Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Esam Yahya | -- | 2291 | 2023-07-28 02:55:00 | | | |
| 2 | Sirius Huang | Meta information modification | 2291 | 2023-07-28 03:40:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yahya, E.B.; Elarbash, S.S.; Bairwan, R.D.; Mohamed, M.M.I.; Khan, N.B.; Harlina, P.W.; Abdul Khalil, H.P.S. Nanocellulose Functional Material. Encyclopedia. Available online: https://encyclopedia.pub/entry/47375 (accessed on 07 February 2026).
Yahya EB, Elarbash SS, Bairwan RD, Mohamed MMI, Khan NB, Harlina PW, et al. Nanocellulose Functional Material. Encyclopedia. Available at: https://encyclopedia.pub/entry/47375. Accessed February 07, 2026.
Yahya, Esam Bashir, Suhail Salem Elarbash, Rahul Dev Bairwan, Montaha Mohamed Ibrahim Mohamed, Niaz Bahadur Khan, Putri Widyanti Harlina, H. P. S. Abdul Khalil. "Nanocellulose Functional Material" Encyclopedia, https://encyclopedia.pub/entry/47375 (accessed February 07, 2026).
Yahya, E.B., Elarbash, S.S., Bairwan, R.D., Mohamed, M.M.I., Khan, N.B., Harlina, P.W., & Abdul Khalil, H.P.S. (2023, July 28). Nanocellulose Functional Material. In Encyclopedia. https://encyclopedia.pub/entry/47375
Yahya, Esam Bashir, et al. "Nanocellulose Functional Material." Encyclopedia. Web. 28 July, 2023.
Copy Citation
Nanocellulose is generally defined as cellulose particles or fibers with dimensions in the nanometer range. It can be derived from different cellulose sources, such as wood pulp, agricultural residues, or bacterial cultures, using various methods including mechanical treatment, enzymatic hydrolysis, or chemical processes.
microbial
nanocellulose
plant fibers
1. Introduction
Nanocellulose refers to cellulose fibers that have been broken down into nanoscale dimensions, typically ranging from a few nanometers to a few hundred nanometers in diameter [1]. Cellulose is the most abundant organic polymer found in nature and is the primary structural component of plant cell walls. The general structure of nanocellulose is derived from the hierarchical structure of cellulose, which is a linear polymer composed of repeating glucose units. In its native form, cellulose consists of long chains of glucose molecules linked together by β-1,4-glycosidic bonds [2][3]. Nanocellulose can be derived from various sources, including wood pulp, agricultural waste, and certain bacteria [4]. Table 1 presents a brief comparison between these three types. Cellulose, in general, possesses unique properties, such as high strength, high aspect ratio, low density, large surface area, and excellent biodegradability [5]. These characteristics make nanocellulose an attractive material for a wide range of applications in various industries.
Table 1. Types and properties of different nanocellulose types depending on the physical characteristics.
| Characteristic/s | CNF | CNC | BNC | Ref |
|---|---|---|---|---|
| Degree of polymerization | ≥500 | 500–15,000 | 300–10,000 | [6][7] |
| Crystallinity (%) | 50–65 | 72–80 | 84–89 | [8] |
| Length (µm) | 0.5–2 µm | 0.05–0.5 µm | >1 µm | [6][9] |
| Width (nm) | 10–50 | 3–15 | 12–22 | [10][11] |
| Diameter (nm) | 5–50 | 2–20 | 10–50 | [12][13] |
| Young Modulus (GPa) | 50–100 | 140–160 | 15–130 | [14] |
| Purity | Low | Low | High | [15][16] |
2. Structure and Properties of Plant-Based Nanocellulose
Cellulose, hemicellulose, and lignin are the constituents of plant cell walls. Cellulose and hemicelluloses are the primary components, constituting around 34%–75% of the primary cell wall and 50%–80% of the secondary cell wall [17]. Lignin (10%–25% by dry weight) binds the cellulose (30%–45% by dry weight) and hemicellulose (20%–25% by dry weight) confirming the strength and stiffness of the cell wall [18][19][20]. There are different types of nanocellulose, including CNC and CNF. The source of CNF and CNC might be the same, but they differ in the physical properties. CNCs are obtained by hydrolyzing cellulose fibers to remove non-crystalline regions, resulting in highly crystalline and rigid nanoscale rods [21]. CNCs are rod-like structures with a high aspect ratio, meaning they have a long and narrow shape. Typically, CNCs have diameters ranging from a few nanometers to tens of nanometers and lengths ranging from several hundred nanometers to several micrometers [22]. CNCs are highly crystalline, meaning their cellulose chains are tightly packed together in an ordered manner [23]. The surface of CNCs contains hydroxyl (-OH) groups, which can provide sites for chemical modifications and interactions with other materials. CNFs, on the other hand, are produced by disintegrating cellulose fibers through mechanical or enzymatic processes, leading to long and flexible nanofibers [24]. CNFs are long and flexible fibrillar structures. The diameters of CNFs are typically in the range of a few nanometers to tens of nanometers. Their lengths can vary from micrometers to several micrometers, depending on the production method [25]. CNFs also possess a high degree of crystallinity, although they may have a slightly lower crystalline order compared to CNCs. Similar to CNCs, CNFs have abundant hydroxyl groups on their surface, enabling interactions with other substances [26]. Both CNCs and CNFs are derived from cellulose through various methods, such as acid hydrolysis, mechanical fibrillation, or enzymatic treatments. These processes break down the larger cellulose fibers into nanoscale dimensions while retaining the characteristic crystalline structure of cellulose. Nanocellulose fibers can vary depending on factors such as the cellulose source, processing methods, and post-treatment modifications. Additionally, nanocellulose fibers can further assemble into networks or form self-supporting films, offering additional structural complexity and functionality. Figure 2 illustrates the structure of plant nanocellulose from the origin to the cellulose molecules.
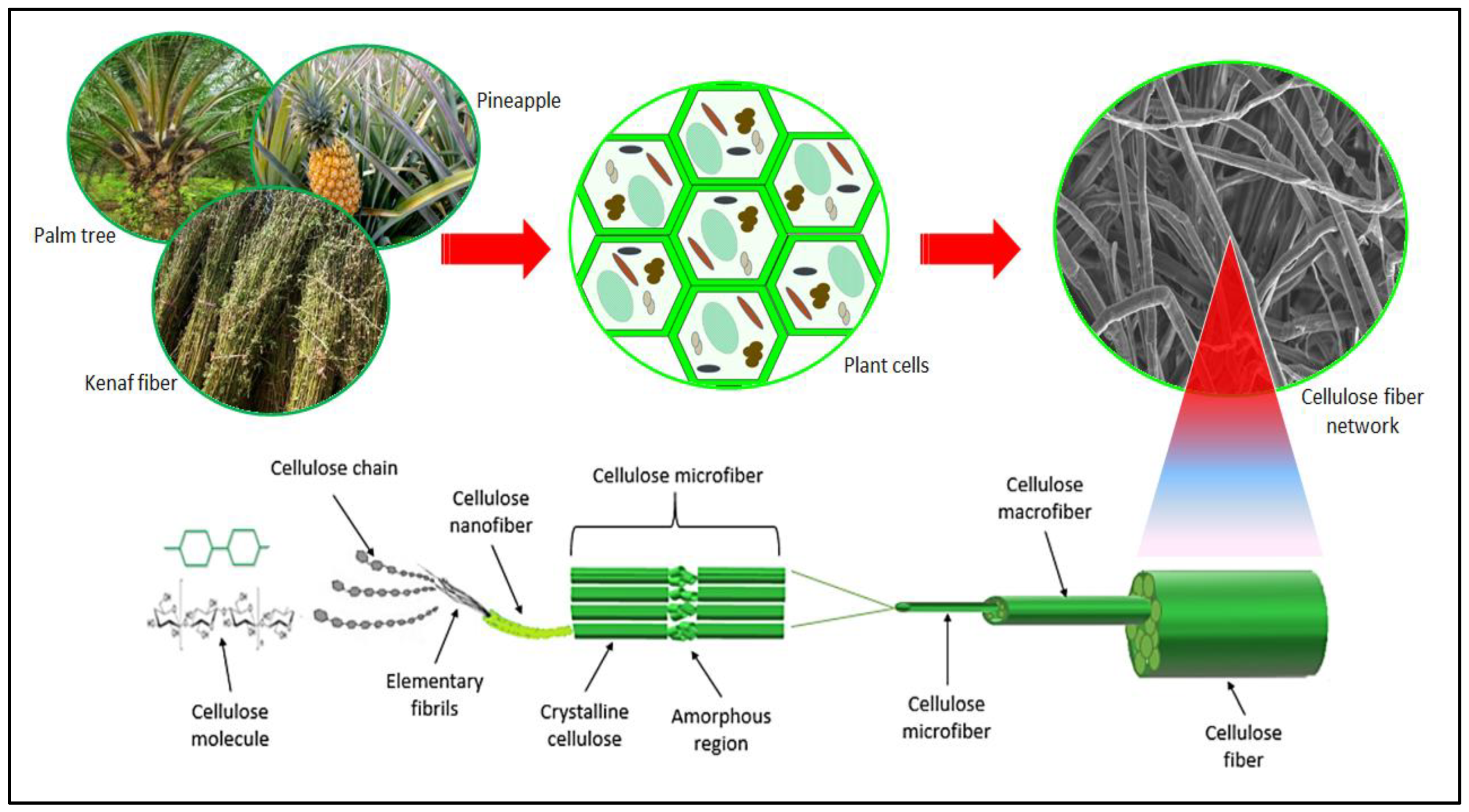
Figure 2. Schematic drawing of plant-based nanocellulose and its role in the formation of plant cell walls (from cellulose fiber to cellulose molecule).
In recent years, there has been significant interest in the isolation of nanocellulose, driven by its versatility in a broad range of applications spanning both medical and non-medical fields. Numerous techniques have been used to produce and to enhance the production of nanocellulose, resulting in material with slightly different properties. Plant cellulose is found in conjunction with other components, primarily hemicellulose and lignin. Therefore, when isolating cellulose, it becomes necessary to eliminate or remove these compounds [27]. Lignin forms ester linkages and hydrogen bonds with hemicellulose and cellulose, and it plays a crucial role in maintaining the structural integrity of cellulosic fibers along with cellulose. As a result, except for cotton, natural or vegetable fibers are commonly referred to as lignocellulosic fibers due to the presence of lignin in their cell walls [28]. The intricate bonding of lignin with hemicellulose creates a matrix that surrounds cellulose molecules, making them highly resilient and resistant to network disruption. The sugar monomers forming long chains are interconnected, leading to robust intermolecular forces between them. Additionally, the cellulose molecule’s high linearity contributes to the tightly packed arrangement of cellulosic fibers [29]. The polymer-like structure and linkages of the polysaccharide contribute to the strong intermolecular forces between the chains of the fiber. Furthermore, the cellulose molecule’s pronounced linearity accounts for the crystalline characteristics observed in cellulosic fibers [29].
3. Structure and Properties of Microbial-Based Nanocellulose
Microbial nanocellulose is the type of nanocellulose produced by microorganisms, which exhibit high crystallinity compared with plant nanocellulose [30]. Microbial nanocellulose as a raw material possesses some unique and advanced mechanical properties, unique structural properties with high crystallinity, high water holding capacity, high purity (as compared to cellulose produced by plants) in addition to its excellent biodegradability [30]. Bacterial cellulose is only created as a nanomaterial naturally by various types of bacteria as an exopolysaccharide. Although it has the same molecular formula as plant celluloses, bacterial cellulose is free from lignin, pectin and hemicelluloses, which make it present in high purity, and it can be isolated by using less energy [31]. Microbial nanocellulose production cost may limit the extensive production of bacterial nanocellulose but its unique properties attract more efforts in order to enhance the development of new approaches to develop its production. Bacterial nanocellulose is the most famous type of microbial nanocellulose, which is produced extracellularly by several bacterial genera including Komagataeibacter, Agrobacterium, Aerobacter, Achromobacter, Alcaligenes, Azotobacter, Pseudomonas, Dickeya, Rhodobacter, Rhizobium, Sarcina, Salmonella and even Escherichia [32][33][34]. Acetobacter xylinus, which is a gram-negative strain of acetic-acid-producing bacteria, is the most efficient and researched producer of bacterial nanocellulose and has been reported as the most promising in terms of nanocellulose yield [35]. Most of these bacteria are aerobic and synthesize nanocellulose extracellularly as nano-fibrils with an average diameter ranging from 10 to 100 nm that are able to self-assemble into a 3D layered pellicle with a large water content of about 98% [30].
Microbial cellulose from bacteria can only be produced at nanoscale. The cellulose produced is highly pure, environmentally friendly and also requires less energy than plant cellulose for purification. In the fermenting process, the micro-organisms attach to cellulose fibers or simply move in the media freely and produce highly swollen gel-like structures [36]. Bacterial cellulose is secreted as a thin ribbon-shaped fibril (leather-like white pellicle), 100 nm wide and 2–3 nm long nanofibrils; bacterial cellulose produced through biosynthesis of cellulose is mainly built up of several bundles of microfibrils extracellularly [37]. The biosynthesis of bacterial nanocellulose starts with the creation of a β-1,4-glucan chain, which is done by polymerization of glucose units and then followed by the production and crystallization of the synthesized cellulose chain. Bacterial nanocellulose has been reported to form inside the bacterial cell, between the cytoplasm and the outer membranes of the bacterial cells [38]. After the biosynthesis of nanocellulose inside the cells, it is then spun from the cell by cellulose-exporting components in the cell membrane to form protofibrils only 2 to 4 nm in diameter. It was found that these protofibrils assemble a ribbon-shaped micro-fibril of approximately 80 nm [39]. In the purification process, while removing the culture media and waste, the microorganisms die. In order to produce good-quality cellulose, either microbial cellulose is washed repeatedly in a hot solution of sodium hydroxide and water until it reaches a neutral pH or through other methods, such as gamma radiation [40]. Although identical in chemical structure to plant-based cellulose, microbial nanocellulose is distinctly characterized by its readily extractable nanofiber network, degradability, excellent tensile strength due to high degrees of polymerization and crystallinity (80%–90%), and the possibility to control these and other physical properties including porosity during biosynthesis [41]. The bacterial cellulose is relatively pure and in crystalline form and no chemical treatments are required to isolate it as obtained from plants. The best advantage of bacterial cellulose is that it can be produced in a variety of shapes and textures, such as particles, whiskers, filaments, films, membranes, etc., and to culture bacteria in situ it can also utilize fruit syrup to grow and reproduce [42].
4. Structure and Properties of Other Sources of Nanocellulose
Macroalgae or seaweed are multicellular marine organisms that contain huge amounts of different polysaccharides, such as agar, alginates, fucoidan, carrageenan, agarose and cellulose [43]. The production of macroalgae-based nanocellulose has increased in the past few years with the increased production of macroalgae due to the growing market demands [44][45]. It has been reported that cellulose is present in most red and brown algae, such as Rhodophyta phylum and the Phaeophyceae class, respectively [46]. Some red algae, such as Gelidium amansii, are rich in carbohydrates (basically cellulose and agar), which form around 75% [47]. Albuquerque et al. [48] stated that the amount of carbohydrate present in Gracilaria birdie (different red algae) is around 73% and 8% protein. Meanwhile, the brown algae Sargassum muticum, Saccorhiza polyschides, and Sargassum filipendula were found to have carbohydrate content in the vicinity of 45%–52% [49][50]. Green algae also contain nanocellulose. Ulva lactuca was found to contain approximately 54.3% of carbohydrate, which is high for green algae applicable for nanocellulose isolation [46]. It has been also reported that some insects, such as silkworms and beetles, produce natural fibers that contain cellulose [51][52] and these fibers can be processed and treated to obtain nanocellulose. For example, silk fibers produced by silkworms contain fibroin, a protein that can be selectively removed to obtain cellulose-based nanofibers [53]. Insect-based nanocellulose has potential applications in biomedical materials and high-performance textiles. These animal-derived materials contain collagen, which can be chemically treated to isolate and convert cellulose into nanocellulose [54]. It is worth noting that the production and use of animal-based nanocellulose is still in the early stages of research and development. The majority of commercial nanocellulose products are derived from plant-based sources due to their abundance and cost-effectiveness. However, as the field progresses, animal-based nanocellulose may find niche applications in specialized industries.
5. Applications of Nanocellulose
Nanocellulose, with its unique properties and versatile nature, holds great potential for various applications across different industries. Nanocellulose finds valuable applications in the biomedical field, including tissue engineering, wound healing, drug delivery systems, and scaffolds for regenerative medicine. Its attractiveness for these applications stems from its biocompatibility, biodegradability, and capacity to mimic the properties of the extracellular matrix [55][56]. Nanocellulose has been also used in wound healing applications due to its excellent moisture-retention capabilities, mechanical strength, and antimicrobial properties [57]. It can promote wound healing by providing a moist environment, preventing infection, and facilitating the migration and proliferation of skin cells [58]. Other researchers have used nanocellulose as a carrier for controlled drug delivery [59]. Its high surface area and ability to encapsulate and release therapeutic agents in a controlled manner make it an ideal material for targeted and sustained drug delivery systems. Nanocellulose can also protect sensitive drugs from degradation and improve their stability [60]. Nanocellulose-based films or membranes can be used as a platform for biosensors [61]. Their high surface area, biocompatibility, and ability to immobilize biomolecules make them suitable for detecting and sensing various biological analytes, such as glucose, proteins, and DNA [62]. These are just a few examples of the wide range of biomedical applications of nanocellulose. Nanocellulose can enhance the properties of packaging materials, making them more sustainable, lightweight, and mechanically strong [63]. It can improve barrier properties against gases and liquids, providing better preservation and protection for food and other sensitive products. Nanocellulose has been incorporated into packaging films to improve their barrier properties against gases, such as oxygen and moisture [64]. Nanocellulose films act as an effective barrier, reducing the permeability of gases and prolonging the shelf life of packaged products [65]. This can help preserve the freshness and quality of food and other sensitive products [66]. Nanocellulose-based coatings can be applied to packaging materials to enhance their performance [67]. Nanocellulose-based packaging materials have a lower carbon footprint and contribute to reducing plastic waste and pollution. Nanocellulose-based packaging can be integrated with sensors or indicators to provide real-time information about the quality or condition of the packaged products [68]. Nanocellulose can be employed in environmental applications, including adsorbents for pollutant removal, oil spill cleanup materials, and sustainable alternatives to single-use plastics. Nanocellulose-based membranes and filters can effectively remove contaminants from water, such as heavy metals [69], dyes [70], and organic pollutants [71]. Nanocellulose-based sorbents offer an eco-friendly alternative to traditional sorbents, aiding in the remediation of oil-contaminated environments [72]. Air filtration has been also benefited from nanocellulose-based materials. Nanocellulose-based air filters can capture and remove particulate matter, allergens, and pollutants from the air [73]. These filters have high filtration efficiency, low pressure drop, and can be produced from sustainable sources, providing cleaner indoor and outdoor air quality [74]. These environmental applications of nanocellulose highlight its potential for mitigating environmental challenges, promoting sustainability, and contributing to a cleaner and healthier planet. Nanocellulose can be also used in several other applications, such as electronic devices, including flexible displays, sensors, and energy storage systems [75]. Its high surface area and electrical conductivity make it a promising material for these applications [76]. The film-forming properties of nanocellulose can help reduce the appearance of fine lines and wrinkles, resulting in a more youthful complexion. It is important to note that the safety and regulatory aspects of nanocellulose in cosmetic products are still being evaluated, and compliance with relevant regulations and guidelines is crucial.
References
- Khalil, H.A.; Jummaat, F.; Yahya, E.B.; Olaiya, N.; Adnan, A.; Abdat, M.; NAM, N.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043.
- Durand, H.; Smyth, M.; Bras, J. Nanocellulose: A new biopolymer for biomedical application. In Biopolymers for Biomedical and Biotechnological Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 129–179.
- Rizal, S.; Alfatah, T.; Abdul Khalil, H.; Yahya, E.B.; Abdullah, C.; Mistar, E.M.; Ikramullah, I.; Kurniawan, R.; Bairwan, R. Enhanced Functional Properties of Bioplastic Films Using Lignin Nanoparticles from Oil Palm-Processing Residue. Polymers 2022, 14, 5126.
- Ilyas, R.; Sapuan, S.; Norrrahim, M.N.F.; Yasim-Anuar, T.A.T.; Kadier, A.; Kalil, M.S.; Atikah, M.; Ibrahim, R.; Asrofi, M.; Abral, H. Nanocellulose/starch biopolymer nanocomposites: Processing, manufacturing, and applications. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–88.
- Xu, Y.; Xu, Y.; Chen, H.; Gao, M.; Yue, X.; Ni, Y. Redispersion of dried plant nanocellulose: A review. Carbohydr. Polym. 2022, 294, 119830.
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869.
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393.
- Mishra, R.; Sabu, A.; Tiwari, S. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978.
- Moon, R.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959.
- Bettaieb, F.; Khiari, R.; Dufresne, A.; Mhenni, M.F.; Belgacem, M.N. Mechanical and thermal properties of Posidonia oceanica cellulose nanocrystal reinforced polymer. Carbohydr. Polym. 2015, 123, 99–104.
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406.
- Islam, M.N.; Rahman, F. Production and modification of nanofibrillated cellulose composites and potential applications. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–141.
- Vieira, D. Obtenção e Caracterização de Nanocelulose a Partir de Fibras de Chorisia Speciosa St. Hil. Master’s Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2015.
- Das, R.; Lindström, T.; Sharma, P.R.; Chi, K.; Hsiao, B.S. Nanocellulose for sustainable water purification. Chem. Rev. 2022, 122, 8936–9031.
- Randhawa, A.; Dutta, S.D.; Ganguly, K.; Patil, T.V.; Patel, D.K.; Lim, K.-T. A review of properties of nanocellulose, its synthesis, and potential in biomedical applications. Appl. Sci. 2022, 12, 7090.
- Madsen, B.; Gamstedt, E.K. Wood versus plant fibers: Similarities and differences in composite applications. Adv. Mater. Sci. Eng. 2013, 2013, 564346.
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. 2022, 285, 119258.
- Louis, A.C.F.; Venkatachalam, S. Energy efficient process for valorization of corn cob as a source for nanocrystalline cellulose and hemicellulose production. Int. J. Biol. Macromol. 2020, 163, 260–269.
- Oun, A.A.; Rhim, J.-W. Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 2018, 25, 2143–2149.
- Rai, R.; Dhar, P. Biomedical engineering aspects of nanocellulose: A review. Nanotechnology 2022, 33, 362001.
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668.
- Leong, S.L.; Tiong, S.I.X.; Siva, S.P.; Ahamed, F.; Chan, C.-H.; Lee, C.L.; Chew, I.M.L.; Ho, Y.K. Morphological control of cellulose nanocrystals via sulfuric acid hydrolysis based on sustainability considerations: An overview of the governing factors and potential challenges. J. Environ. Chem. Eng. 2022, 10, 108145.
- Zhao, X.; Bhagia, S.; Gomez-Maldonado, D.; Tang, X.; Wasti, S.; Lu, S.; Zhang, S.; Parit, M.; Rencheck, M.L.; Korey, M. Bioinspired design toward nanocellulose-based materials. Mater. Today 2023, 66, 409–430.
- Surendran, G.; Sherje, A.P. Cellulose nanofibers and composites: An insight on basics and biomedical applications. J. Drug Deliv. Sci. Technol. 2022, 75, 103601.
- Meftahi, A.; Momeni Heravi, M.E.; Barhoum, A.; Samyn, P.; Najarzadeh, H.; Alibakhshi, S. Cellulose Nanofibers: Synthesis, Unique Properties, and Emerging Applications. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 233–262.
- Bourmaud, A.; Mayer-Laigle, C.; Baley, C.; Beaugrand, J. About the frontier between filling and reinforcement by fine flax particles in plant fibre composites. Ind. Crop. Prod. 2019, 141, 111774.
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45.
- Hurtado, P.L.; Rouilly, A.; Vandenbossche, V.; Raynaud, C. A review on the properties of cellulose fibre insulation. Build. Environ. 2016, 96, 170–177.
- Schiros, T.N.; Antrobus, R.; Farías, D.; Chiu, Y.-T.; Joseph, C.T.; Esdaille, S.; Sanchirico, G.K.; Miquelon, G.; An, D.; Russell, S.T. Microbial nanocellulose biotextiles for a circular materials economy. Environ. Sci. Adv. 2022, 1, 276–284.
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972.
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa-Jr, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072.
- Stanisławska, A. Bacterial nanocellulose as a microbiological derived nanomaterial. Adv. Mater. Sci. 2016, 16, 45–57.
- Reshmy, R.; Philip, E.; Thomas, D.; Madhavan, A.; Sindhu, R.; Binod, P.; Varjani, S.; Awasthi, M.K.; Pandey, A. Bacterial nanocellulose: Engineering, production, and applications. Bioengineered 2021, 12, 11463.
- Skočaj, M. Bacterial nanocellulose in papermaking. Cellulose 2019, 26, 6477–6488.
- Cottet, C.; Ramirez-Tapias, Y.A.; Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased materials from microbial biomass and its derivatives. Materials 2020, 13, 1263.
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623.
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12.
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257.
- Pacheco, G.; de Mello, C.V.; Chiari-Andréo, B.G.; Isaac, V.L.B.; Ribeiro, S.J.L.; Pecoraro, É.; Trovatti, E. Bacterial cellulose skin masks—Properties and sensory tests. J. Cosmet. Dermatol. 2018, 17, 840–847.
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than meets the eye in bacterial cellulose: Biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 2014, 14, 10–32.
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545.
- Zaki, M.; Abdul Khalil, H.P.S.; Sabaruddin, F.; Bairwan, R.; Oyekanmi, A.A.; Alfatah, T.; Danish, M.; Mistar, E.; Abdullah, C. Microbial treatment for nanocellulose extraction from marine algae and its applications as sustainable functional material. Bioresour. Technol. Rep. 2021, 16, 100811.
- Bar-Shai, N.; Sharabani-Yosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering. Sci. Rep. 2021, 11, 11843.
- Oyekanmi, A.A.; Saharudin, N.; Hazwan, C.M.; HPS, A.K.; Olaiya, N.G.; Abdullah, C.K.; Alfatah, T.; Gopakumar, D.A.; Pasquini, D. Improved hydrophobicity of macroalgae biopolymer film incorporated with kenaf derived CNF using silane coupling agent. Molecules 2021, 26, 2254.
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288.
- Jeong, T.S.; Choi, C.H.; Lee, J.Y.; Oh, K.K. Behaviors of glucose decomposition during acid-catalyzed hydrothermal hydrolysis of pretreated Gelidium amansii. Bioresour. Technol. 2012, 116, 435–440.
- Albuquerque, J.C.S.; Araújo, M.L.H.; Rocha, M.V.P.; de Souza, B.W.S.; de Castro, G.M.C.; Cordeiro, E.M.S.; de Sousa Silva, J.; Benevides, N.M.B. Acid hydrolysis conditions for the production of fine chemicals from Gracilaria birdiae alga biomass. Algal Res. 2021, 53, 102139.
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207.
- Fraly Erbabley, N.Y.G.; Junianto, J. Chemical characteristics and phytochemicals of the brown alga Sargassum filipendulla from kelanit waters of southeast Maluku. Egypt. J. Aquat. Biol. Fish. 2020, 24, 535–547.
- Ennab, R.M.; Aljabali, A.A.; Charbe, N.B.; Barhoum, A.; Alqudah, A.; Tambuwala, M.M. Nanocelluloses in Wound Healing Applications. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–28.
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105.
- Calvo, V.; González-Domínguez, J.M.; Benito, A.M.; Maser, W.K. Synthesis and Processing of Nanomaterials Mediated by Living Organisms. Angew. Chem. 2022, 134, e202113286.
- Noremylia, M.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. Int. J. Biol. Macromol. 2022, 206, 954–976.
- Fang, B.; Wan, Y.-Z.; Tang, T.-T.; Gao, C.; Dai, K.-R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098.
- Yahya, E.B.; Amirul, A.; HPS, A.K.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; AK, A.S.; Adnan, A. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612.
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivlér, P.; Skog, M.; Rinklake, I.; Shamasha, R. Nanocellulose composite wound dressings for real-time pH wound monitoring. Mater. Today Bio 2023, 19, 100574.
- Zhang, M.; Guo, N.; Sun, Y.; Shao, J.; Liu, Q.; Zhuang, X.; Twebaze, C.B. Nanocellulose aerogels from banana pseudo-stem as a wound dressing. Ind. Crop. Prod. 2023, 194, 116383.
- Puppala, N.V.; Doddipatla, P.; Mohannath, G. Use of nanocellulose in the intracellular delivery of biological and non-biological drugs: A review. Cellulose 2023, 30, 1335–1354.
- Bellmann, T.; Thamm, J.; Beekmann, U.; Kralisch, D.; Fischer, D. In situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs. Pharmaceutics 2023, 15, 559.
- de Assis, S.C.; Morgado, D.L.; Scheidt, D.T.; de Souza, S.S.; Cavallari, M.R.; Ando Junior, O.H.; Carrilho, E. Review of Bacterial Nanocellulose-Based Electrochemical Biosensors: Functionalization, Challenges, and Future Perspectives. Biosensors 2023, 13, 142.
- Eldhose, M.; Roy, R.; George, C.; Joseph, A. Sensing and Biosensing Applications of Nanocellulose. In Handbook of Biopolymers; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–26.
- Vilarinho, F.; Sanches Silva, A.; Vaz, M.F.; Farinha, J.P. Nanocellulose in green food packaging. Crit. Rev. Food Sci. Nutr. 2018, 58, 1526–1537.
- Matsumoto, M.; Kitaoka, T. Ultraselective gas separation by nanoporous metal− organic frameworks embedded in gas-barrier nanocellulose films. Adv. Mater. 2016, 28, 1765–1769.
- Aulin, C.; Karabulut, E.; Tran, A.; Wågberg, L.; Lindström, T. Transparent nanocellulosic multilayer thin films on polylactic acid with tunable gas barrier properties. ACS Appl. Mater. Interfaces 2013, 5, 7352–7359.
- Parvathy, S.; Hema, S.; Sajith, M.; Sulthan, R.; Sreelekshmi, C.; Sambhudevan, S.; Shankar, B. A Review on Barrier Properties of Nanocellulose and Polylactic acid Composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2022; p. 012017.
- Rizal, S.; Olaiya, F.G.; Saharudin, N.; Abdullah, C.; NG, O.; Mohamad Haafiz, M.; Yahya, E.B.; Sabaruddin, F.; Ikramullah; Abdul Khalil, H.P.S. Isolation of textile waste cellulose nanofibrillated fibre reinforced in polylactic acid-chitin biodegradable composite for green packaging application. Polymers 2021, 13, 325.
- Ginja, G.A.; da Costa, J.P.d.C.; Gounella, R.H.; Izquierdo, J.E.E.; Carmo, J.P.; Fonseca, F.J.; Cavallari, M.R.; Junior, O.H.A.; de Souza, S.S. A Humidity Sensor Based on Bacterial Nanocellulose Membrane (BNC). IEEE Sens. J. 2023, 23, 3485–3492.
- Suman; Kardam, A.; Gera, M.; Jain, V. A novel reusable nanocomposite for complete removal of dyes, heavy metals and microbial load from water based on nanocellulose and silver nano-embedded pebbles. Environ. Technol. 2015, 36, 706–714.
- Tasrin, S.; Mohamed Madhar Fazil, S.; Senthilmurugan, S.; Selvaraju, N. Facile preparation of nanocellulose embedded polypyrrole for dye removal: Unary and binary process optimization and seed toxicity. Int. J. Environ. Sci. Technol. 2021, 18, 365–378.
- Maqbool, Q.; Barucca, G.; Sabbatini, S.; Parlapiano, M.; Ruello, M.L.; Tittarelli, F. Transformation of industrial and organic waste into titanium doped activated carbon–cellulose nanocomposite for rapid removal of organic pollutants. J. Hazard. Mater. 2022, 423, 126958.
- Yin, Z.; Cheng, Y.; Deng, Y.; Li, Z.; Liu, K.; Li, M.; Chen, X.; Xue, M.; Ou, J.; Lei, S. Functional and versatile colorful superhydrophobic nanocellulose-based membrane with high durability, high-efficiency oil/water separation and oil spill cleanup. Surf. Coat. Technol. 2022, 445, 128714.
- Xiong, Z.; Lin, J.; Li, X.; Bian, F.; Wang, J. Hierarchically structured nanocellulose-implanted air filters for high-efficiency particulate matter removal. ACS Appl. Mater. Interfaces 2021, 13, 12408–12416.
- Sun, Z.; Huang, Z.; Guo, L.; Hu, S.; Wang, H.; Meng, L.; Tang, M.; Qi, H. Acetylated tunicate nanocellulose-based high-efficient air filter media with antibacterial property. J. Membr. Sci. 2023, 669, 121307.
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Yang, W.; Tjong, J.; Sain, M. Current state of applications of nanocellulose in flexible energy and electronic devices. Front. Chem. 2020, 8, 420.
- Fan, W.; Wang, J.; Zhang, Z.; Li, Z. Synergistic enhancement of UV-resistance and electrical conductivity of waterborne polyurethane composite with combination of functionalized 2D graphene oxide and 1D nanocellulose. Prog. Org. Coat. 2021, 151, 106017.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
28 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




