Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Malthe Fredsgaard | -- | 2508 | 2023-07-31 08:40:13 | | | |
| 2 | Jessie Wu | + 3 word(s) | 2511 | 2023-07-31 09:43:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fredsgaard, M.; Kaniki, S.E.K.; Antonopoulou, I.; Chaturvedi, T.; Thomsen, M.H. Potential Anti-H1N1 Effects of Phenolic Compounds from Salicornia. Encyclopedia. Available online: https://encyclopedia.pub/entry/47417 (accessed on 08 February 2026).
Fredsgaard M, Kaniki SEK, Antonopoulou I, Chaturvedi T, Thomsen MH. Potential Anti-H1N1 Effects of Phenolic Compounds from Salicornia. Encyclopedia. Available at: https://encyclopedia.pub/entry/47417. Accessed February 08, 2026.
Fredsgaard, Malthe, Samba Evelyne Kabemba Kaniki, Io Antonopoulou, Tanmay Chaturvedi, Mette Hedegaard Thomsen. "Potential Anti-H1N1 Effects of Phenolic Compounds from Salicornia" Encyclopedia, https://encyclopedia.pub/entry/47417 (accessed February 08, 2026).
Fredsgaard, M., Kaniki, S.E.K., Antonopoulou, I., Chaturvedi, T., & Thomsen, M.H. (2023, July 31). Potential Anti-H1N1 Effects of Phenolic Compounds from Salicornia. In Encyclopedia. https://encyclopedia.pub/entry/47417
Fredsgaard, Malthe, et al. "Potential Anti-H1N1 Effects of Phenolic Compounds from Salicornia." Encyclopedia. Web. 31 July, 2023.
Copy Citation
The saltwater-tolerant plants in the Salicornia genus belonging to the Amaranthaceae family are widely recognized and researched as producers of clinically applicable phytochemicals. The plants in the Salicornia genus contain flavonoids, flavonoid glycosides, and hydroxycinnamic acids, including caffeic acid, ferulic acid, chlorogenic acid, apigenin, kaempferol, quercetin, isorhamnetin, myricetin, isoquercitrin, and myricitrin, which have all been shown to support the antiviral, virucidal, and symptom-suppressing activities.
phenolic compounds
antiviral nutraceuticals
flavonoids
H1N1
enzymatic inhibition
1. Introduction
The increasing focus on the interplay between public health and bioactive compounds extracted from biomass guides researchers to shift to the most prominent groups of bioactive compounds, phenolic compounds [1]. A group of secondary metabolites known as phenolic compounds are characterized by the presence of at least one (-OH) groups attached to a benzene ring, and they often possess additional functional groups such as carboxyl, methyl, ethyl, and ketone groups. These compounds can form various structures such as homodimers, heterodimers, or polymers, including diferulic acid, chlorogenic acid, or condensed tannins [2]. Phenolic compounds have been found to possess different in vitro and in vivo properties; e.g., anti-inflammatory, anticarcinogenic, antidiabetic, pain relieving, and neuroprotective [3][4][5][6]. Though the concentrations and the presence of phenolic compounds have been studied and documented in various fruits [7], nuts [8], and berries [9][10], the scientific interest in phenolic compounds from halophytic biomasses is new. Utilizing the residual biomass at the end of the annual cultivation cycle of edible halophytes could introduce a novel, holistic, circular economy view of biobased nutraceuticals, as compounds found in end-of-cycle halophyte fractions are known for their pharmacological properties [11][12][13][14].
Salicornia spp. are edible plants in the Amaranthacae family and are otherwise also known as glasswort and marsh samphire, amongst others, and are known to contain a broad array of phenolic compounds [15][16][17][18][19]. Ferulic acid is one of the many phenolic compounds found in Salicornia spp., and other selected phenolic compounds found include caffeic acid, chlorogenic acid, apigenin, kaempferol, quercetin, isorhamnetin, myricetin, isoquercitrin, and myricitrin [15][20][21][22][23][24]. Species belonging to the Salicornia genus have been underutilized as medicinal herbs in recent decades, even though they have been traditionally consumed for their flavorful aerial parts in salads and seafood dishes. Additionally, they have been used as a green salt alternative, which results in a lower risk of cardiovascular diseases compared to regular salt [25][26][27][28][29][30][31][32]. The average person’s daily intake of fruit and vegetables has decreased with the introduction of processed foods containing high amounts of simple sugars, starch, sodium, and fat. As fruits, vegetables, and herbs are the primary sources of phenolic compounds, this change in diet towards heavily processed food rich in macronutrients also decreased the intake, and thereby the nutraceutical effects, of phenolic compounds, thus potentially increasing the risk of lifestyle-related diseases and severe viral infections [14][33][34][35][36]. Limongelli et al. reviewed phenolic compounds from the Salicornia genus and their health-promoting effects. In their review, they stated that the species in the Salicornia genus possess antitumor, antihypertensive, antibacterial, neuroprotective, and antidiabetic activities. However, antiviral effects were not mentioned [37]. High sodium diets have been proven to induce vascular dysfunction, and in a study by Panth et al., a diet with a similar high sodium concentration provided from extracts of Salicornia europaea L. did not induce vascular dysfunction in rats. This effect was thought to be due to ferulic acid, which was associated with nitric oxide regulation in a study by Suzuki et al. [26][38]. Salicornia spp. possess many medicinal properties attributed to the phenolic content, many of which contain multiple inhibitory pathways towards enzymes related to pathogenesis, some completely unrelated to the antiviral protein inhibitory effect [39][40]. Among the many pharmacological applications of Salicornia spp., these are plants of great interest as traditional medicine against hepatitis, constipation, nephropathy, and diarrhea, and are proven to possess antihyperglycemic and antihyperlipidemic activities, thus making Salicornia spp. effective as a potential glucose- and lipid-regulating agent to subjects fed with high-glucose and high-lipid diets [27][41][42]. Of the phenolic compounds reviewed are two known and generally recognized as safe (GRAS) by The United States Food and Drug Administration (USFDA) and can be administered in concentrations of 500 mg/serving (quercetin); 150 mg/kg and 1500 mg/kg in food and chewing gum, respectively (isoquercitrin). USFDA is an administrative entity that discusses chemical stability, manufacturing procedures, metabolism, toxicology, clinical studies, and the reason for granting GRAS status to specific substances. At the time of publication, 1040 chemical compounds, extracts, fiber residues, proteins, etc., are registered as GRAS, and 68 with a pending decision by USFDA [43]. The other phenolic compounds in this research are not yet GRAS compounds but have been used by independent researchers worldwide for in vitro and in vivo experiments in less than toxic concentrations for their beneficial nutraceutical and pharmaceutical properties and have been deemed safe in amounts obtained through dietary consumption [44][45].
2. Influenza A Strain
The influenza virus is a part of the Orthomyxovirus family that contains four types; influenza A to influenza D, with only influenza A to influenza C being able to infect humans, with the influenza A (H1N1) strain of influenza A being the most predominant of all. H1N1 contains the active site proteins, as seen on the H1N1 virion in Figure 1, hemagglutinin (HA), neuraminidase (NA), and M2 ion channels, which antiviral therapeutics can inhibit. Multiple subtypes of influenza have been described, including 18 different subtypes of HA and 11 subtypes of NA, classified in 162 influenza A subtypes, with a theoretical amount of 198 being possible [46][47]. NA and HA are surface glycoprotein spikes, usually in a ratio of 4 NA to 1 HA.
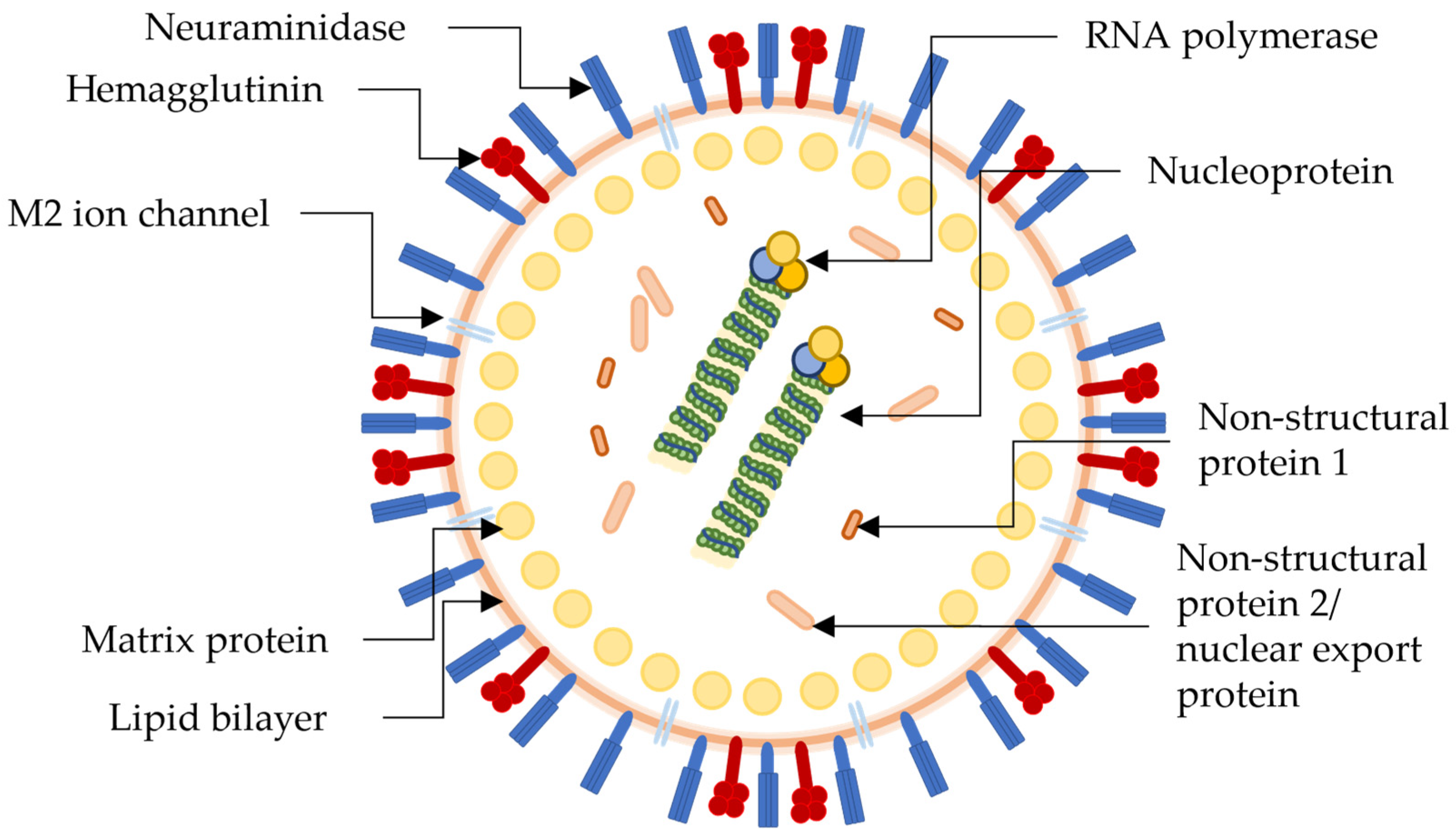
Figure 1. Pictorial representation of the structure and biology of a generic influenza virion with antiretroviral drug targets. Not specific to the H1N1 virus.
Liu et al. concluded that the inhibitory effects of flavonoids towards NA were highly structure-dependent and established a general order of potency: aurones > flavonoles > isoflavones > flavanonoles and flavanoles. For the optimal inhibitory effect of NA, specific hydroxyl group placements were established at positions 4′-OH and 7-OH, the carbonyl group on C4=O, and a set of double-bound carbon C2=C3; see Figure 2 for reference. Compounds from Salicornia spp. in Table 1 that have these properties are kaempferol, apigenin, myricetin, quercetin, myricitrin, and isoquercitrin. The most potent NA inhibitory effects from the study of 25 flavonoids by Liu et al. were from the flavones apigenin, luteolin, not having the 3-OH hydroxyl group, and the aurones flavonoid group [48].
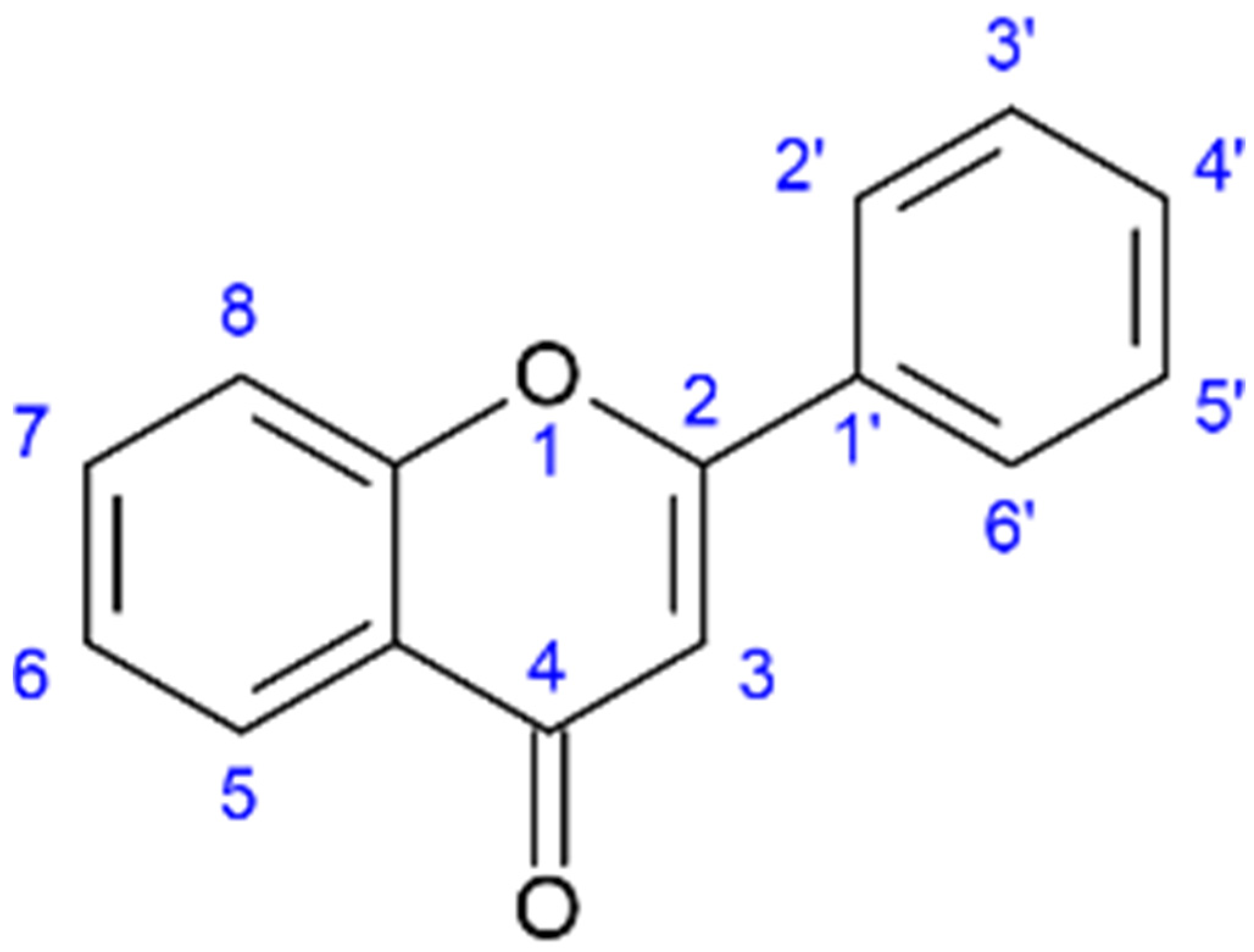
Figure 2. General structure of the flavone backbone (2-phenyl-1,4-benzopyrone) and numbered sites for attaching, amongst others, hydroxyl, methyl, and sugar groups.
Isoquercitrin is the 3-O-glucoside form of quercetin, making the compound more polar than the aglycone and with higher bioavailability [40]. Even though the structural differences between glycosylated flavonoids can be minor, a clear difference between the closely related isomers isoquercitrin and hyperoside could be observed between the antiviral effects of the compounds when a green fluorescent protein (GFP) reporter was inserted into H1N1, and the modified virus was mixed with the flavonoids at 25 µM and compared to a negative assay control (mock). An insignificant effect was seen between the effect from the mock and hyperoside, but isoquercitrin resulted in almost complete inhibition of H1N1-infected chicken embryos. The H1N1 inhibiting effect provided by isoquercitrin was likely the primary reason for the H1N1 inhibiting effect seen by a water extract of Nelumbo nucifera Gaertn. [49]. As isoquercitrin has been found in great concentrations in S. fruticosa L., see Table 1, a purified fraction of isoquercitrin from the Salicornia species could also possess high inhibition of H1N1 in chicken embryos. Isoquercitrin has been shown to exhibit the suppression of hemagglutinin (HA) and NA and inhibit cytopathic effects on both H1N1 and H3N2, making isoquercitrin a multi-acting therapeutic agent [50]. The anti-H1N1 effect of isoquercitrin has also been linked to the suppression of reactive oxygen species (ROS), acidic vesicular organelle formation, and the polymerase basic protein 2 (PB2) protein [51]. Kim et al. observed a significant synergistic effect when isoquercitrin was combined with amantadine in low concentrations and did not induce resistant vira. Isoquercitrin showed a higher inhibition rate of the H1N1 virus than (-)-epigallocatechin gallate and resveratrol, which have previously been known to have a high inhibition rate of the H1N1 virus when compared to other antiviral phenolic compounds [52].
During the past few years, ferulic acid has been determined to play an essential role as a nutraceutical and against viral infections, including H1N1 with an IC50 inhibition at 140 µM towards NA [53]. In addition to low cytotoxicity, ferulic acid and caffeic acid show utility for regulating the activity of the immune system [54][55]. Sodium ferulate activated toll-like receptors (TLR) 7 and 9, enhancing survival in H1N1-infected mice, contributing to antiviral defense [56]. This effect could be secondary to the induction of the heme oxygenase 1 gene (HMOX1) and possibly reflect ferulic acid’s additional benefits. TLR7 and TLR9 are proteins responsible for detecting viral nucleic acids, initiating swift signaling pathways that play a crucial role in generating interferons, thereby enhancing the body’s antiviral defenses [56]. Another potential impact of ferulic acid is its inhibiting effect on functions such as virus replication. During the past few years, ferulic acid has been determined to play an essential role against viral infections, including H1N1, murine coronavirus (MHV-A59), and human rhinovirus 14 (HRV-14), as a component of phenolic extract from plants with exceptional and identified action [53][57][58][59][60][61][62][63]. Ferulic acid is also a cytochrome P450 1A2 enzyme (CYP1A2) inhibitor, positively increasing its half-life and averting serious drug interactions [64]. CYP1A2 is involved in the metabolism of xenobiotics in the body involved in drug metabolism. Feruloylated oligosaccharides (FOs) are nutraceuticals derived from arabinoxylans, with ferulic acid esterified to l-arabinofuranosyl side chain oligosaccharides. FOs possess the physiological functions of both ferulic acid and oligosaccharides. Unlike pure oligosaccharides, FOs still release ferulic acid after fermentation by human gut microorganisms, which can introduce prebiotic effects in the gut microbiota [65]. Ferulic acid released in the colon can be absorbed in the body, exerting various physiological functions as free ferulic acid. The anti-inflammatory impact of antioxidant nutraceuticals, such as phenolics from Salicornia spp., might also quell the excessive inflammatory reaction within lung parenchyma evoked by viral H1N1 [62][66]. McCarty et al. suggested that a very low daily dosage ranging from 500 to 1000 mg of ferulic acid could aid in the control of H1N1 by decreasing infection response by suppressing viral spread and dampening proinflammatory signaling, which promotes an influx of inflammatory cells [67].
Chlorogenic acids are a group of esters formed between caffeic and quinic acids and contain chlorogenic acid (ChA), neochlorogenic acid (neo-ChA), and cryptochlorogenic acid (crypto-ChA), amongst others. These three compounds are closely related isomers, only differentiated by the orientation of the quinic acid group. ChAs have been recognized as an effective NA inhibitor by Ding et al., who proposed it as a nutraceutical that can decrease the time of the infection and be safely administered through the diet in low concentrations; e.g., by ingesting wild lowbush blueberries [68]. Ding et al. proved that the synthetic medicine oseltamivir at 2 µM was less inhibiting on H1N1 than 100 µM ChA, and the cellular toxicity of ChA was 0% at levels below 200 µM [68].
Dayem et al. investigated the in vivo pharmaceutical effect of five flavonoids and their effect on H1N1-infected mice. Trials were set up with the administration of flavonoids as pre-treatment before virus inoculation, co-treatment with simultaneous viral inoculation and flavonoid administration, and post-treatment flavonoid administration after virus inoculation [69]. It was found that isorhamnetin suppressed the covalent binding of lipids to peptides and the lipidation of microtubule-associated protein 1 light chain 3 beta (MAP1LC3B), hence decreasing virus-induced overexpression of autophagy-related genes of healthy cells (autophagy-related gene proteins 5 and 7 (atg-5 and atg-7)) protecting the infected host. Isorhamnetin was proven to inhibit both NA and HA at a concentration of 50 µM, indicating the potential use of isorhamnetin as an anti-H1N1 medication [69].
As seen in Table 1, many of the phenolic compounds selected for the review have been tested for various inhibition mechanisms in silico and in vitro. Typical for many of the selected phenolic compounds is the ability to inhibit the strain A/PR/8/34 (H1N1) in an infected MDCK cell line, and the NAI of that strain has also been investigated in multiple studies. From this, the inhibition efficiency of the compounds, tested on the MDCK cell line with A/PR/8/34 (H1N1), was shown to be isoquercitrin > caffeic acid > quercetin, and the NAI of the selected compounds on A/PR/8/34 (H1N1) was shown to be apigenin > kaempferol > quercetin > myricetin. Molecular docking simulations also proved the effect on NAI with similar results to the in vitro NAI results, showing the most potent inhibitors to be isoquercitrin > ferulic acid > kaempferol > quercetin. Furthermore, ChA is seen to possess good NAI and could, together with isoquercitrin, be viable options for NAI.
Table 1. Selected phenolic compounds detected in Salicornia spp. reviewed for their H1N1 inhibitory properties. Data are presented as mean values and standard deviations measured by triplicates.
| Compound | H1N1 Inhibition Target | Inhibition Activity | Ref. |
|---|---|---|---|
| Caffeic acid | A/PR/8/34 (H1N1) infected MDCK | IC50 inhibition: 81.6 ± 8.3 µM | [70] |
| A/Osaka/2024/09 (H1N1) infected MDCK | IC50 inhibition: 98.8 µM | [70] | |
| A/Osaka/71/11 (H1N1) infected MDCK | IC50 inhibition: 97.1 µM | [70] | |
| Ferulic acid | NA | Docking energy: −7.1 kcal/mol | [53] |
| A/Malaysia/Muar/33/09 NA (H1N1) inhibition (NAI) in vitro assay | IC50 inhibition: 140 µM | [53] | |
| EC50 of A/Malaysia/Muar/33/09 (H1N1) in infected MDCK cells | EC50 inhibition: 1.32 ± 0.08 µM | [53] | |
| Chlorogenic acid | EC50 of A/PR/8/34 (H1N1) in infected MDCK cells | EC50 inhibition: 44.87 µM | [68] |
| NAI by fluorescence-based in vitro assay | IC50 inhibition: 22.13 ± 1.07 µM | [68] | |
| Apigenin | EC50 of A/PR/8/34 (H1N1) in infected MDCK cells | EC50 inhibition: 56.7 ± 11.1 µM | [71] |
| EC50 of A/Toyama/129/11 (H1N1) in infected MDCK cells | EC50 inhibition: 65.9 ± 32.2 µM | [71] | |
| EC50 of A/Toyama/26/11 (H1N1) in infected MDCK cells | EC50 inhibition: 30.0 ± 17.4 µM | [71] | |
| A/PR/8/34 (H1N1) NAI in vitro assay | IC50 inhibition: 31.6 ± 0.9 µM | [48] | |
| Kaempferol | NA | Docking energy: −6.8 kcal/mol | [72] |
| NAI by RT-PCR in vitro assay | Significant inhibition at 50 µM | [69] | |
| A/PR/8/34 (H1N1) NAI in vitro assay | IC50 inhibition: 58.6 ± 0.6 µM | [48] | |
| Quercetin | A/PR/8/34 (H1N1) infected MDCK | IC50 inhibition: 274.6 ± 3.3 µM | [70] |
| A/PR/8/34 (H1N1) plaque formation inhibition in vitro assay | IC50 inhibition: 1.40 µM | [73] | |
| NAI by RT-PCR in vitro assay | Significant inhibition at 50 µM | [69] | |
| NA | Docking energy: −6.8 kcal/mol | [72] | |
| A/PR/8/34 (H1N1) NAI in vitro assay | IC50 inhibition: 58.4 ± 3.8 µM | [48] | |
| Isorhamnetin | HI by RT-PCR in vitro assay | Significant inhibition at 50 µM | [69] |
| NAI by RT-PCR in vitro assay | Significant inhibition at 50 µM | [69] | |
| Myricetin | A/PR/8/34 (H1N1) NAI in vitro assay | IC50 inhibition: 82.6 ± 8.9 µM | [48] |
| A/PR/8/34 (H1N1) plaque formation inhibition in vitro assay | IC50 inhibition: 0.90 µM | [73] | |
| Isoquercitrin | HI RAW 264.7 cell line in vitro assay | Complete inhibition: 5 µM | [50] |
| NAI by fluorescence-based in vitro assay | IC50 inhibition: 37.1 ± 0.6 µM | [50] | |
| PB2 | Docking energy: −8.0 kcal/mol | [51] | |
| NA | Docking energy: −8.6 kcal/mol | [74] | |
| A/PR/8/34 (H1N1) infected MDCK | IC50 inhibition: 10.6 ± 0.4 µM | [51] | |
| A/WS/33 (H1N1) infected MDCK | IC50 inhibition: 21.4 ± 2.4 µM | [51] | |
| Myricitrin | A/PR/8/34 (H1N1) infected MDCK | 255.6% increase in cell viability at 224.0 µM | [75] |
MDCK: Madin-Darby Canine Kidney cells, IC50: Half maximal inhibitory concentration, EC50: Half maximal effective concentration, HI: Hemagglutination inhibition, NA: Neuraminidase, NAI: Neuraminidase inhibition, RT-PCR: Reverse transcription polymerase chain reaction, PB2: polymerase basic protein 2.
References
- Rashmi, H.B.; Negi, P.S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109208.
- Herrmann, K. Occurrence and Content of Hydroxycinnamic and Hydroxybenzoic Acid Compounds in Foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347.
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74.
- Giordano, R.; Aliotta, G.E.; Johannesen, A.S.; Voetmann-Jensen, D.; Laustsen, F.H.; Andersen, L.A.; Rezai, A.; Fredsgaard, M.; Lo Vecchio, S.; Arendt-Nielsen, L.; et al. Effects of Salicornia-Based Skin Cream Application on Healthy Humans’ Experimental Model of Pain and Itching. Pharmaceuticals 2022, 15, 150.
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin Bioactive Effects: Moving from Preclinical Evidence to Potential Clinical Applications. BMC Complement. Med. Ther. 2020, 20, 241.
- Wang, Q.; Wei, H.C.; Zhou, S.J.; Li, Y.; Zheng, T.T.; Zhou, C.Z.; Wan, X.H. Hyperoside: A Review on Its Sources, Biological Activities, and Molecular Mechanisms. Phyther. Res. 2022, 36, 2779–2802.
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044.
- Taş, N.G.; Gökmen, V. Phenolic Compounds in Natural and Roasted Nuts and Their Skins: A Brief Review. Curr. Opin. Food Sci. 2017, 14, 103–109.
- Li, W.; Hydamaka, A.W.; Lowry, L.; Beta, T. Comparison of Antioxidant Capacity and Phenolic Compounds of Berries, Chokecherry and Seabuckthorn. Cent. Eur. J. Biol. 2009, 4, 499–506.
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial Properties of Phenolic Compounds from Berries. J. Appl. Microbiol. 2001, 90, 494–507.
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140.
- Kim, J.Y.; Cho, J.-Y.; Moon, J.-H.; Choi, G.-C.; Lee, K.-D.; Ham, K.-S.; Kim, S.-J. Change of Phenylpropanoic Acid and Flavonol Contents at Different Growth Stage of Glasswort (Salicornia herbacea L.). Food Sci. Biotechnol. 2014, 23, 685–691.
- Hulkko, L.S.S.; Rocha, R.M.; Trentin, R.; Fredsgaard, M.; Chaturvedi, T.; Custódio, L.; Thomsen, M.H. Bioactive Extracts from Salicornia ramosissima J. Woods Biorefinery as a Source of Ingredients for High-Value Industries. Plants 2023, 12, 1251.
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120.
- Zengin, G.; Aumeeruddy-Elalfi, Z.; Mollica, A.; Yilmaz, M.A.; Mahomoodally, M.F. In Vitro and in Silico Perspectives on Biological and Phytochemical Profile of Three Halophyte Species—A Source of Innovative Phytopharmaceuticals from Nature. Phytomedicine 2018, 38, 35–44.
- Ben Farhat, M.; Beji-Serairi, R.; Selmi, S.; Saidani-Tounsi, M.; Abdelly, C. Salicornia fruticosa L. and Portulaca oleracea L. Antioxidants as Affected by Domestic Cooking Processes. Int. J. Gastron. Food Sci. 2022, 27, 100462.
- Won, K.J.; Lee, K.P.; Baek, S.; Cui, L.; Kweon, M.H.; Jung, S.H.; Ryu, Y.K.; Hong, J.M.; Cho, E.A.; Shin, H.S.; et al. Desalted Salicornia europaea Extract Attenuated Vascular Neointima Formation by Inhibiting the MAPK Pathway-Mediated Migration and Proliferation in Vascular Smooth Muscle Cells. Biomed. Pharmacother. 2017, 94, 430–438.
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744.
- Wang, X.; Bai, J.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. A Comparative Metabolomics Analysis of the Halophyte Suaeda Salsa and Salicornia europaea. Environ. Geochem. Health 2021, 43, 1109–1122.
- Cybulska, I.; Zembrzuska, J.; Brudecki, G.; Thomsen, M.H. Optimizing Methods to Characterize Caffeic, Ferulic, and Chlorogenic Acids in Salicornia sinus-persica and Salicornia bigelovii Extracts by Tandem Mass Spectrometry (LC-MS/MS). BioResources 2021, 16, 5508–5523.
- Lee, D.; Park, J.Y.; Lee, S.; Kang, K.S. In Vitro Studies to Assess the α-Glucosidase Inhibitory Activity and Insulin Secretion Effect of Isorhamnetin 3-O-Glucoside and Quercetin 3-O-Glucoside Isolated from Salicornia herbacea. Processes 2021, 9, 483.
- Ameixa, O.M.C.C.; Rebelo, J.; Silva, H.; Pinto, D.C.G.A. Gall Midge Baldratia salicorniae Kieffer (Diptera: Cecidomyiidae) Infestation on Salicornia europaea L. Induces the Production of Specialized Metabolites with Biotechnological Potential. Phytochemistry 2022, 200, 113207.
- Kim, K.-S.; Park, S.-H. Isolation and Identification of Antioxidant Flavonoids from Salicornia herbacea L. Appl. Biol. Chem. 2004, 47, 120–123.
- Kong, C.S.; Lee, J.I.; Kim, Y.A.; Kim, J.A.; Bak, S.S.; Hong, J.W.; Park, H.Y.; Yea, S.S.; Seo, Y. Evaluation on Anti-Adipogenic Activity of Flavonoid Glucopyranosides from Salicornia herbacea. Process Biochem. 2012, 47, 1073–1078.
- Ekanayake, S.; Egodawatta, C.; Attanayake, R.N.; Perera, D. From Salt Pan to Saucepan: Salicornia, a Halophytic Vegetable with an Array of Potential Health Benefits. Food Front. 2023, 4, 641–676.
- Panth, N.; Park, S.-H.; Kim, H.; Kim, D.-H.; Oak, M.-H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176.
- Rhee, M.H.; Park, H.-J.; Cho, J.Y. Salicornia herbacea: Botanical, Chemical and Pharmacological Review of Halophyte Marsh Plant. J. Med. Plants Res. 2009, 3, 548–555.
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Caçador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima j. Woods. Antioxidants 2021, 10, 1312.
- Hrynkiewicz, K.; Patz, S.; Ruppel, S. Salicornia europaea L. as an Underutilized Saline-Tolerant Plant Inhabited by Endophytic Diazotrophs. J. Adv. Res. 2019, 19, 49–56.
- Zhang, S.; Wei, M.; Cao, C.; Ju, Y.; Deng, Y.; Ye, T.; Xia, Z.; Chen, M. Effect and Mechanism of Salicornia bigelovii Torr. Plant Salt on Blood Pressure in SD Rats. Food Funct. 2015, 6, 920–926.
- Cho, H.D.; Lee, J.H.; Jeong, J.H.; Kim, J.Y.; Yee, S.T.; Park, S.K.; Lee, M.K.; Seo, K.-i. Production of Novel Vinegar Having Antioxidant and Anti-Fatigue Activities from Salicornia herbacea L. J. Sci. Food Agric. 2016, 96, 1085–1092.
- Patel, S. Salicornia: Evaluating the Halophytic Extremophile as a Food and a Pharmaceutical Candidate. 3 Biotech 2016, 6, 1–10.
- Cole, G.M.; Lim, G.P.; Yang, F.; Teter, B.; Begum, A.; Ma, Q.; Harris-White, M.E.; Frautschy, S.A. Prevention of Alzheimer’s Disease: Omega-3 Fatty Acid and Phenolic Anti-Oxidant Interventions. Neurobiol. Aging 2005, 26, 133–136.
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501.
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible Role of Diet in Cancer: Systematic Review and Multiple Meta-Analyses of Dietary Patterns, Lifestyle Factors, and Cancer Risk. Nutr. Rev. 2017, 75, 405–419.
- Bertoia, M.L.; Mukamal, K.J.; Cahill, L.E.; Hou, T.; Ludwig, D.S.; Mozaffarian, D.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies. PLOS Med. 2015, 12, e1001878.
- Limongelli, F.; Crupi, P.; Clodoveo, M.L.; Corbo, F.; Muraglia, M. Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules 2022, 27, 7954.
- Suzuki, A.; Kagawa, D.; Fujii, A.; Ochiai, R.; Tokimitsu, I.; Saito, I. Short- and Long-Term Effects of Ferulic Acid on Blood Pressure in Spontaneously Hypertensive Rats. Am. J. Hypertens. 2002, 15, 351–357.
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100.
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, Toxicology, and Metabolism. Food Chem. Toxicol. 2014, 68, 267–282.
- Kim, S.; Lee, E.Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia europaea L. Molecules 2021, 26, 2252.
- Park, S.H.; Ko, S.K.; Choi, J.G.; Chung, S.H. Salicornia herbacea Prevents High Fat Diet-Induced Hyperglycemia and Hyperlipidemia in ICR Mice. Arch. Pharm. Res. 2006, 29, 256–264.
- U.S. Food and Drug Administration. GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&sort=FDA_s_Letter&order=DESC&showAll=true&type=basic&search= (accessed on 23 February 2023).
- Murakami, A. Dose-Dependent Functionality and Toxicity of Green Tea Polyphenols in Experimental Rodents. Arch. Biochem. Biophys. 2014, 557, 3–10.
- Lambert, J.D.; Sang, S.; Yang, C.S. Possible Controversy over Dietary Polyphenols: Benefits vs Risks. Chem. Res. Toxicol. 2007, 20, 583–585.
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 25 January 2023).
- Wu, X.; Wu, X.; Sun, Q.; Zhang, C.; Yang, S.Y.; Li, L.; Jia, Z. Progress of Small Molecular Inhibitors in the Development of Anti-Influenza Virus Agents. Theranostics 2017, 7, 826–845.
- Liu, A.L.; Wang, H.-D.; Lee, S.M.Y.; Wang, Y.T.; Du, G.H. Structure-Activity Relationship of Flavonoids as Influenza Virus Neuraminidase Inhibitors and Their in Vitro Anti-Viral Activities. Bioorganic Med. Chem. 2008, 16, 7141–7147.
- Cho, W.K.; Yang, H.J.; Ma, J.Y. Lotus (Nelumbo Nucifera Gaertn.) Leaf Water Extracts Suppress Influenza a Viral Infection via Inhibition of Neuraminidase and Hemagglutinin. J. Funct. Foods 2022, 91, 105019.
- Cho, W.-K.; Lee, M.-M.; Ma, J.Y. Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase. Int. J. Mol. Sci. 2022, 23, 13112.
- Nile, S.H.; Kim, D.H.; Nile, A.; Park, G.S.; Gansukh, E.; Kai, G. Probing the Effect of Quercetin 3-Glucoside from Dianthus superbus L. against Influenza Virus Infection—In Vitro and in Silico Biochemical and Toxicological Screening. Food Chem. Toxicol. 2020, 135, 110985.
- Kim, Y.; Narayanan, S.; Chang, K.O. Inhibition of Influenza Virus Replication by Plant-Derived Isoquercetin. Antivir. Res. 2010, 88, 227–235.
- Hariono, M.; Abdullah, N.; Damodaran, K.V.; Kamarulzaman, E.E.; Mohamed, N.; Hassan, S.S.; Shamsuddin, S.; Wahab, H.A. Potential New H1N1 Neuraminidase Inhibitors from Ferulic Acid and Vanillin: Molecular Modelling, Synthesis and in Vitro Assay. Sci. Rep. 2016, 6, 38692.
- Ma, Z.; Hong, Q.; Wang, Y.; Liang, Q.; Tan, H.; Xiao, C.; Tang, X.; Shao, S.; Zhou, S.; Gao, Y. Ferulic Acid Induces Heme Oxygenase-1 via Activation of ERK and Nrf2. Drug Discov. Ther. 2011, 5, 299–305.
- Wang, X.; Stavchansky, S.; Kerwin, S.M.; Bowman, P.D. Structure-Activity Relationships in the Cytoprotective Effect of Caffeic Acid Phenethyl Ester (CAPE) and Fluorinated Derivatives: Effects on Heme Oxygenase-1 Induction and Antioxidant Activities. Eur. J. Pharmacol. 2010, 635, 16–22.
- Zhu, Y.; Shao, Y.; Qu, X.; Guo, J.; Yang, J.; Zhou, Z.; Wang, S. Sodium Ferulate Protects against Influenza Virus Infection by Activation of the TLR7/9-MyD88-IRF7 Signaling Pathway and Inhibition of the NF-ΚB Signaling Pathway. Biochem. Biophys. Res. Commun. 2019, 512, 793–798.
- Enkhtaivan, G.; Maria John, K.M.; Ayyanar, M.; Sekar, T.; Jin, K.J.; Kim, D.H. Anti-Influenza (H1N1) Potential of Leaf and Stem Bark Extracts of Selected Medicinal Plants of South India. Saudi J. Biol. Sci. 2015, 22, 532–538.
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural Product-Derived Phytochemicals as Potential Agents against Coronaviruses: A Review. Virus Res. 2020, 284, 197989.
- Kim, H.Y.; Shin, H.S.; Park, H.; Kim, Y.C.; Yun, Y.G.; Park, S.; Shin, H.J.; Kim, K. In Vitro Inhibition of Coronavirus Replications by the Traditionally Used Medicinal Herbal Extracts, Cimicifuga Rhizoma, Meliae Cortex, Coptidis Rhizoma, and Phellodendron Cortex. J. Clin. Virol. 2008, 41, 122–128.
- Spasova, M.; Philipov, S.; Nikolaeva-Glomb, L.; Galabov, A.S.; Milkova, T. Cinnamoyl- and Hydroxycinnamoyl Amides of Glaucine and Their Antioxidative and Antiviral Activities. Bioorganic Med. Chem. 2008, 16, 7457–7461.
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Inhibition of the Main Protease of SARS-CoV-2 (Mpro) by Repurposing/Designing Drug-like Substances and Utilizing Nature’s Toolbox of Bioactive Compounds. Comput. Struct. Biotechnol. J. 2022, 20, 1306–1344.
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic Acid From Plant Biomass: A Phytochemical With Promising Antiviral Properties. Front. Nutr. 2022, 8, 1358.
- Salman, S.; Shah, F.H.; Idrees, J.; Idrees, F.; Velagala, S.; Ali, J.; Khan, A.A. Virtual Screening of Immunomodulatory Medicinal Compounds as Promising Anti-SARS-CoV-2 Inhibitors. Future Virol. 2020, 15, 267–275.
- Appiah-Opong, R.; Commandeur, J.N.M.; van Vugt-Lussenburg, B.; Vermeulen, N.P.E. Inhibition of Human Recombinant Cytochrome P450s by Curcumin and Curcumin Decomposition Products. Toxicology 2007, 235, 83–91.
- Gong, L.; Wang, H.; Wang, T.; Liu, Y.; Wang, J.; Sun, B. Feruloylated Oligosaccharides Modulate the Gut Microbiota in Vitro via the Combined Actions of Oligosaccharides and Ferulic Acid. J. Funct. Foods 2019, 60, 103453.
- Wang, X.; Zhang, M.; Zhao, Y.; Wang, H.; Liu, T.; Xin, Z. Pentadecyl Ferulate, a Potent Antioxidant and Antiproliferative Agent from the Halophyte Salicornia herbacea. Food Chem. 2013, 141, 2066–2074.
- McCarty, M.F.; DiNicolantonio, J.J. Nutraceuticals Have Potential for Boosting the Type 1 Interferon Response to RNA Viruses Including Influenza and Coronavirus. Prog. Cardiovasc. Dis. 2020, 63, 383–385.
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral Activity of Chlorogenic Acid against Influenza A (H1N1/H3N2) Virus and Its Inhibition of Neuraminidase. Sci. Rep. 2017, 7, 45723.
- Dayem, A.A.; Choi, H.Y.; Kim, Y.B.; Cho, S.-G. Antiviral Effect of Methylated Flavonol Isorhamnetin against Influenza. PLoS ONE 2015, 10, e0121610.
- Kanazawa, R.; Morimoto, R.; Horio, Y.; Sumitani, H.; Isegawa, Y. Inhibition of Influenza Virus Replication by Apiaceae Plants, with Special Reference to Peucedanum Japonicum (Sacna) Constituents. J. Ethnopharmacol. 2022, 292, 115243.
- Kai, H.; Obuchi, M.; Yoshida, H.; Watanabe, W.; Tsutsumi, S.; Park, Y.K.; Matsuno, K.; Yasukawa, K.; Kurokawa, M. In Vitro and in Vivo Anti-Influenza Virus Activities of Flavonoids and Related Compounds as Components of Brazilian Propolis (AF-08). J. Funct. Foods 2014, 8, 214–223.
- Sadati, S.M.; Gheibi, N.; Ranjbar, S.; Hashemzadeh, M.S. Docking Study of Flavonoid Derivatives as Potent Inhibitors of Influenza H1N1 Virus Neuraminidas. Biomed. Rep. 2019, 10, 33–38.
- Kaihatsu, K. Potential Anti-Influenza Virus Agents Based on Coffee Ingredients and Natural Flavonols. Nat. Prod. Chem. Res. 2014, 2, 1000129.
- Albeshri, A.; Baeshen, N.A.; Bouback, T.A.; Aljaddawi, A.A. Evaluation of Cytotoxicity and Antiviral Activity of Rhazya Stricta Decne Leaves Extract against Influenza A/PR/8/34 (H1N1). Saudi J. Biol. Sci. 2022, 29, 103375.
- Motlhatlego, K.E.; Mehrbod, P.; Fotouhi, F.; Abdalla, M.A.; Eloff, J.N.; McGaw, L.J. Anti-Influenza A Virus Activity of Two Newtonia Species and the Isolated Compound Myricetin-3-o-Rhamnoside. BMC Complement. Med. Ther. 2021, 21, 92.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
577
Revisions:
2 times
(View History)
Update Date:
31 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




