Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nikolay Dimitrov Kanazirski | -- | 2049 | 2023-07-27 20:40:40 | | | |
| 2 | Dean Liu | Meta information modification | 2049 | 2023-07-28 05:30:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kanazirski, N.; Vladova, D.; Neychev, D.; Raycheva, R.; Kanazirska, P. Effect of Er:YAG Laser Exposure. Encyclopedia. Available online: https://encyclopedia.pub/entry/47370 (accessed on 07 February 2026).
Kanazirski N, Vladova D, Neychev D, Raycheva R, Kanazirska P. Effect of Er:YAG Laser Exposure. Encyclopedia. Available at: https://encyclopedia.pub/entry/47370. Accessed February 07, 2026.
Kanazirski, Nikolay, Diyana Vladova, Deyan Neychev, Ralitsa Raycheva, Petya Kanazirska. "Effect of Er:YAG Laser Exposure" Encyclopedia, https://encyclopedia.pub/entry/47370 (accessed February 07, 2026).
Kanazirski, N., Vladova, D., Neychev, D., Raycheva, R., & Kanazirska, P. (2023, July 27). Effect of Er:YAG Laser Exposure. In Encyclopedia. https://encyclopedia.pub/entry/47370
Kanazirski, Nikolay, et al. "Effect of Er:YAG Laser Exposure." Encyclopedia. Web. 27 July, 2023.
Copy Citation
In order to make a comprehensive assessment of the effect of Er:YAG lasers, histomorphological techniques were used to measure the reduction in amorphous layer thickness after Er:YAG laser treatment, both with and without the placement of dental screw implants from different manufacturers.
Er:YAG laser
implantology
amorphous smear layer
1. Introduction
Restoring the function and esthetics of the dentition has become an attainable goal in modern dentistry thanks to the major advancements in dental implantology. Placing dental implants has become a routine procedure in modern dental practice. A thorough understanding of the mechanisms of osseointegration and the factors influencing this process is essential to achieve positive outcomes in implantological treatment. The term osseointegration was introduced by Brånemark in 1969. Osseointegration was initially defined at the light microscopic level as “a direct structural and functional connection between living bone and surface living bone and the surface of a loadbearing implant” [1]. A large number of authors have worked on the mechanisms of osseointegration [2][3]. In recent years, the definition of osseointegration has changed, with the following definition being formulated: “a process in which a clinically asymptomatic rigid fixation of alloplastic material is achieved and maintained in bone during functional loading” (Brånemark 1985) [1][4].
Osseointegration is the direct structural and functional connection between living bone tissue and the implant, and it is considered to be an indicator of the success of implant placement. Osseointegration represents a specific stage of the evolution of the implant–tissue interface that results from the interaction of bone and bioinert materials [5].
To describe the complex interaction of biomaterials and tissues, Kasemo and Lausmaa used the term “implant-tissue interface”, which refers to a qualitatively new structure formed as a result of the interaction between the materials and the biological system [6].
The process of osseointegration depends on multiple factors that act during the four phases of the healing process. The framework used for the phases of osseointegration was defined based on an interpolation of the concept expressed by Stadelmann et al. [7] regarding the physiology and healing of chronic bone wounds. There are four phases of the healing process after implant placement: hemostasis, inflammation, proliferation and remodeling. According to a study by Terheyden et al. [8], the first signs of remodeling after dental implant placement were observed after about 6 weeks, which was two weeks later than in an animal model. The formation of titanium oxide on the implant surface after placement creates a prerequisite for osseointegration [4]. Ti-alloys are popular candidates for load-bearing implant applications owing to their high strength-to-weight ratio, good biocompatibility and excellent corrosion resistance, regardless of whether they are a pure Ti, Ti-6Al-4V, Ti-Nb alloy or any other alloy. Chakkravarthy et al. reported that Ti-30Nb-2Zr, which is a promising next-generation biomedical implant material, was fabricated in the form of a porous scaffold using the selective laser melting (SLM) additive manufacturing route [9][10]. Titanium nanoparticles (Ti-NP), as a material with suitable mechanical properties, can be a used for the production of dental ceramics with higher strength properties via combination with lithium disilicate [11].
According to Brånemark [1], osseointegration refers to a phenomenon in which the implant becomes so ankylosed with the bone that they cannot be separated without fracture.
Various factors that influence the rate and success of osseointegration can be categorized as those related to implant characteristics, such as the physical and chemical macro- and micro-design of the implants; as bone characteristics, such as the quantity and quality of bone and the local and systemic conditions of the patient; or the time and protocol used for the functional loading of the dental implant [12]. To ensure proper healing of the placed implant, it is necessary to guarantee good primary stability. Another important aspect is the need to control micro-movements. Primary stability is defined as the biometric stability immediately after implant placement, and it is a direct result of mechanical engagement of the implant with the adjacent bone [13]. It is a result of the frictional interaction between the implant and the bone. The formula defining this interaction is as follows:
F = k·N, as:
Implant surface design has evolved to meet oral rehabilitation challenges in both healthy and compromised bone. Many studies aim to comprehensively discuss currently available implant surface modifications that are commonly used in implantology in terms of their impact on osseointegration and biofilm formation, which is critical in helping clinicians to choose the most suitable materials to improve the success and survival of implantation [16].
The use of implants with modified surfaces aims to accelerate osseointegration, which is evidenced by achieving control of the stability of the placed implants. The microrelief of the implant surface determines the good connection of the implant surface to the fibrin skeleton, as well as the course of contact osteogenesis and the increase in the total area of the region of the implant–tissue interface. Modification methods are divided into two main groups: subtractive methods, in which defects on the implant surface are created by removing an amount of material, and additive methods, in which positive roughness is created by adding material.
Subtractive methods are as follows: machining, etching, abrasive jet methods, SLA (Sandblasted with Large grift Al2 O3, followed by Acid etching) and laser texturing.
Additive methods are as follows: titanium plasma spray, hydroxyapatite coating, anodic oxidation and brushite coating.
Changing the surface chemistry of implants involves a classic combination of abrasive jet treatment and subsequent high temperature etching.
Modern dentistry faces the challenge of providing rapid treatment results within a short period without compromising quality. The placement of dental implants typically involves the use of rotary techniques and drills to create a bone bed for implant placement. During this procedure, water cooling is used, and the revolutions per minute (RPM) value is carefully chosen to avoid overheating the bone and denaturing its protein structures. In addition, the use of a rotary technique to create a bone bed results in a smear layer, which can affect primary stability and may lead to changes even beyond day 12. Following the introduction of Er:Yag lasers, the rapid development of surgical technologies has enabled the creation of implant sites using alternative techniques, such as laser surgery. However, attempts to use lasers alone for osteotomy have revealed certain drawbacks, including prolonged treatment duration and inaccuracies in calibrating the osteotomy hole. Nevertheless, using the Er:Yag laser at a wavelength of 2940 nm offers benefits such as proper decontamination of tissues, the absence of a smear layer on the osteotomy surface and reduced bleeding. These advantages provide opportunities for accelerated osseointegration and early prosthetic treatment.
The combination of conventional rotary techniques and laser osteotomy demonstrates synergistic effects, making it possible to integrate lasers into the conventional implantation protocol. Early loading of dental implants placed using the two-stage technique becomes feasible through the combined use of rotary and laser techniques. This combined technique aims to create the conditions required for accelerated osseointegration, which is crucial to improving masticatory function and facilitating esthetic rehabilitation for the patient.
When placing a screw dental implant, proper preparation of the recipient site is crucial. The method of recipient site preparation determines the possibility of early loading based on the achieved primary stability of the implants. This stability depends on both the type of bone located at the implantation site (according to the Misch classification) and the technique used. Several approaches can be employed to attain primary stability. The conventional and most commonly used method is the rotary technique with water cooling.
In several in vitro studies using bovine models, it has been reported that one of the major factors influencing successful osseointegration is the temperature generated during recipient site preparation. The authors discuss the importance of not exceeding the temperature limit of 47 °C to prevent bone necrosis [1][17]. Various measures are being explored to reduce heat generation during osteotomy, including drill geometry and design, bone density and cortical thickness, single-stage or reciprocating drilling, use of reusable drills, internal or external cooling and the pressure applied by the operator during osteotomy [18].
The use of ultrasound for osteotomy has the disadvantage of being relatively slower than the standard rotary technique. However, one benefit of using ultrasound is that it enables much faster tissue regeneration than the conventional technique [19].
Laser technology provides another option for preparing the implant site. Among all types of lasers, the Er:Yag laser is the most suitable for this purpose. This type of laser allows researchers to work on bone structures without causing carbonization due to its pulse operation and effective cooling during the procedure [20]. The selection of the correct wavelength and laser parameters is of paramount importance in terms of minimizing the risk of thermal damage to the bone [20][21]. According to studies conducted by some authors, the three aforementioned techniques used to preparing the implant site are comparable, at least during the early stage of the healing process [22][23]. Achieving temperature control via various osteotomy techniques can be challenging. All of these factors contribute to the possibility of influencing the duration of osseointegration [24].
2. Histological Analysis
Group A—The survey of horizontal and vertical histosections located across the contact surface of the bed showed a rough bone surface with an irregular periphery along the incision edges and numerous microcracks resulting from the osteotomy. The cracks were filled with bone fragments and soft tissue, collectively forming an amorphous layer that covered the trepanation surface. The amorphous layer blocked the Volkmann’s and Haversian canals. The osteotomy edges beneath the amorphous layer displayed varying degrees of destructive changes, resulting in a porous surface with low-grade-to-absent thermoalteration (Figure 1).
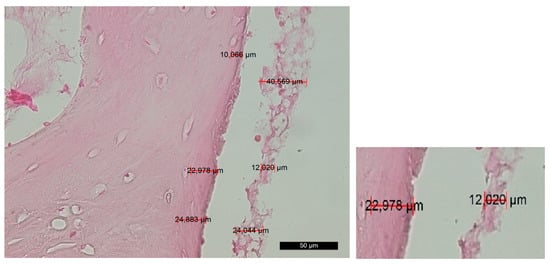
Figure 1. Changes in the contact surface in group A—magnification of 50 µm and an example of the measurement.
Group B—The survey of histosections across the contact surface of the implant site revealed linear and distinct trepanation edges, which were free of bone and soft-tissue fragments. Volkmann’s and Haversian canals were open. The amorphous layer was irregular, vague or fragmented, while, in some places, it was completely absent. This layer can be further subdivided into two distinct sublayers—the superficial layer (PL) and the deep layer (DL). The superficial layer showed signs of carbonization in certain areas, along with remnants of bone and soft tissue. The deep layer exhibited mild traumatic damage that resulted from the preliminary mechanical treatment (Figure 2).
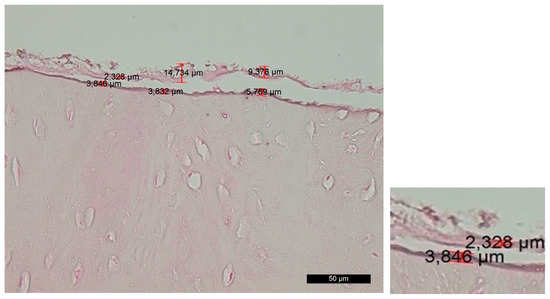
Figure 2. Changes in the contact surface in group B—magnification of 50 µm and an example of the measurement.
Group C—The histological findings were almost identical to those observed in group A. The main difference was the smaller thickness of the amorphous layer on the contact surface due to the slight compression that occurred during implant placement. As an exception, there were certain areas on the surface that exhibited characteristics of compression trauma, which were included in the amorphous layer. The boundaries of this layer were not clearly defined (Figure 3).
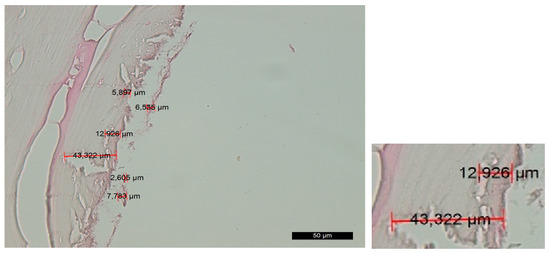
Figure 3. Changes in the contact surface in group C—magnification of 50 µm and an example of the measurement.
Group D—The histological findings were relatively similar to those of experimental group B. Smooth linear edges were predominantly observed adjacent to the trepanation surface. No isolated and/or layered amorphous masses of bone or soft-tissue fragments were found. The surface layer appeared to be wel structured, with almost no signs of compression trauma, alteration and carbonization. The Volkmann’s and Haversian canals extended directly to the surface, allowing direct contact between the cells within them (including osteoblasts and osteoclasts) and the implant surface. The thickness of the amorphous layer was extremely small, and in many areas, it was practically absent (Figure 4).
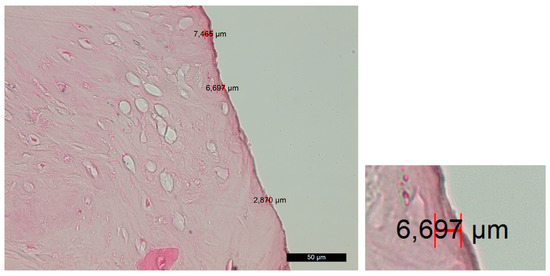
Figure 4. Changes in the contact surface in group D—magnification of 50 µm and an example of the measurement.
Implant site preparation using the standard rotary technique was performed within the normal time frame. Surface treatment with the Er:YAG laser took two-to-three minutes per osteotomy opening, which is not a statistically significant prolongation compared to laser preparation alone, which took 25 min on average. No signs of bone carbonization, melting or cracking were observed in any of the groups.
Histomorphological measurements showed the following amorphous layer thicknesses by group: group A—21.813 to 222.13 µm; group B—6.08 µm to 43.64 µm; group C—5.90 to 54.52 µm; and group D—1.29 to 7.98 µm.
References
- Brånemark, P.I. Introduction to osseointegration. In Tissue Integrated Prostheses: Osseointegration in Clinical Dentistry, 1st ed.; Brånemark, P.I., Ed.; Quintessence Publishing Co.: Chicago, IL, USA, 1985; pp. 11–76.
- Zarb, G.; Albrektsson, T. Osseointegration: A requiem for the periodontal ligament. Int. J. Periodontics. Rest. Dent. 1991, 11, 88–91.
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, 9.
- Jayesh, R.S.; Dhinakarsamy, V. Osseointegration. J. Pharm. Bioall. Sci. 2015, 7, 226–229.
- Siebers, M.; Brugge, P.; Walboomers, X.; Jansen, J. Integrins as linker proteins between osteoblasts and bone replacing materials. A Crit. Rev. Biomater. 2005, 26, 137–146.
- Kasemo, B.; Lausmaa, J. Surface scoence aspects on inorganic biomaterials. CRC Crit. Rev. Biocomp. 1986, 2, 335–380.
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26–38.
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of cells. Clin. Oral Impl. Res. 2011, 23, 1127–1135.
- Chakkravarthy, V.; Sujin, P.J.; Lakshmanan, M.; Manojkumar, P.; Narayan, L.R.; Kumaran, M. Additive manufacturing of novel Ti-30Nb-2Zr biomimetic scaffolds for successful limb salvage Author links open overlay pane. Mater. Today Proc. 2022, 64, 1711–1716.
- Chakkravarthy, V.; Manojkumar, P.; Lakshmanan, M.; Eswar Prasad, K.; Dafale, R.; Chitra Vadhana, V.; Narayan, R.L. Comparing bio-tribocorrosion of selective laser melted Titanium-25% Niobium and conventionally manufactured Ti-6Al-4 V in in-flammatory conditions. J. Alloys Compd. 2023, 952, 169852.
- Rajaei, A.; Kazemian, M.; Khandan, A. Investigation of mechanical stability of lithium disilicate ceramic reinforced with titanium nanoparticles. Nanomed. Res. J. 2022, 7, 350–359.
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary Concepts in Osseointegration of Dental Implants: A Review. BioMed Res. Int. 2022, 2022, 11.
- Al-Sabbagh, M.; Eldomiaty, W.; Khabbaz, Y. Can Osseointegration Be Achieved Without Primary Stability? Dent. Clin. N. Am. 2019, 63, 461–473.
- Joos, G. Theoretical Physics, 3rd ed.; Dover Publications: New York, NY, USA, 1987; pp. 50–51.
- Peev, S. Bone Augmentation in Dental Implantology, 1st ed.; Quintessence Pub Co.: Varna, Bulgaria, 2016; pp. 20–21.
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641.
- Eriksson, A.R.; Albrektsson, T. Assessment of bone viability after heat trauma. Scand. J. Plast. Reconstr. Surg. 1984, 18, 261–268.
- Soldatos, N.; Pham, H.; Fakhouri, W.D.; Ngo, B.; Lampropoulos, P.; Tran, T.; Weltman, R. Temperature Changes during Implant Osteotomy Preparations in Human Cadaver Tibiae Comparing MIS® Straight Drills with Densah® Burs. Genes 2022, 13, 1716.
- Vercellotti, T.; Nevins, M.L.; Kim, D.M.; Nevins, M.; Wada, K.; Schenk, R.K.; Fiorellini, J.P. Osseus response following resective therapy with piezosurgey. Int. Periodontics Restor. Dent. 2005, 25, 543–549.
- Moslemi, N.; Shahnaz, A.; Masoumi, S.; Torabi, S.; Akbari, S. Laser-assisted osteotomy for implant site preparation: A literature review. Implant. Dent. 2017, 26, 129–136.
- Marotti, J.; Neto, P.T.; de Campos, T.T.; Aranha, A.C.C.; Weingart, D.; Wolfart, S.; Haselhuhn, K. Recent Patents of Lasers in Implant Dentistry. Recent Pat. Biomed. Eng. 2011, 4, 103–109.
- Stübinger, S.; Biermeier, K.; Bächi, B.; Ferguson, S.J.; Sader, R.; von Rechenberg, B. Comparison of Er:YAG laser, piezoelectric, and drill osteotomy for dental implant site preparation: A biomechanical and histological analysis in sheep. Lasers Surg. Med. 2010, 42, 652–661.
- Wu, X.; Deng, F.; Wang, Z.; Zhao, Z.; Wang, J. Biomechanical and histomorphometric analyses of the osseointegration of microscrews with different surgical techniques in beagle dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 106, 644–650.
- Jeong, S.M.; Yoo, J.H.; Fang, Y.; Choi, B.-H.; Son, J.-S.; Oh, J.-H. The effect of guided flapless implant procedure on heat generation from implant drilling. J. Craniomaxillofac. Surg. 2014, 42, 725–729.
More
Information
Subjects:
Surgery
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
492
Revisions:
2 times
(View History)
Update Date:
28 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




