Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christophe Wiart | -- | 1973 | 2023-07-31 11:14:15 | | | |
| 2 | Peter Tang | Meta information modification | 1973 | 2023-07-31 11:18:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wiart, C.; Shorna, A.A.; Rahmatullah, M.; Nissapatorn, V.; Seelan, J.S.S.; Rahman, H.; Rusdi, N.A.; Mustaffa, N.; Elbehairy, L.; Sulaiman, M. Scorodocarpus borneensis (Baill.) Becc.. Encyclopedia. Available online: https://encyclopedia.pub/entry/47425 (accessed on 08 February 2026).
Wiart C, Shorna AA, Rahmatullah M, Nissapatorn V, Seelan JSS, Rahman H, et al. Scorodocarpus borneensis (Baill.) Becc.. Encyclopedia. Available at: https://encyclopedia.pub/entry/47425. Accessed February 08, 2026.
Wiart, Christophe, Afsana Amin Shorna, Mohammed Rahmatullah, Veeranoot Nissapatorn, Jaya Seelan Sathya Seelan, Homathevi Rahman, Nor Azizun Rusdi, Nazirah Mustaffa, Layane Elbehairy, Mazdida Sulaiman. "Scorodocarpus borneensis (Baill.) Becc." Encyclopedia, https://encyclopedia.pub/entry/47425 (accessed February 08, 2026).
Wiart, C., Shorna, A.A., Rahmatullah, M., Nissapatorn, V., Seelan, J.S.S., Rahman, H., Rusdi, N.A., Mustaffa, N., Elbehairy, L., & Sulaiman, M. (2023, July 31). Scorodocarpus borneensis (Baill.) Becc.. In Encyclopedia. https://encyclopedia.pub/entry/47425
Wiart, Christophe, et al. "Scorodocarpus borneensis (Baill.) Becc.." Encyclopedia. Web. 31 July, 2023.
Copy Citation
Scorodocarpus borneensis (Baill.) Becc. is attracting increased attention as a potential commercial medicinal plant product in Southeast Asia.
garlic tree
nutraceutical
organosulfurs
Scorodocapus borneensis
1. Introduction
Approximately 14,500 species of flowering plants are estimated to exist in Malaysia (including Sarawak and Sabah), of which 2500 are medicinal [1]. Out of these, four have captured global attention for their therapeutic potentials: Calophyllum teysmannii var inophyloides (King) P.F. Stevens from Sarawak as a source of antiretroviral agents [2]; Mitragyna speciosa Korth. for opioid addiction [3]; Labisia pumila Benth. & Hook. f. as a female aphrodisiac [4]; and Eurycoma longifolia Jack as a male aphrodisiac, with the latter probably being hazardous [5]. Rainforest medicinal plants in Malaysia and Southeast Asia are on the verge of extinction due to incessant burning and logging for palm oil [6]; in addition, there is a need to document and examine their potential utilization in pharmaceutical/nutraceutical/functional cosmetic applications before their extinction.
Infections resulting from Pseudomonas aeruginosa, carbapenem-resistant Acinetobacter baumannii, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus (MRSA) might soon become completely untreatable [7]. In 2009, a multidrug-resistant yeast Candida uris emerged in Japan with a death rate of approximately 50%. This yeast resists azoles, echinocandin, and amphotericin B, stays alive for seven days on inanimate surfaces, and evades ICU’s sanitation protocols [8]. If left unchecked, 10 million people could die yearly from untreatable microbial infections by 2050 [9].
2. Taxonomy, Habitat, Distribution, Ecology, and Botanical Description
S. borneensis is a massive timber tree belonging to the Olacaceae A. L. de Jussieu ex R. Brown (1818) family in the order Santalales R. Br. ex Bercht. & J. Presl (1820). It grows in the rainforests of Thailand, Malaysia, and Indonesia and reaches approximately 60 m in height. This plant has a unique and stout odor of garlic that can be perceived up to 100 m away. Its bark is dark brown, and flaky, and the inner bark is reddish-orange and sappy. The leaves are simple, alternate, and exstipulate. The petioles are 1.5–2 cm long. The blades are elliptic, glossy, 4–9 cm × 10–22 cm, dark green, cuneate at the base, acuminate at the apex, and marked with 5–6 pairs of secondary nerves. The racemes are axillary and approximately 4 cm long. The calyx is minute, tubular, and vaguely 4–5-lobed. The corolla is tubular, white, hairy, 4–5-lobed, and approximately 1 cm long. A total of 8–10 sessile stamens with filamentous anthers are present. The ovary is slender, hairy and approximately 5 mm long. The drupes are somewhat globose, green, and comprise a woody endocarp approximately 5 cm across (Figure 1) carved with blood vessel-like lines sheltering a pungent, spongy, and oily seed with the size and appearance of a somewhat light brown ping pong ball [10]. The transversal section of the germinating seeds presents purple patches (personal observation).

Figure 1. Endocarp of S. borneensis.
3. Medicinal Uses
Henry Nicholas Ridley listed the plant in his “Malay Materia Medica” (J. Straits Medical Assn. 5, 122) under the local name “kulim” and wrote the following: “A large tree every part of which smells strongly of onions”. The fresh seeds are used for medicine in Malaysia and Indonesia. In Peninsular Malaysia, a decoction of seeds is ingested to prevent or treat kidney failure and fresh pounded seeds are applied to ringworms. Other uses for the seeds include high blood pressure, stroke, heart diseases, and food poisoning, as well as a substitute for garlic, from which the Malay name “bawang hutan” literally meaning garlic of the forest. In Sabah, the Murut people use the seeds for food (local name: Sedau) [11]. In Kalimantan, the tree is called “kayu bawang” meaning wood garlic and the fruits are eaten in place of garlic [12] and used prevent meat and oil from decay [13]. In Sumatra, the locals use the seeds for food purposes and for intestinal worms [14].
4. Antibacterial and Antifungal Activity of Extracts
There is a great need for affordable antibacterial agents to confront the emergence of resistant bacteria in clinical practice, sanitation, food preservation, veterinary medicine, and for the treatment of infected crops [7]. The seeds and leaves of S. borneensis contain antimicrobial principle.
The methanol extract of leaves (60 µL of 50% w/v in 5 mm well) inhibited the growth of MRSA, E. coli, and C. albicans while delaying the bacterial decay of red tilapia filets [13].The petroleum ether extract of fresh seeds inhibited the growth of B. cereus, P. aeruginosa, C. albicans, and A. ochraceus.
A preparation made of 50 mg of this oil mixed with 1 g of paraffin was able to protect rodents against Microsporium sp. skin infection as well as ringworm [14]. Essential oil of leaves (yield 0.3%; 20 µL/5 mm well) in 6 mm agar wells of inhibited the growth of S. sobrinus, S. nutans, C. albicans, S. aureus, and S.typhi [15][16]. A dichloromethane extract of leaves inhibited the replication of the Hepatitis B virus [17]. Indonesian workers have attempted to identify active principles [18].
The ethyl acetate extract of bark (100 µL of a 10% w/v/6 mm well) inhibited the growth of S. aureus and E. coli [19]. The mechanism of actions of these extracts are not yet known.
5. Cytotoxicity and Brine Shrimp Toxicity of Extracts
Organosulfur compounds from garlic in vitro inhibited the growth of lymphocytic leukemia cells [20]. Likewise, extracts of S. borneensis are cytotoxic for leukemia cells. The methanol extracts of seeds, leaves, and bark inhibited T-Lymphoblastic leukemia CEM-SS cells, while the n-hexane extract of seeds inhibited the growth of mouse lymphocytic leukemia L1210 cells [14]. In a subsequent study, ethyl acetate extract of bark was toxic for brine shrimps [12].
6. Termiticidal Activity of Extracts
Coptotermes curvignathus and Coptotermes gestroi account for significant losses in fruit trees, timber, coconut, rubber tree plantations and paddy fields in Southeast Asia, for which environmentally friendly termiticides are desperately needed. n-Hexane and ethyl acetate extract of bark were toxic for C. curvignathus with the LC50 values of 0.01 and 0.02% (w/v), respectively [21]. The acetone extract of wood exhibited repellent activity against Coptotermes gestroi [22].
7. Radical-Scavenging Activity of Extracts
The involvement of free radicals in the pathophysiology of cardiovascular, metabolic, and neurodegenerative diseases is well-established [23] and the chemo-preventive effect of garlic (Allium sativum L.) organosulfur is owed, at least in part, to radical-scavenging effects [24]. The methanol extract from leaves, bark, and seeds [13], ethyl acetate extract of bark [19], the ethanol extract of seeds [25], and the n-hexane extracts of seeds scavenged DPPH free radicals [22]. The essential oil of leaves displayed meek radical-scavenging activities [16][17]. The anti-free radical activities of organosulfur compounds in this plant need to be examined. The organosulfur compounds in general can protect cells against free radicals by interacting with oxidative stressors and affecting the function of redox-sensitive cysteine proteins [24].
8. Organosulfur Compounds
The seeds of S. borneensis radiate an intense garlic odor due to the volatile organosulfur compounds (Figure 2) first identified by Kubota and coworkers (1994) [26].
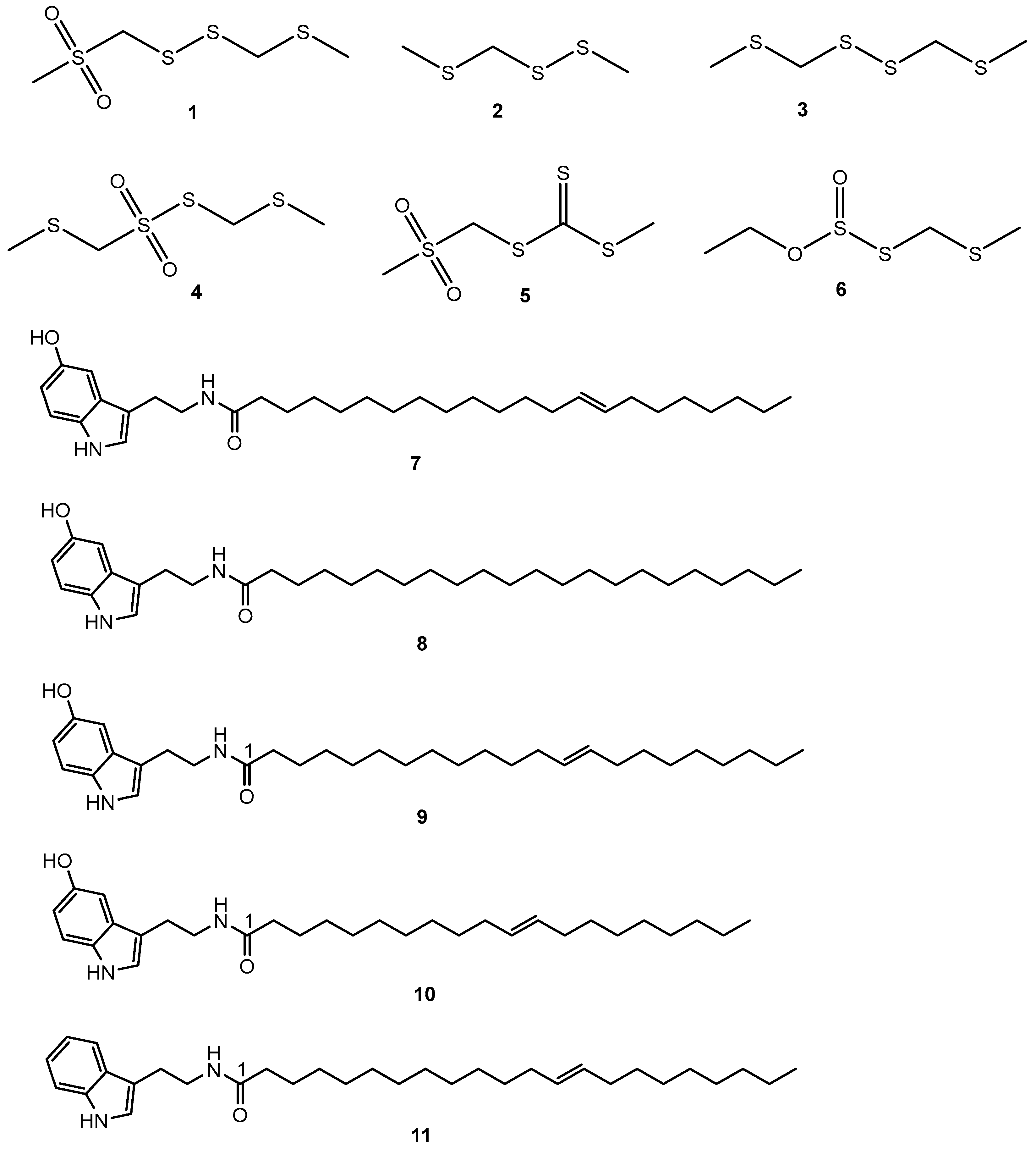
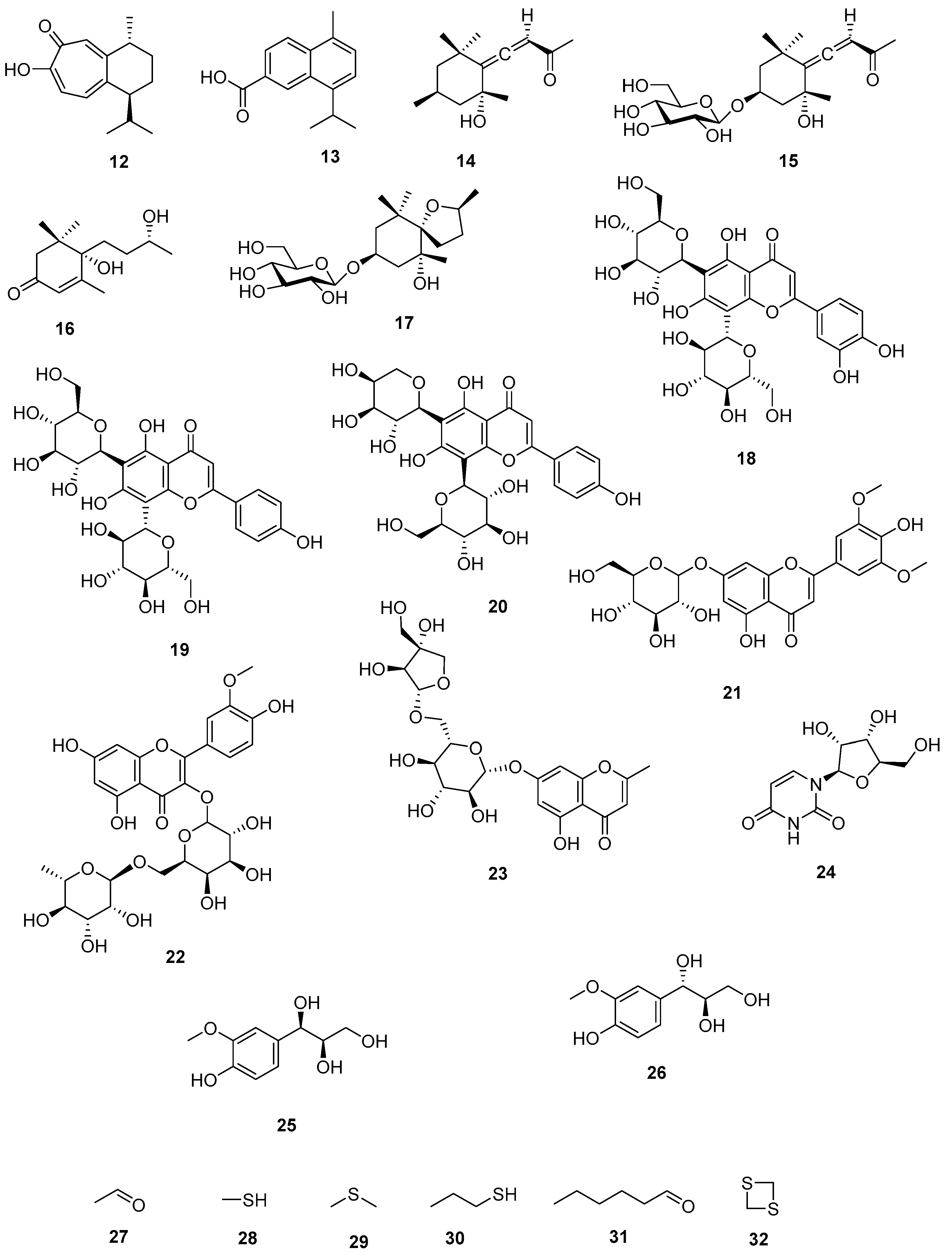
Figure 2. Secondary metabolites identified from S. borneensis.
Methylthiomethyl(methylsulfonyl)methyl disulfide (1), methyl methylthiomethyl disulfide (2), and bis-(methylthiomethyl)disulfide (3). Methylthiomethyl(methylsulfonyl)methyl disulfide (1) has an odor threshold as low as 1.6 ppm and inhibited the growth of S. aureus (FDA 209P), Micrococcus luteus (PCI 1002), Bacillus subtilis (PCI 219), Mycobacterium smegmatis (ATCC 607), Escherichia coli (NIHJ), Candida albicans (KF 1), Saccharomyces cerevisae (ATCC 9763), Mucor racemosus (IFO 4581), and Aspergillus niger (KF 105), while being inactive for P. aeruginosa (IFO 3080) [26].
Bis-(methylthiomethyl) disulfide (3) inhibited the growth of S. aureus (FDA 209P), Bacillus subtilis (PCI 219), Mycobacterium smegmatis (ATCC 607), Escherichia coli (NIHJ), Candida albicans (KF 1), Saccharomyces cerevisae (ATCC 9763), Mucor racemosus (IFO 4581), and Aspergillus niger (KF 105), while being inactive for M. luteus (PCI 1002). In this experiment, methyl methylthiomethyl disulfide (2) was inactive against all the strains tested. Being a major constituent, bis-(methylthiomethyl) disulfide (3) has been suggested to be used as a flavoring agent and food preservative [26][27][28][29]. Subsequently, bis-(methylthiomethyl)disulfide (3) inhibited the growth of Bacillus cereus, Pseudomonas aeruginosa, Aspergillus ochraceus, Saccharomyces lipolytica, Candida lipolytica, and Saccharomyces lypolitica, Penicillium sp., Acremonium sp., Microsporium sp., and Pseudoscaellia boedes [14].
Lim et al., 1998 [29] further identified from the seeds 2,4,5,7-tetrathiaoctane 4,4-dioxide (4) and 5-thioxo-2,4,6-trithiaheptane 2,2-dioxide (5) both antibacterial and antifungal as well as O-ethyl-S-methylthiomethyl thiosulfite (6). These linear organosulfur compounds are not common in flowering plants. Bis(methylthiomethyl) disulfide (3) is only known to be produced by Gallesia integrifolia (Spreng.) Harms in the family Phytolaccaceae [30] in the order Caryophyllales Juss. ex Bercht. & J. Presl (1820), which is a neighbor to the order Santalales in the Clade Malvids. Organosulfur compounds different from those of S. borneensis are found in members of the genus Allium L. (family Amaryllidaceae, Clade Monocots). Lim et al., 1998 [29] proposed a biosynthetic pathway for S. borneensis organosulfur compounds similar to that of plants in the genus Allium L.; however, Kubota et al., 1998 [31] provided evidence for (Rs)-3-[(methylthio)methylsulfinyl]-l-alanine and S-[(methylthio)methyl]-l-cysteine as the precursors of methyl methylthiomethyl disulfide (2) and bis(methylthiomethyl) disulfide (3), respectively.
Thrombolytic agents are needed to prevent strokes, which are one of the major causes of death globally. Organosulfur compounds in the seeds of S. borneensis inhibit platelet aggregation. Methylthiomethyl (methylsulfonyl)methyl disulfide (1) (2,4,5,7-tetrathiaoctane 2,2-dioxide), methyl methylthiomethyl disulfide (2) (2,4,5-trithiahexane I), bis-(methylthiomethyl) disulfide (2,4,5,7-tetrathiaoctane II) (3), and 2,4,5,7-tetrathiaoctane 4,4-dioxide (4) inhibited the aggregation of rabbit platelets induced by collagen [32]. Bis-(methylthiomethyl) disulfide (3) inhibited the growth of T-Lymphoblastic leukemia CEM-SS cells [14]. The antimicrobial and cytotoxic modes of action of these organosulfur compounds are unknown and could involve, at least in part, the disruption of DNA and cellular membranes [23].
9. Indole Alkaloids
The seeds of S. borneensis, especially when they germinate, yield long-chain and purple-colored indole alkaloids (Figure 2) of a very uncommon constitution in flowering plants such as 13-docosenoyl serotonin (7), scorodocarpine A (8), B (9), and C (10) [14] as well as dehydroxy scorodocarpine B (11) [14][33][34]. These alkaloids tend to inhibit the growth of leukemia cells in vitro. 13-Docosenoyl serotonin (7) inhibited the growth of T-Lymphoblastic leukemia CEM-SS cells [14], while scorodocarpine B (9) and dehydroxyl scorodocarpine B (11) were cytotoxic towards L1210 mouse lymphocytic leukemia cells [19] and scavenged 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals [33]. Being indole alkaloids with long chains, these rare alkaloids might have neurotrophic properties [35].
10. Sesquiterpenes
The seeds contain scodopin (12) and the bark cadalene-β-carboxylic acid (13) [22][35] (Figure 2). Scodopin (12) inhibited the growth of B. cereus and P. aeruginosa, and was cytotoxic for T-Lymphoblastic leukemia CEM-SS cells [14]. Cadalene-β-carboxylic acid (13) is toxic to brine shrimps [22].
11. Megastigmanes
Grasshopper ketone (14), icariside B1 (15), blumenol B (16), and scorospiroside (17) have been identified from the leaves Figure 2). Grasshopper ketone (14) decreased cytokine production by mice splenocytes challenged with in concanavalin A while at concentrations ranging from 0.1 to 100 µg/mL inhibited the production of nitric oxide, interleukin-6, interleukin-1β, and tumor necrosis factor-α by RAW 264.7 cells challenged with lipopolysaccharides. Grasshopper ketone (14) inhibited the growth of cress shoots at concentrations greater than 10 μmol/L. At 600 ppm, blumenol B (16) inhibited the growth of Miscanthus floridulus [36][37][38][39][40].
12. Flavonoid Glycosides
A phytochemical analysis of the leaves resulted in the identification of lucenin-2 (luteolin 6,8-di-C-glucoside) (18), vicenin-2 (apigenin 6,8-di-C-glucoside) (19), isoschaftoside (apigenin 6-C-arabinosyl-8-C-glucoside) (20), tricin 7-O-glucoside (21), and isorhamnetin 3-O-robinobioside (22) [40] (Figure 2). Lucenin-2 (18) inhibited the growth of P. aeruginosa (ATCC 27853), E. coli (ATCC 11229), and K. pneumoniae (ATCC 27736) with the MIC values of 8, 64, and 64 μg/mL, respectively [41]. Vicenin-2 (19) displayed antiglycation [42], anti-inflammatory [43], antiseptic [44], antiosteoporosis [45] properties, and proved to be of potential value against prostate cancer [46] and colon cancer [47]. Isoschaftoside (20) is phytotoxic [48], hepatoprotective [49], and diuretic [50]. Isorhamnetin 3-O-robinobioside (22) is antigenotoxic [51], and immunostimulant [52][53].
13. Miscellaneous
5,7-Dihydroxy-2-methylchromone-7-O-β-D-apiosyl (1,6)-β-D-glucoside (23) was identified from the leaves as well as uridine (24) Threo-guaiacylglycerol (25) and erythro-guaiacylglycerol (26) [40]. Kubota et al., 1994 [26] identified via GC-MS analysis (by comparison with standards) from the essential oil of the fresh seeds (89 mg/100 g) ethanal (96.4%) (27) and traces of methanethiol (28), dimethyl sulfide (29), propane thiol (30), dimethyl sulfide (31), (E)-2-hexanal (32), and 1.3 dithietane (33) (Figure 2).
14. Toxicity, Side Effects, and Drug Interaction
There are no preclinical studies available on the toxicity of the fruits of S. borneensis. Petroleum ether extract of seeds had an intraperitoneal lethal dose 50% (LD50) value of 275 mg/Kg in mice and, when mixed at 50 mg in 1 g of paraffin, it did not irritate the skin of rabbits [14]. The use of the seeds for food by the Malays, Indonesians, and other ethnic groups in North Borneo since the dawn of time might be an indication of a lack of toxicity when taken at a dietary dose; however, acute or chronic toxicity studies, including drug interaction studies are needed, especially regarding the thrombolytic activities of organosulfur compounds [32].
References
- Kulip, J.; Fan, L.N.; Manshoor, N.; Julius, A.; Said, I.M.; Gisil, J.; Joseph, J.A.; Tukin, W.F. Medicinal plants in Maliau Basin, Sabah, Malaysia. J. Trop. Biol. Conserv. 2010, 6, 21–33.
- Fuller, R.W.; Bokesch, H.R.; Gustafson, K.R.; McKee, T.C.; Cardellina, I.I.J.H.; McMahon, J.B.; Cragg, G.M.; Soejarto, D.D.; Boyd, M.R. HIV-inhibitory coumarins from latex of the tropical rainforest tree Calophyllum teysmannii var. inophylloide. Bioorganic Med. Chem. Lett. 1994, 4, 1961–1964.
- Boyer, E.W.; Babu, K.M.; Adkins, J.E.; McCurdy, C.R.; Halpern, J.H. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction 2008, 103, 1048–1050.
- Norhayati, M.N.; George, A.; Nik Hazlina, N.H.; Azidah, A.K.; Intan Idiana, H.; Law, K.S.; Shaiful Bahari, I.; Wan Zahiruddin, W.M.; Liske, E.; Azreena, A. Efficacy and safety of Labisia pumila var alata water extract among pre-and postmenopausal women. J. Med. Food. 2014, 17, 929–938.
- Faisal, G.G.; Alahmad, B.E.; Mustafa, N.S.; Najmuldeen, G.F.; Althunibat, O.; Azzubaidi, M.S. Histopathological effects of Eurycoma longifolia Jack extract (Tongkat Ali) on the prostate of rats. J. Asian Sci. Res. 2013, 3, 843–851.
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen FBrühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545.
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187.
- Hu, S.; Zhu, F.; Jiang, W.; Wang, Y.; Quan, Y.; Zhang, G.; Gu, F.; Yang, Y. Retrospective analysis of the clinical characteristics of Candida auris infection worldwide from 2009 to 2020. Front. Microbiol. 2021, 12, 658329.
- Álvarez-Pérez, S.; García, M.E.; Anega, B.; Blanco, J.L. Antifungal Resistance in Animal Medicine: Current State and Future Challenges. In Fungal Diseases in Animals; Springer: Cham, Switzerland, 2021; pp. 163–179.
- Kodoh, J. Surveys of non-timber forest products traded in tamu, Sabah, Malaysia. Sepilok Bull. 2005, 3, 27–36.
- Kustiawan, P.M.; Siregar, K.A.A.K.; Saleh, L.O.; Batistuta, M.A.; Setiawan, I.M. A review of botanical characteristics, chemical composition, pharmacological activity and use of Scorodocarpus borneensis. Biointerface Res. Appl. Chem. 2021, 12, 8324–8334.
- Dewi, Y.S.K.; Simamora, C.J.K.; Fadly, D. Antioxidant and antimicrobial activities of methanolic extracts of Scorodocarpus borneensis Becc. Syst. Rev. Pharm. 2020, 11, 246–252.
- Heriyanto, N.M.; Garsetiasih, R. Potensi pohon kulim (Scorodocarpus borneensis Becc.) di kelompok hutan Gelawan Kampar, Riau. Bul. Plasma Nutfah 2004, 10, 37–42.
- Wiart, C. Antimicrobial and Cytotoxic Compounds of Scorodocarpus borneensis (Olacaceae) and Glycosmis calcicola (Rutaceae). Ph.D. Thesis, Universiti Putra Malaysia, Kuala Lumpur, Malaysia, 2001.
- Kuspradini, H.; Sinta Putri, A.; Edi Sukaton, E.; Tohru Mitsunaga, T. Bioactivity of essential oils from leaves of Dryobalanops lanceolata, Cinnamomum burmannii, Cananga odorata and Scorodocarpus borneensis. Agric. Sci. Procedia 2016, 9, 411–418.
- Kuspradini, H.; Egra, S.; Wulandari, I.; Putri, A.S. Chemical composition and bioactivity of essential oil from the leaves of Scorodocarpus borneensis Becc. (Olacaceae) grown in Indonesia. Asia Life Sci. 2018, 27, 343–353.
- Wahyuni, T.S.; Permanasari, A.A.; Widyawaruyanti, A.; Hotta, H.; Aoki-Utsubo, C.; Hafid, A.F. Antiviral activity of Indonesian medicinal plants against hepatitis B virus. Pharmacogn. J. 2020, 12, 1108–1114.
- Sudrajat, S.; Kartika, R.; Kustiawan, W. Analysis phytochemical compounds of ethyl acetate extract garlic tree, Scorodocarpus borneensis Becc. as a source of bioactive ingredients. Int. J. Sci. Technol. Res. 2019, 8, 183–186.
- Kartika, R.; Bustanussalam, B.; Simanjuntak, P. Identification of cadalene-β-carboxylic acid from barks of bawang hutan (Scorodocarpus borneensis Becc.). Ann. Bogor. 2012, 16, 19–22.
- Wiart, C. Medicinal Plants of Southeast Asia, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 2002.
- Smith, T.; Majid, F.; Eckl, V.; Reynolds, C.M. Herbal supplement sales in US increase by record-breaking 17.3% in 2020. HerbalGram 2021, 131, 52–65.
- Kadir, R.; Hassan, B. Toxicity and repellent effects of wood extractives of five Malaysian wood species on Asian subterranean termite Coptotermes gestroi Wasmann. Eur. J. Wood Prod. 2020, 78, 1249–1262.
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863.
- Shukla, Y.; Kalra, N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007, 247, 167–181.
- Dewi, Y.S.; Purwayantie, P. Phytochemical and antioxidant activity from fruits of Kulim (Scorodocarpus borneensis Becc.). In Proceedings of the Second International on Food and Agriculture, Bali, Indonesia, 2–3 November 2019; Volume 2, pp. 519–524.
- Kubota, K.; Matsumoto, M.; Ueda, M.; Kobayashi, A. New antimicrobial compound from Scorodocarpus borneensis Becc. Biosci. Biotechnol. Biochem. 1994, 58, 430–431.
- Kubota, K.; Kobayashi, A. Sulfur compounds in wood garlic (Scorodocarpus borneensis Becc.) as versatile food components. In Sulfur Compounds in Foods; ACS Symposium Series, No. 564; American Chemical Society: Washington, DC, USA, 1994; pp. 238–246.
- Kubota, K.; Ohhira, S.; Kobayashi, A. Identification and antimicrobial activity of the volatile flavor constituents from Scorodocarpus borneensis Becc. Biosci. Biotechnol. Biochem. 1994, 58, 644–646.
- Lim, H.; Kubota, K.; Kobayashi, A.; Sugawara, F. Sulfur-containing compounds from Scorodocarpus borneensis and their antimicrobial activity. Phytochemistry 1998, 48, 787–790.
- Akisue, M.; Wasicky, R.; Akisue, R.; De Oliveira, F. Gallesia integrifolia (Sprengel) harms-essential oil of leaves. Rev. Farm. Bioquímica Univ. Sao Paulo 1984, 20, 145–147.
- Kubota, K.; Hirayama, H.; Sato, Y.; Kobayashi, A.; Sugawara, F. Amino acid precursors of the garlic-like odour in Scorodocarpus borneensis. Phytochemistry 1998, 49, 99–102.
- Lim, H.; Kubota, K.; Kobayashi, A.; Seki, T.; Ariga, T. Inhibitory effect of sulfur-containing compounds in Scorodocarpus borneensis Becc. on the aggregation of rabbit platelets. Biosci. Biotechnol. Biochem. 1999, 63, 298–301.
- Kartika, R.; Barus, T.; Surbakti, R.; Simanjuntak, P. Structure characterization of alkaloid scorodocarpines derivative from fruits of Scorodocarpus borneensis Becc. (Olacaceae). Asian J. Chem. 2014, 26, 6047–6049.
- Wiart, C.; Martin, M.T.; Awang, K.; Hue, N.; Serani, L.; Laprévote, O.; Païs, M.; Rahmani, M. Sesquiterpenes and alkaloids from Scorodocarpus borneensis. Phytochemistry 2001, 58, 653–656.
- Coowar, D.; Bouissac, J.; Hanbali, M.; Paschaki, M.; Mohier, E.; Luu, B. Effects of indole fatty alcohols on the differentiation of neural stem cell derived neurospheres. J. Med. Chem. 2004, 47, 6270–6282.
- Kim, M.J.; Jeong, S.M.; Kang, B.K. Anti-inflammatory effects of grasshopper ketone from Sargassum fulvellum ethanol extract on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. J. Microbiol. Biotechnol. 2019, 9, 820–826.
- Kato-Noguchi, H.; Tamura, K.; Sasaki, H.; Suenaga, K. Identification of two phytotoxins, blumenol A and grasshopper ketone, in the allelopathic Japanese rice variety Awaakamai. J. Plant Physiol. 2012, 169, 682–685.
- Tseng, M.H.; Kuo, Y.H.; Chen, Y.M.; Chou, C.H. Allelopathic potential of Macaranga tanarius (L.). Muell.—Arg. J. Chem. Ecol. 2003, 29, 1269–1286.
- Kang, B.K.; Kim, M.J.; Ahn, D.H. In vivo and in vitro inhibitory activity of an ethanolic extract of Sargassum fulvellum and its component grasshopper ketone on atopic dermatitis. Int. Immunopharmacol. 2016, 40, 176–183.
- Abe, F.; Yamauchi, T. Megastigmanes and flavonoids from the leaves of Scorodocarpus borneensis. Phytochemistry 1993, 33, 1499–1501.
- Basile, A.; Giordano, S.; López-Sáez, J.A.; Cobianchi, R.C. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry 1999, 52, 1479–1482.
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem. Toxicol. 2014, 69, 55–62.
- Marrassini, C.; Davicino, R.; Acevedo, C.; Anesini, C.; Gorzalczany, S.; Ferraro, G. Vicenin-2, a potential anti-inflammatory constituent of Urtica circularis. J. Nat. Prod. 2011, 74, 1503–1507.
- Tan, W.S.; Arulselvan, P.; Ng, S.F.; Mat Taib, C.N.; Sarian, M.N.; Fakurazi, S. Improvement of diabetic wound healing by topical application of vicenin-2 hydrocolloid film on Sprague Dawley rats. BMC 2019, 19, 20.
- Zhang, Z.; Zhao, Q.; Liu, T.; Zhao, H.; Wang, R.; Li, H.; Zhang, Y.; Shan, L.; He, B.; Wang, X.; et al. Effect of vicenin-2 on ovariectomy-induced osteoporosis in rats. Biomed. Pharmacother. 2020, 129, 10474.
- Nagaprashantha, L.D.; Vatsyayan, R.; Singhal, J.; Fast, S.; Roby, R.; Awasthi, S.; Singhal, S.S. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem. Pharmacol. 2011, 82, 1100–1109.
- Yang, D.; Zhang, X.; Zhang, W.; Rengarajan, T. Vicenin-2 inhibits Wnt/β-catenin signaling and induces apoptosis in HT-29 human colon cancer cell line. Drug Des. Dev. Ther. 2018, 56, 1303–1310.
- Hooper, A.M.; Tsanuo, M.K.; Chamberlain, K.; Tittcomb, K.; Scholes, J.; Hassanali, A.; Khan, Z.R.; Pickett, J.A. Isoschaftoside, a C-glycosylflavonoid from Desmodium uncinatum root exudate, is an allelochemical against the development of Striga. Phytochemistry 2010, 71, 904–908.
- Su, Y.; Kang, Y.; Yi, J.; Lin, Q.; Zhang, C.; Lin, Z.; Yan, Z.; Qu, J.; Liu, J. Isoschaftoside reverses nonalcoholic fatty liver disease via activating autophagy in vivo and in vitro. eCAM 2022, 12, 135.
- Gomes, A.C.C.; Sampaio, L.D.S.; Silva, P.A.D.; Lamas, M.E.; Sakuragui, C.M.; Barreto Junior, C.B.; Simas, N.K.; Kuster, R.M. In vitro effect of isoschaftoside isolated from Syngonium podophyllum on pig kidney Na+, K+-ATPase. Química Nova 2014, 37, 1606–1609.
- Boubaker, J.; Sghaier, M.B.; Skandrani, I.; Ghedira, K.; Chekir-Ghedira, L. Isorhamnetin 3-O-robinobioside from Nitraria retusa leaves enhance antioxidant and antigenotoxic activity in human chronic myelogenous leukemia cell line K562. BMC 2012, 12, 135.
- Ajaghaku, D.L.; Akah, P.A.; Ilodigwe, E.E.; Nduka, S.O.; Osonwa, U.E.; Okoye, F.B.C. Upregulation of CD4+ T-lymphocytes by isomeric mixture of quercetin-3-O-rutinoside and quercetin-3-o-robinobioside isolated from Millettia aboensis. Immunol. Investig. 2018, 47, 372–388.
- Brochado, C.D.O.; Almeida, A.P.D.; Barreto, B.P.; Costa, L.P.; Ribeiro, L.S.; Pereira, R.L.D.C.; Koatz, V.L.G.; Costa, S.S. Flavonol robinobiosides and rutinosides from Alternanthera brasiliana (Amaranthaceae) and their effects on lymphocyte proliferation in vitro. J. Braz. Chem. Soc. 2003, 14, 449–451.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
31 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




