipour, F. Natural Antimicrobial Peptides from Bacteria: Characteristics and Potential Applications to Fight against Antibiotic Resistance. J. Appl. Microbiol. 2012, 113, 723–736.
- Monzón-Atienza, L.; Bravo, J.; Fernández-Montero, Á.; Charlie-Silva, I.; Montero, D.; Ramos-Vivas, J.; Galindo-Villegas, J.; Acosta, F. Dietary Supplementation of Bacillus velezensis Improves Vibrio anguillarum Clearance in European Sea Bass by Activating Essential Innate Immune Mechanisms. Fish Shellfish Immunol. 2022, 124, 244–253.
- Valero, Y.; Arizcun, M.; Cortés, J.; Ramírez-Cepeda, F.; Guzmán, F.; Mercado, L.; Esteban, M.Á.; Chaves-Pozo, E.; Cuesta, A. NK-Lysin, Dicentracin and Hepcidin Antimicrobial Peptides in European Sea Bass. Ontogenetic Development and Modulation in Juveniles by Nodavirus. Dev. Comp. Immunol. 2020, 103, 103516.
- Schiffrin, E.J.; Blum, S. Interactions between the Microbiota and the Intestinal Mucosa. Eur. J. Clin. Nutr. 2002, 56, S60–S64.
- Makridis, P.; Kokou, F.; Bournakas, C.; Papandroulakis, N.; Sarropoulou, E. Isolation of Phaeobacter sp. from Larvae of Atlantic Bonito (Sarda sarda) in a Mesocosmos Unit, and Its Use for the Rearing of European Seabass Larvae (Dicentrarchus labrax L.). Microorganisms 2021, 9, 128.
- Touraki, M.; Karamanlidou, G.; Karavida, P.; Chrysi, K. Evaluation of the Probiotics Bacillus subtilis and Lactobacillus plantarum Bioencapsulated in Artemia nauplii against Vibriosis in European Sea Bass Larvae (Dicentrarchus labrax, L.). World J. Microbiol. Biotechnol. 2012, 28, 2425–2433.
- Schaeck, M.; Duchateau, L.; Van den Broeck, W.; Van Trappen, S.; De Vos, P.; Coulombet, C.; Boon, N.; Haesebrouck, F.; Decostere, A. Vibrio lentus Protects Gnotobiotic Sea Bass (Dicentrarchus labrax L.) Larvae against Challenge with Vibrio harveyi. Vet. Microbiol. 2016, 185, 41–48.
- Öztürk, F.; Esendal, Ö.M. Usage of Lactobacillus rhamnosus as a Probiotic in Sea Bass (Dicentrarchus labrax). J. Anatol. Environ. Anim. Sci. 2020, 5, 93–99.
- El-Sayed, H.S.; Ibrahim, H.A.H.; Beltagy, E.A.; Khairy, H.M. Effects of Short Term Feeding of Some Marine Microalgae on the Microbial Profile Associated with Dicentrarchus labrax Post Larvae. Egypt. J. Aquat. Res. 2014, 40, 251–260.
- Guardiola, F.A.; Porcino, C.; Cerezuela, R.; Cuesta, A.; Faggio, C.; Esteban, M.A. Impact of Date Palm Fruits Extracts and Probiotic Enriched Diet on Antioxidant Status, Innate Immune Response and Immune-Related Gene Expression of European Seabass (Dicentrarchus labrax). Fish Shellfish Immunol. 2016, 52, 298–308.
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic Bacteria as Biological Control Agents in Aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671.
- Wuertz, S.; Schroeder, A.; Wanka, K.M. Probiotics in Fish Nutrition—Long-Standing Household Remedy or Native Nutraceuticals? Water 2021, 13, 1348.
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429.
- Frawley, E.R.; Fang, F.C. The Ins and Outs of Bacterial Iron Metabolism. Mol. Microbiol. 2014, 93, 609–616.
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in Iron Metabolism: From Mechanism to Therapy Potential. Trends Mol. Med. 2016, 22, 1077–1090.
- Lalloo, R.; Moonsamy, G.; Ramchuran, S.; Görgens, J.; Gardiner, N. Competitive Exclusion as a Mode of Action of a Novel Bacillus cereus Aquaculture Biological Agent. Lett. Appl. Microbiol. 2010, 50, 563–570.
- Khan, A.; Doshi, H.V.; Thakur, M.C. Bacillus spp.: A Prolific Siderophore Producer. Bacilli Agrobiotechnol. 2017, 14, 309–323.
- Assan, D.; Kuebutornye, F.K.A.; Hlordzi, V.; Chen, H.; Mraz, J.; Mustapha, U.F.; Abarike, E.D. Effects of Probiotics on Digestive Enzymes of Fish (Finfish and Shellfish); Status and Prospects: A Mini Review. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110653.
- Ringø, E.; Van Doan, H.; Lee, S.H.; Soltani, M.; Hoseinifar, S.H.; Harikrishnan, R.; Song, S.K. Probiotics, Lactic Acid Bacteria and Bacilli: Interesting Supplementation for Aquaculture. J. Appl. Microbiol. 2020, 129, 116–136.
- Hamza, A.; Fdhila, K.; Zouiten, D.; Masmoudi, A.S. Virgibacillus proomii and Bacillus mojavensis as Probiotics in Sea Bass (Dicentrarchus labrax) Larvae: Effects on Growth Performance and Digestive Enzyme Activities. Fish Physiol. Biochem. 2016, 42, 495–507.
- Frouël, S.; Le Bihan, E.; Serpentini, A.; Lebel, J.M.; Koueta, N.; Nicolas, J.L. Preliminary Study of the Effects of Commercial Lactobacilli Preparations on Digestive Metabolism of Juvenile Sea Bass (Dicentrarchus labrax). Microb. Physiol. 2008, 14, 100–106.
- Tovar, D.; Zambonino, J.; Cahu, C.; Gatesoupe, F.J.; Vázquez-Juárez, R.; Lésel, R. Effect of Live Yeast Incorporation in Compound Diet on Digestive Enzyme Activity in Sea Bass (Dicentrarchus labrax) Larvae. Aquaculture 2002, 204, 113–123.
- Tovar-Ramírez, D.; Zambonino Infante, J.; Cahu, C.; Gatesoupe, F.J.; Vázquez-Juárez, R. Influence of Dietary Live Yeast on European Sea Bass (Dicentrarchus labrax) Larval Development. Aquaculture 2004, 234, 415–427.
- Chouayekh, H.; Farhat-Khemakhem, A.; Karray, F.; Boubaker, I.; Mhiri, N.; Abdallah, M.B.; Alghamdi, O.A.; Guerbej, H. Effects of Dietary Supplementation with Bacillus amyloliquefaciens US573 on Intestinal Morphology and Gut Microbiota of European Sea Bass. Probiotics Antimicrob. Proteins 2022, 15, 30–43.
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521.
- Chistiakov, D.A.; Hellemans, B.; Volckaert, F.A.M. Review on the Immunology of European Sea Bass Dicentrarchus labrax. Vet. Immunol. Immunopathol. 2007, 117, 1–16.
- Serradell, A.; Torrecillas, S.; Makol, A.; Valdenegro, V.; Fernández-Montero, A.; Acosta, F.; Izquierdo, M.S.; Montero, D. Prebiotics and Phytogenics Functional Additives in Low Fish Meal and Fish Oil Based Diets for European Sea Bass (Dicentrarchus labrax): Effects on Stress and Immune Responses. Fish Shellfish Immunol. 2020, 100, 219–229.
- El-Shazly, M.; Wu, H.-C.; Cheyadmi, S.; Chadli, H.; Nhhala, H.; Yamlahi, B.E.; Maadoudi, M.E.; Kounnoun, A.; Cacciola, F.; Ez-Zaaim, A.; et al. Primary and Secondary Physiological Stress Responses of European Sea Bass (Dicentrarchus labrax) Due to Rearing Practices under Aquaculture Farming Conditions in M’diq Bay, Moroccan Mediterranean: The Case of Sampling Operation for Size and Weight Measurement. Life 2022, 13, 110.
- Torrecillas, S.; Rivero-Ramírez, F.; Izquierdo, M.S.; Caballero, M.J.; Makol, A.; Suarez-Bregua, P.; Fernández-Montero, A.; Rotllant, J.; Montero, D. Feeding European Sea Bass (Dicentrarchus labrax) Juveniles with a Functional Synbiotic Additive (Mannan oligosaccharides and Pediococcus acidilactici): An Effective Tool to Reduce Low Fishmeal and Fish Oil Gut Health Effects? Fish Shellfish Immunol. 2018, 81, 10–20.
- Lamari, F.; Mahdhi, A.; Chakroun, I.; Esteban, M.A.; Mazurais, D.; Amina, B.; Gatesoupe, F.J. Interactions between Candidate Probiotics and the Immune and Antioxidative Responses of European Sea Bass (Dicentrarchus labrax) Larvae. J. Fish Dis. 2016, 39, 1421–1432.
- Rollo, A.; Sulpizio, R.; Nardi, M.; Silvi, S.; Orpianesi, C.; Caggiano, M.; Cresci, A.; Carnevali, O. Live Microbial Feed Supplement in Aquaculture for Improvement of Stress Tolerance. Fish Physiol. Biochem. 2006, 32, 167–177.
- Silvi, S.; Nardi, M.; Sulpizio, R.; Orpianesi, C.; Caggiano, M.; Carnevali, O.; Cresci, A. Effect of the Addition of Lactobacillus delbrueckii Subsp. delbrueckii on the Gut Microbiota Composition and Contribution to the Well-Being of European Sea Bass (Dicentrarchus labrax L.). Microb. Ecol. Health Dis. 2009, 20, 53–59.
- Carnevali, O.; de Vivo, L.; Sulpizio, R.; Gioacchini, G.; Olivotto, I.; Silvi, S.; Cresci, A. Growth Improvement by Probiotic in European Sea Bass Juveniles (Dicentrarchus labrax, L.), with Particular Attention to IGF-1, Myostatin and Cortisol Gene Expression. Aquaculture 2006, 258, 430–438.
- Aerts, J.; Schaeck, M.; De Swaef, E.; Ampe, B.; Decostere, A. Vibrio lentus as a Probiotic Candidate Lowers Glucocorticoid Levels in Gnotobiotic Sea Bass Larvae. Aquaculture 2018, 492, 40–45.
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A Review on the Application of Bacillus as Probiotics in Aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828.
- Baldissera, M.D.; Souza, C.F.; Parmeggiani, B.; Leipnitz, G.; Verdi, C.M.; Santos, R.C.V.; Stefani, L.M.; Baldisserotto, B. The Disturbance of Antioxidant/Oxidant Balance in Fish Experimentally Infected by Aeromonas caviae: Relationship with Disease Pathophysiology. Microb. Pathog. 2018, 122, 53–57.
- Salem, A.M.; Ibrahim, H.A. Effects of Dietary Marine Bacillus subtilis HS1 Probiotic, Chitosan Prebiotic and Two Marine Synbiotics Mixtures on the Growth and Oxidative Stress of the European Seabass (Dicentrarchus labrax) Larvae. Egypt J. Aquat. Biol. Fish 2022, 26, 1119–1138.
- Tovar-Ramírez, D.; Mazurais, D.; Gatesoupe, J.F.; Quazuguel, P.; Cahu, C.L.; Zambonino-Infante, J.L. Dietary Probiotic Live Yeast Modulates Antioxidant Enzyme Activities and Gene Expression of Sea Bass (Dicentrarchus labrax) Larvae. Aquaculture 2009, 300, 142–147.
- Schaeck, M.; Reyes-López, F.E.; Vallejos-Vidal, E.; Van Cleemput, J.; Duchateau, L.; Van den Broeck, W.; Tort, L.; Decostere, A. Cellular and Transcriptomic Response to Treatment with the Probiotic Candidate Vibrio lentus in Gnotobiotic Sea Bass (Dicentrarchus labrax) Larvae. Fish Shellfish Immunol. 2017, 63, 147–156.
- Oliva-Teles, A. Nutrition and Health of Aquaculture Fish. J. Fish Dis. 2012, 35, 83–108.
- Eissa, E.-S.H.; Baghdady, E.S.; Gaafar, A.Y.; El-Badawi, A.A.; Bazina, W.K.; Al-Kareem, O.M.A.; El-Hamed, N.N.B.A. Assessing the Influence of Dietary Pediococcus acidilactici Probiotic Supplementation in the Feed of European Sea Bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on Farm Water Quality, Growth, Feed Utilization, Survival Rate, Body Composition, Blood Biochemical Parameters, and Intestinal Histology. Aquac. Nutr. 2022, 2022, 1–11.
- Picchietti, S.; Fausto, A.M.; Randelli, E.; Carnevali, O.; Taddei, A.R.; Buonocore, F.; Scapigliati, G.; Abelli, L. Early Treatment with Lactobacillus delbrueckii Strain Induces an Increase in Intestinal T-Cells and Granulocytes and Modulates Immune-Related Genes of Larval (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2009, 26, 368–376.
- Abelli, L.; Randelli, E.; Carnevali, O.; Picchietti, S. Stimulation of Gut Immune System by Early Administration of Probiotic Strains in Dicentrarchus labrax and Sparus aurata. Ann. N. Y. Acad. Sci. 2009, 1163, 340–342.
- Román, L.; Real, F.; Padilla, D.; Aamri, F.E.; Déniz, S.; Grasso, V.; Acosta, F. Cytokine Expression in Head-Kidney Leucocytes of European Sea Bass (Dicentrarchus labrax L.) after Incubation with the Probiotic Vagococcus fluvialis L-21. Fish Shellfish Immunol. 2013, 35, 1329–1332.
- Mladineo, I.; Bušelic, I.; Hrabar, J.; Radonic, I.; Vrbatovic, A.; Jozic, S.; Trumbic, Ž. Autochthonous Bacterial Isolates Successfully Stimulate In Vitro Peripheral Blood Leukocytes of the European Sea Bass (Dicentrarchus labrax). Front. Microbiol. 2016, 7, 1244.
- Nadal, A.L.; Ikeda-Ohtsubo, W.; Sipkema, D.; Peggs, D.; McGurk, C.; Forlenza, M.; Wiegertjes, G.F.; Brugman, S. Feed, Microbiota, and Gut Immunity: Using the Zebrafish Model to Understand Fish Health. Front. Immunol. 2020, 11, 114.
- Xia, Y.; Wang, M.; Gao, F.; Lu, M.; Chen, G. Effects of Dietary Probiotic Supplementation on the Growth, Gut Health and Disease Resistance of Juvenile Nile Tilapia (Oreochromis niloticus). Anim. Nutr. 2020, 6, 69–79.
- Butt, R.L.; Volkoff, H. Gut Microbiota and Energy Homeostasis in Fish. Front. Endocrinol. 2019, 10, 9.




