You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandra Bearz | -- | 1595 | 2023-07-27 10:49:37 | | | |
| 2 | Catherine Yang | -1 word(s) | 1594 | 2023-07-27 11:02:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Stanzione, B.; Del Conte, A.; Bertoli, E.; De Carlo, E.; Revelant, A.; Spina, M.; Bearz, A. ROS1 for Advanced Non Small Cell Lung Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/47351 (accessed on 24 December 2025).
Stanzione B, Del Conte A, Bertoli E, De Carlo E, Revelant A, Spina M, et al. ROS1 for Advanced Non Small Cell Lung Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/47351. Accessed December 24, 2025.
Stanzione, Brigida, Alessandro Del Conte, Elisa Bertoli, Elisa De Carlo, Alberto Revelant, Michele Spina, Alessandra Bearz. "ROS1 for Advanced Non Small Cell Lung Cancer" Encyclopedia, https://encyclopedia.pub/entry/47351 (accessed December 24, 2025).
Stanzione, B., Del Conte, A., Bertoli, E., De Carlo, E., Revelant, A., Spina, M., & Bearz, A. (2023, July 27). ROS1 for Advanced Non Small Cell Lung Cancer. In Encyclopedia. https://encyclopedia.pub/entry/47351
Stanzione, Brigida, et al. "ROS1 for Advanced Non Small Cell Lung Cancer." Encyclopedia. Web. 27 July, 2023.
Copy Citation
ROS proto-oncogene 1 (ROS1) rearrangements occur in 0.9–2.6% of patients with non small cell lung cancer (NSCLC), conferring sensitivity to treatment with specific tyrosine-kinase inhibitors (TKI). Crizotinib, a first-generation TKI, was the first target-therapy approved for the first-line treatment of ROS1-positive NSCLC. Entrectinib, a multitarget inhibitor with an anti-ROS1 activity 40 times more potent than crizotinib and better activity on the central nervous system (CNS), received approval for treatment-naive patients.
ROS1
non small cell lung cancer
target therapy
1. Introduction
Lung cancer is considered the first cause of death from cancer worldwide [1]. Non-small cell lung cancer (NSCLC), which accounts for around 84% of all lung cancer cases and is frequently diagnosed at an advanced stage, has a poor prognosis and low survival rates. NSCLC is not a single entity but rather represents a variety of distinct illnesses, based on its molecular characteristics and, in some circumstances, on the expression of particular targetable oncogenic drivers [2]. ROS1 is a tyrosine kinase receptor belonging to the insulin receptor family. Its gene is on chromosome 6. It is made up of an extracellular N-terminal domain, a single trans-membrane domain, and an intracellular C-terminal region that contains the kinase domain [3][4]. Wild-type ROS1′s role is not completely known, but it seems to be involved in differentiation of epithelial tissues during embryogenesis. Chromosomal rearrangements involving ROS1 were first identified in glioblastoma, but they have been identified also in cholangiocarcinoma, gastric cancer, ovarian cancer, soft-tissue sarcomas, breast cancer, lung cancer and many other tumor types [5]. Several gene fusion partners have been discovered. The most frequent fusion gene is CD74–ROS1, representing 44% of cases, followed by EZRs–ROS1, SDC4–ROS1 and SLC34A2. The upregulation of the SHP-2 phosphatase, MAPK/ERK pathway, PI3K/AKT/mTOR pathway, and JAK/STAT pathway, which control cellular survival, growth, and proliferation, is a characteristic of all fusion genes. These genes are oncogenic and have constitutive, ligand-independent catalytic activity [6]. The prognostic role of these different fusion genes has been explored in several studies but clear conclusions cannot be outlined. Interestingly, oncogenic co-mutations can be found in 36% of ROS1 positive NSCLC, in particular EGFR or KRAS mutations, MET amplification or ALK translocation [7]. ROS1 rearrangements are present in 0.9–2.6% of NSCLCs. ROS1-positive NSCLCs share some clinicopathological characteristics with ALK-positive NSCLCs, although they are different entities. In fact, ROS1 rearrangements are more frequently found in adenocarcinomas (with a predominance of solid, papillary, acinar, cribriform, and mucinous histology patterns), women, young patients and light- or never-smokers. Furthermore, ROS1-positive tumors are usually diagnosed in an advanced stage (III-IV), with brain metastases and lymph-node involvement; they are also associated with major thromboembolic risk, included trombotic microangiopathy or disseminated intravascular coagulation (DIC) [8][9][10][11][12][13][14]. Central nervous system (CNS) involvement is frequent not only at diagnosis (36% of patients), but also as the first-site of progression (47% of patients) [11]. All metastatic lung cancers should be tested for the presence of ROS1 rearrangements, regardless of clinical characteristics. In particular, ROS1 should be sought not only in adenocarcinomas, but also in tumors with mixed histology or squamous-cell carcinomas, because an adenocarcinoma component cannot be excluded, especially in never-smokers or younger (<50 years) patients [15]. The techniques that can be used to detect the presence of ROS1 are immunohistochemistry (IHC), fluorescence in situ hybridation (FISH), reverse-transcriptase-polymerase-chain-reaction (RT-PCR) and next generation sequencing (NGS). These techniques are not always available in all cancer treatment centers, but the use of them is essential for a correct diagnosis and an optimal setting of the therapeutic path. IHC is usually used as a screening technique [16][17]. It has lower costs than other techniques, and a high sensitivity, but a variable specificity, ranging from 70% to 90%. Its limits are the lack of globally accepted scores, the difficulty in interpretation of results and the need to use other techniques to confirm results, in the case of positivity or questionable results. In particular, a weak ROS1 expression can be found in hyperplastic type II pneumocytes, alveolar macrophages and osteoclasts, making the evaluation of the outcome more difficult. IHC false-positives are more common in EGFR-mutated lepidic or acinar adenocarcinomas. Actually, there are three commercially available anti-ROS1 antibodies. When a positive IHC is found in contrast with a negative FISH, due to the presence of other oncogenic driver mutations, the use of a further technique is necessary, usually NGS. The gold standard to identify ROS1 rearrangements is represented by FISH, thanks to its high specificity and sensitivity [18]. FISH testing can be performed either on histological sections or cytological specimens; it requires low input of material and it is possible to have results in a short time. When more than 15% of the cells display separation of the 3’ and 5’ probes or a distinct 3’ signal (centromeric), the sample is deemed positive. Its limits are the need of an experienced pathologist due to the difficulty level to interpret the results, impossibility to know ROS1 fusion partners, and need of sufficient amount of tumor cells (more than 50) [19]. RT-PCR, despite its high sensitivity and specificity, is rarely used for its high costs and technical difficulties. In particular, this technique requires different steps, such as RNA extraction, complementary DNA synthesis, quantitative PCR and analysis, with a high risk of variations [20][21][22]. NGS allows to test simultaneously many predictive biomarkers, with high sensitivity and specificity [23][24]. It is possible to identify several ROS1 fusion partners as well as other oncogenic molecular targets, by using tumor DNA or RNA. NGS can be performed on tumor tissue or plasma. The cons of NGS are long lead times and high costs, limiting its use in routine clinical practice, especially in small hospital centers. ROS1 turned out like a driver for targeted therapies, changing the natural history of this disease. Different tyrosine kinase inhibitors are now available in ROS1-rearranged NSCLC, and several clinical trials are ongoing in this setting [25][26]. In Figure 1, the timeline of main discoveries in ROS1-rearranged NSCLC is reported, from the first identification of ROS1-rearrangements to the most recently introduced drugs.
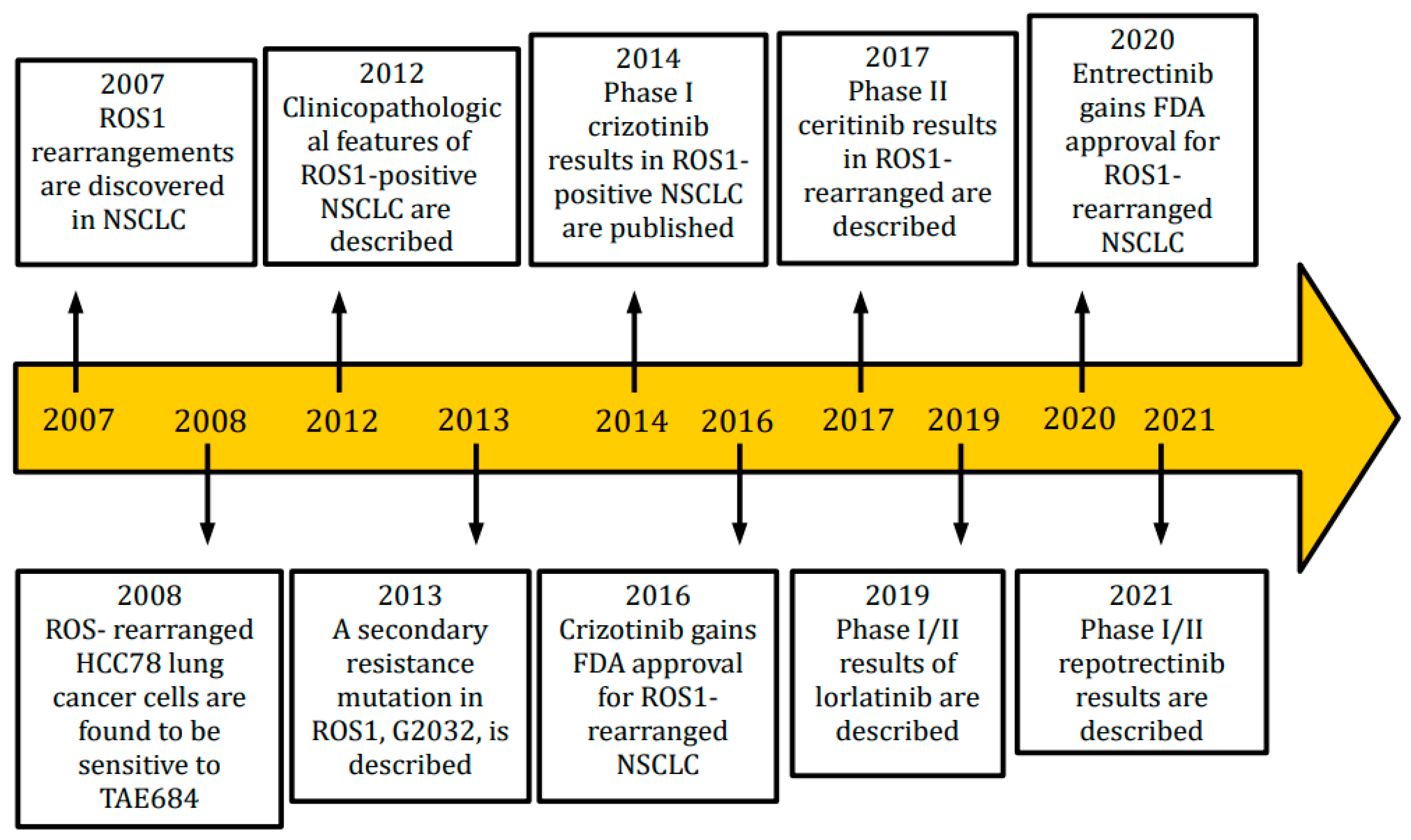
Figure 1. Timeline of main findings in ROS1-positive NSCLC.
2. Entrectinib vs. Crizotinib
Crizotinib and entrectinib are being compared head-to-head in patients with ROS1-positive non small cell lung cancer, including those who have brain metastases, in a phase III randomized controlled trial (NCT04603807) that has been accepting patients since 2021 [27]. The comparative efficacy and safety of the two medications in a clinical trial environment will be directly demonstrated by this investigation. Until the results of this study are available, the only feasible assessments can only be derived from indirect comparisons. In particular, Tremblay et al. published a paper in which they produced a clinical trial-to-clinical trial simulated treatment comparison (STC) using data from the regulatory approval-supporting trials for crizotinib and entrectinib to compare the efficacy of crizotinib and entrectinib in ROS1-positive non small cell lung cancer [28]. Obviously, this type of analysis is affected by numerous biases and high variability. Although the crizotinib and entrectinib study populations were similar in many trial inclusion/exclusion criteria, some crucial differences may impact the outcome of the indirect comparison. The population of the studies PROFILE 1001 and STARTRK-1, STARTRK-2 and ALKA-372-001 presented substantial differences, particularly with regard to sex, ECOG PS and smoking habit. In particular, PROFILE 1001 included patients with ECOG PS 0–1, while ECOG PS 2 patients were only included under special circumstances. On the contrary, patients in the entrectinib trials had an ECOG PS of 0–2, indicating that some of the participants may have had lower performance status. According to estimates, results are much poorer when ECOG PS is higher. In addition, PROFILE 1001 had a much longer follow-up time as well. Another drawback is that the research involved comparatively few patients, and results were measured differently. Furthermore, data on the presence of CNS metastases were not collected at baseline in the PROFILE 1001 study, in contrast to the entrectinib registrational study. With the above limits, Tremblay et al. found that crizotinib showed nonsignificant ORR advantages over entrectinib both before and after adjustment for key variables. When compared to entrectinib, crizotinib was linked with non-significant but prolonged mPFS and mDOR both before and after correction. The 12-month OS after adjustment showed a similar pattern, despite the fact that the uncorrected analysis indicated a non-significantly higher risk of mortality for crizotinib. Chu et al. published a similar clinical trial-to-clinical trial comparison that included entrectinib pivotal data from the ALKA-372-001, STARTRK-1, and STARTRK-2 studies in ROS1-positive NSCLC and matching-adjusted indirect comparison (MAIC) of crizotinib from PROFILE 1001 and individual patient data (IPD) for entrectinib [29]. This MAIC discovered that entrectinib may significantly outperform crizotinib in terms of ORR results but found no statistically significant difference between the two drugs’ PFS or OS outcomes. The differences between these indirect comparisons are the evaluation of the frequency of brain metastases, which is present only in the MAIC, and the type of data used; the MAIC used an earlier crizotinib and entrectinib data cut, whereas STC evaluated the updated OS from PROFILE 1001 and a larger sample size with at least 6 months’ follow-up from the integrated entrectinib analysis. While awaiting the data related to the phase III trial with the face-to-face comparison of the two drugs, in daily clinical practice, it could be hypothesized that crizotinib is preferred in the absence of brain metastases and entrectinib prescribed in the case of CNS localizations, exploiting its greater ability to penetrate through the blood-brain barrier. Table 1 summarizes outcomes of the main trials of crizotinib and entrectinib in ROS1-positive NSCLC.
Table 1. Comparison of major outcomes for crizotinib and entrectinib.
| Clinical Trial | Phase | ORR | Median PFS | Median OS |
|---|---|---|---|---|
| PROFILE 1001 [30][31] | 1b | 72% | 19.3 months | 51.4 months |
| EUCROSS [32] | 2 | 70% | 15.9 months | 20 months |
| METROS [33] | 2 | 65% | 22.8 months | - |
| AcSè [34] | 2 | 69% | 5.5 months | 17 months |
| STARTRK-1 STARTRK-2 ALKA-372-001 [35] |
1/2 | 67.1% | 15.7 months | Not reached |
| STARTRK-NG [36] | 1/2 | 57.7% | Not reached | - |
References
- Siegel, R.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Pikor, L.A.; Ramnarine, V.R.; Lam, S.; Lam, W.L. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013, 82, 179–189.
- Acquaviva, J.; Wong, R.; Charest, A. The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer. Biochim. Biophys. Acta 2009, 1795, 37–52.
- Davies, K.D.; Doebele, R.C. Molecular Pathways: ROS1 Fusion Proteins in Cancer. Clin. Cancer Res. 2013, 19, 4040–4045.
- Charest, A.; Wilker, E.W.; McLaughlin, M.E.; Lane, K.; Gowda, R.; Coven, S.; McMahon, K.; Kovach, S.; Feng, Y.; Yaffe, M.B.; et al. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glio-blastoma in mice. Cancer Res. 2006, 66, 7473–7481.
- Jun, H.J.; Johnson, H.; Bronson, R.T.; De Feraudy, S.; White, F.; Charest, A. The Oncogenic Lung Cancer Fusion Kinase CD74-ROS Activates a Novel Invasiveness Pathway through E-Syt1 Phosphorylation. Cancer Res. 2012, 72, 3764–3774.
- Wiesweg, M.; Eberhardt, W.E.; Reis, H.; Ting, S.; Savvidou, N.; Skiba, C.; Herold, T.; Christoph, D.C.; Meiler, J.; Worm, K.; et al. High Prevalence of Concomitant Oncogene Mutations in Prospectively Identified Patients with ROS1-Positive Metastatic Lung Cancer. J. Thorac. Oncol. 2016, 12, 54–64.
- Bergethon, K.; Shaw, A.T.; Ou, S.-H.I.; Katayama, R.; Lovly, C.M.; McDonald, N.T.; Massion, P.P.; Siwak-Tapp, C.; Gonzalez, A.; Fang, R.; et al. ROS1 Rearrangements Define a Unique Molecular Class of Lung Cancers. J. Clin. Oncol. 2012, 30, 863–870.
- Park, E.; Choi, Y.-L.; Ahn, M.-J.; Han, J. Histopathologic characteristics of advanced-stage ROS1-rearranged non-small cell lung cancers. Pathol. Res. Pract. 2019, 215, 152441.
- Drilon, A.; Jenkins, C.; Iyer, S.; Schoenfeld, A.; Keddy, C.; Davare, M.A. ROS1-dependent cancers—Biology, diagnostics and therapeutics. Nat. Rev. Clin. Oncol. 2021, 18, 35–55.
- Patil, T.; Smith, D.E.; Bunn, P.A.; Aisner, D.L.; Le, A.T.; Hancock, M.; Purcell, W.T.; Bowles, D.W.; Camidge, D.R.; Doebele, R.C. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non–Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J. Thorac. Oncol. 2018, 13, 1717–1726.
- Zhu, V.W.; Zhao, J.J.; Gao, Y.; Syn, N.L.; Zhang, S.S.; Ou, S.-H.I.; Bauer, K.A.; Nagasaka, M. Thromboembolism in ALK+ and ROS1+ NSCLC patients: A systematic review and meta-analysis. Lung Cancer 2021, 157, 147–155.
- Shah, A.T.; Bernardo, R.J.; Berry, G.J.; Kudelko, K.; Wakelee, H.A. Two Cases of Pulmonary Tumor Thrombotic Microangi-opathy Associated with ROS1-Rearranged Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, e153–e156.
- Woodford, R.; Lu, M.; Beydoun, N.; Cooper, W.; Liu, Q.; Lynch, J.; Kasherman, L. Disseminated intravascular coagulation complicating diagnosis of ROS1 -mutant non-small cell lung cancer: A case report and literature review. Thorac. Cancer 2021, 12, 2400–2403.
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346.
- Wang, W.; Cheng, G.; Zhang, G.; Song, Z. Evaluation of a new diagnostic immunohistochemistry approach for ROS1 rearrangement in non-small cell lung cancer. Lung Cancer 2020, 146, 224–229.
- Bubendorf, L.; Büttner, R.; Al-Dayel, F.; Dietel, M.; Elmberger, G.; Kerr, K.; López-Ríos, F.; Marchetti, A.; Öz, B.; Pauwels, P.; et al. Testing for ROS1 in non-small cell lung cancer: A review with recommendations. Virchows Arch. 2016, 469, 489–503.
- Sholl, L.M.; Weremowicz, S.; Gray, S.W.; Wong, K.-K.; Chirieac, L.R.; Lindeman, N.I.; Hornick, J.L. Combined use of ALK immuno-histochemistry and FISH for optimal detection of ALK-rearranged lung adenocarcinomas. J. Thorac. Oncol. 2013, 8, 322–328.
- Zito Marino, F.; Rossi, G.; Cozzolino, I.; Montella, M.; Micheli, M.; Bogina, G.; Munari, E.; Brunelli, M.; Franco, R. Multiplex fluorescence in situ hybridisation to detect anaplastic lymphoma kinase and ROS proto-oncogene 1 receptor tyrosine kinase rearrangements in lung cancer cy-tological samples. J. Clin. Pathol. 2020, 73, 96–101.
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722.
- Wong, S.Q.; Li, J.; Tan, A.Y.-C.; Vedururu, R.; Pang, J.-M.B.; Do, H.; Ellul, J.; Doig, K.; Bell, A.; MacArthur, G.A.; et al. Sequence artefacts in a prospective series of forma-lin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med. Genom. 2014, 7, 23.
- Shan, L.; Lian, F.; Guo, L.; Qiu, T.; Ling, Y.; Ying, J.; Lin, D. Detection of ROS1 Gene Rearrangement in Lung Adenocarcinoma: Comparison of IHC, FISH and Real-Time RT-PCR. PLoS ONE 2015, 10, e0120422.
- Wilcock, D.M.; Schmidt, R.L.; Furtado, L.V.; Matynia, A.P.; Deftereos, G.; Sirohi, D. Histologic and Molecular Characterization of Non−Small Cell Lung Carcinoma with Discordant ROS1 Immunohistochemistry and Fluorescence In Situ Hybridization. Appl. Immunohistochem. Mol. Morphol. 2021, 30, 19–26.
- Davies, K.D.; Le, A.T.; Sheren, J.; Nijmeh, H.; Gowan, K.; Jones, K.L.; Varella-Garcia, M.; Aisner, D.L.; Doebele, R.C. Comparison of Molecular Testing Modalities for Detection of ROS1 Rearrangements in a Cohort of Positive Patient Samples. J. Thorac. Oncol. 2018, 13, 1474–1482.
- Gendarme, S.; Bylicki, O.; Chouaid, C.; Guisier, F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Curr. Oncol. 2022, 29, 641–658.
- Guaitoli, G.; Bertolini, F.; Bettelli, S.; Manfredini, S.; Maur, M.; Trudu, L.; Aramini, B.; Masciale, V.; Grisendi, G.; Dominici, M.; et al. Deepening the Knowledge of ROS1 Rearrangements in Non-Small Cell Lung Cancer: Diagnosis, Treatment, Resistance and Concomitant Alterations. Int. J. Mol. Sci. 2021, 22, 12867.
- Clinicaltrials.gov. Randomized, Open Label, Multicenter, Phase III Study of Entrectinib versus Crizotinib in Patients with Locally-Advanced or Metastatic Non-Small Cell Lung Cancer Harboring ROS1 Gene Rearrangements with and without Central Nervous System Metastases. 2021. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04603807 (accessed on 15 June 2023).
- Tremblay, G.; Groff, M.; Iadeluca, L.; Daniele, P.; Wilner, K.; Wiltshire, R.; Bartolome, L.; Usari, T.; Cappelleri, J.C.; Camidge, D.R. Effectiveness of crizotinib versus entrectinib in ROS1-positive non-small-cell lung cancer using clinical and real-world data. Future Oncol. 2022, 18, 2063–2074.
- Chu, P.; Antoniou, M.; Bhutani, M.K.; Aziez, A.; Daigl, M. Matching-adjusted indirect comparison: Entrectinib versus crizotinib in ROS1 fusion-positive non-small cell lung cancer. J. Comp. Eff. Res. 2020, 9, 861–876.
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 371, 1963–1971.
- Shaw, A.; Riely, G.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.; Solomon, B.; Varella-Garcia, M.; Iafrate, A.; Shapiro, G.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126.
- Michels, S.; Massutí, B.; Schildhaus, H.-U.; Franklin, J.; Sebastian, M.; Felip, E.; Grohé, C.; Rodriguez-Abreu, D.; Abdulla, D.S.; Bischoff, H.; et al. Safety and Efficacy of Crizotinib in Patients with Advanced or Metastatic ROS1-Rearranged Lung Cancer (EUCROSS): A European Phase II Clinical Trial. J. Thorac. Oncol. 2019, 14, 1266–1276.
- Landi, L.; Chiari, R.; Tiseo, M.; D’Incà, F.; Dazzi, C.; Chella, A.; Delmonte, A.; Bonanno, L.; Giannarelli, D.; Cortinovis, D.L.; et al. Crizotinib in MET-Deregulated or ROS1-Rearranged Pretreated Non-Small Cell Lung Cancer (METROS): A Phase II, Prospective, Multicenter, Two-Arms Trial. Clin. Cancer Res. 2019, 25, 7312–7319.
- Moro-Sibilot, D.; Cozic, N.; Pérol, M.; Mazières, J.; Otto, J.; Souquet, P.; Bahleda, R.; Wislez, M.; Zalcman, G.; Guibert, S.; et al. Crizotinib in c-MET- or ROS1-positive NSCLC: Results of the AcSé phase II trial. Ann. Oncol. 2019, 30, 1985–1991.
- Dziadziusko, R.; Krebs, M.G.; De Braud, F.; Siena, S.; Drilon, A.; Doebele, R.C.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.-J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion–Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 1253–1263.
- Desai, A.V.; Robinson, G.W.; Gauvain, K.; Basu, E.M.; Macy, M.E.; Maese, L.; Whipple, N.S.; Sabnis, A.J.; Foster, J.H.; Shusterman, S.; et al. Entrectinib in children and young adults with solid or primary CNS tumors harboring NTRK, ROS1 or ALK aberrations (STARTRK-NG). Neuro-Oncology 2022, 24, 1776–1789.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
572
Revisions:
2 times
(View History)
Update Date:
27 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




