Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liana Maria Muresan | -- | 2398 | 2023-07-27 08:45:23 | | | |
| 2 | Fanny Huang | Meta information modification | 2398 | 2023-07-27 11:27:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Muresan, L.M. Reinforcement Materials. Encyclopedia. Available online: https://encyclopedia.pub/entry/47343 (accessed on 13 January 2026).
Muresan LM. Reinforcement Materials. Encyclopedia. Available at: https://encyclopedia.pub/entry/47343. Accessed January 13, 2026.
Muresan, Liana Maria. "Reinforcement Materials" Encyclopedia, https://encyclopedia.pub/entry/47343 (accessed January 13, 2026).
Muresan, L.M. (2023, July 27). Reinforcement Materials. In Encyclopedia. https://encyclopedia.pub/entry/47343
Muresan, Liana Maria. "Reinforcement Materials." Encyclopedia. Web. 27 July, 2023.
Copy Citation
The role of the reinforcement in a composite material is mainly one of increasing the mechanical and anti-corrosion properties of the system, but the intrinsic properties of nanofillers, as well as their size, morphology, chemical functional groups, and their amounts, influence significantly many more properties of nanocomposites.
nanocomposite coatings
reinforcement materials
anti-corrosion properties
1. Introduction
The role of the reinforcement in a composite material is mainly one of increasing the mechanical and anti-corrosion properties of the system, but the intrinsic properties of nanofillers, as well as their size, morphology, chemical functional groups, and their amounts, influence significantly many more properties of nanocomposites. The dispersion of nanofillers in a metallic, ceramic, or polymeric matrix provides coatings with improved characteristics such as hardness, corrosion, and wear resistance, as well as improved thermal stability. Optimum concentration of filler material well-dispersed in the metal matrix can extend the penetration path of the corrosive ions (generating a high tortuosity factor) and prolongs the lifetime of metals [1]. Moreover, the nanoparticles incorporated into polymers showed an increase of the integrity and lifetime of coatings thanks to the filling up of cracks and microcavities in the coatings. However, uniform dispersion is a difficult task due to the agglomeration tendency of the nanomaterials.
Reinforcement materials mostly used are in the form of nanoparticles, nanotubes, nanowires, nanorods, nanoplatelets, nanosheets, nanofilms, or nanocapsules [2]. The most important types of reinforcing materials are summarized in Figure 1.
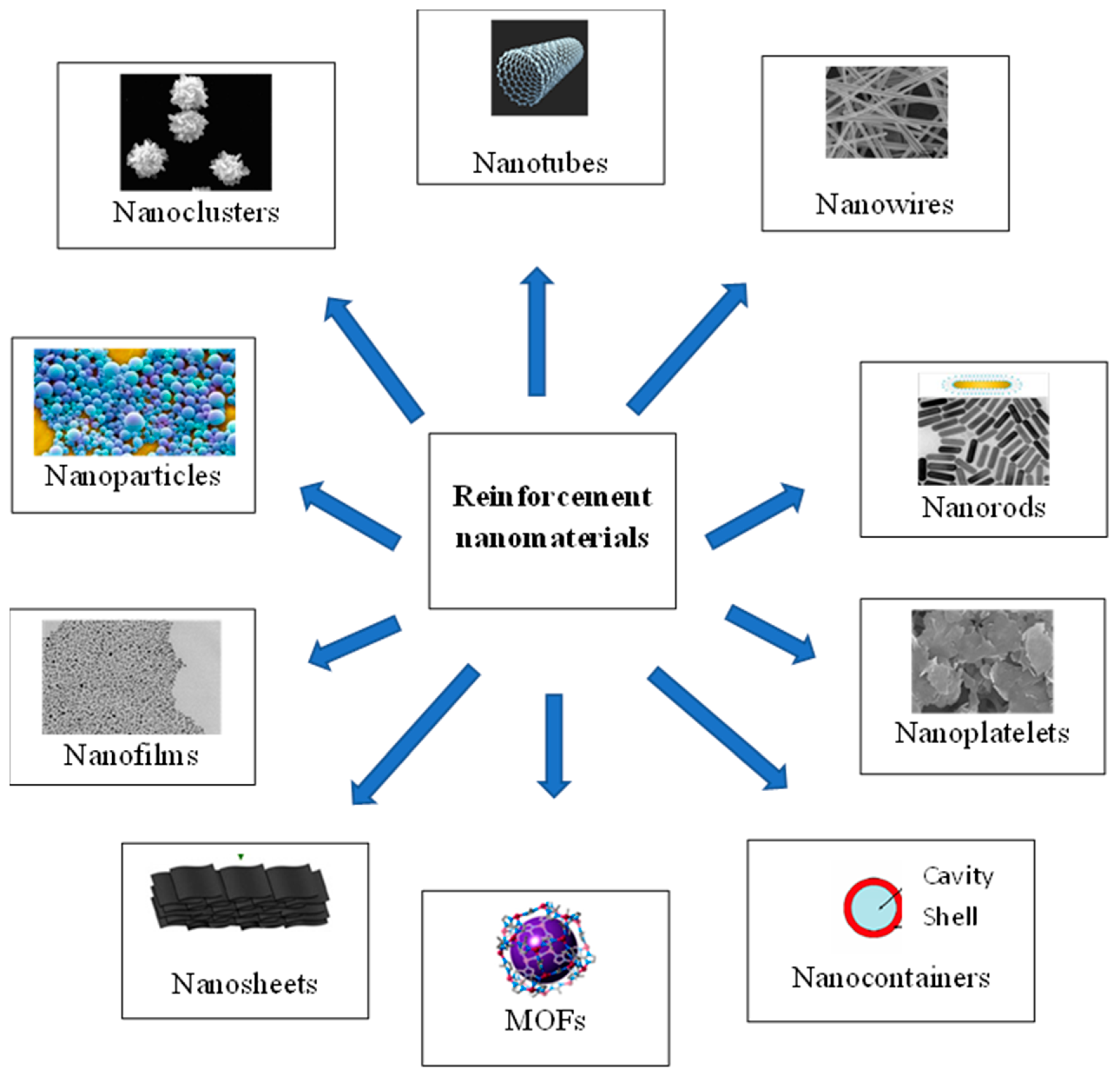
Figure 1. Main types of nano-sized reinforcement materials used for nanocomposite coatings.
2. Nanoparticles (NPs)
Nanoparticles are materials that have size ranges from 1 to 100 nm. NPs of different families, shapes, dimensions, and surface functional groups have been used in developing advanced composite coatings after incorporation in different matrices.
The main difficulties encountered in obtaining nanocomposite coatings with high-protection efficiency incorporating nanoparticles are, generally, the low degree of particles incorporation in the matrix, the agglomeration of the particles and, hence, the effort to ensure a uniform distribution of the fillers in the coating. The smaller the particle size, the higher the agglomeration tendency and difficulty to obtain uniform deposits. Some of these problems can be, at least partially, solved by modifying the surface properties of the particles. Nowadays, various methods have been developed to ensure the stability of the particles in the matrix, such as the use of surface-active agents, surface modifiers, capping agents, dopants etc.
The nanoparticles can be amorphous [3] or crystalline [4] with various geometries: spherical [5], cubic [6], tubular [7], etc. Besides conventional nanomaterials, such as oxides [8][9][10][11], or metals [12][13], new native or functionalized nanoparticles such as carbon nanotubes [14], graphene [15], graphene oxide [16], or organo-grafted nanoparticles [17][18] represent a new generation of nanomaterials used as fillers in composite coatings.
Graphene is a 2D carbon nanomaterial that possesses unique electrical, optical, and mechanical properties but a low dispersibility in matrices due to the lack of surface functional groups. On the contrary, graphene oxide (GO) preserves the exceptional properties of graphene, having additionally abundant surface functional groups and, consequently, good dispersibility and solubility in solvents, which makes it promising nano-scale filler for a next generation of functional composite materials. The functionalization of graphene oxide with a high surface area is found to improve the dispersion degree and, hence, enhances stability and mechanical properties of the coatings [1]. The fine nanoparticles dispersed in coatings can fill in cavities, increase the cross-linking density, and prevent matrix disaggregation during curing, offering solutions to enhancing the integrity and durability of coatings [19].
Besides graphene, other popular oxide nanoparticles used as fillers in composite coatings are TiO2 [10], ZnO [20][21], SiO2 [22], Al2O3 [23], ZrO2 [9], CeO2/ZrO2 [8], and TiO2·CeO2 binary oxides [24]. The grafting of organic molecules on oxide nanoparticles (e.g., PANI on Al2O3 [17]) or ferocene onto CeO2 [18]) and/or doping with inhibitors (e.g., Ce nitrate doped alumina/polyaniline nanoparticles into epoxy coating [17]) help the uniform dispersion and incorporation of NPs in the matrix, providing active protection against corrosion. Recently, core-shell nanosized architectures have been synthesized [25], offering the possibility to place corrosion inhibitors in the core of the system for a controlled release and develop the self-healing concept [26].
A short selection of the reported nanoparticles used in composite corrosion resistant coatings is presented in Table 1.
Table 1. Most important nanoparticles used in nanocomposite corrosion-resistant coatings.
| Nanoparticles | Particles Concentration in the Composite Preparation Step | Advantages | Limitations/Drawbacks | References |
|---|---|---|---|---|
| Carbon nanotubes | 0.4–1% CNT |
|
|
[14][27] |
| Graphene | 100 mg L−1 |
|
|
[28][29] |
| Graphene oxide | 0.1 gL−1 GO 0.5–5 wt.% GO |
|
|
[30][31] |
| Metals | In situ growth |
|
|
[12] |
| Oxides | 5 mM CeO2 1% TiO2 1, 3 and 5 g/L ZrO2 0.1 g/L ZnO 1, 2, 3 wt.% ZnO 3 gL−1 hematite |
|
|
[8][9][10][11][20][21] |
| Organo-grafted nanoparticles | 10 gL−1 Fc-CeO2 GO-Aminothiazole (AT) and GO-2-amino-4-(1-Naphthyl)Thiazole (ANT) |
|
|
[18][32] |
| Core-shell particles |
3 wt.% PANI-MSNs 20 mg SiO2@PANI/1g silicone 5% (f-SiO2@RGO) |
|
|
[25][33][34] |
It can be observed that the NP concentration used to prepare the nanocomposites is variable and depends on the matrix and of the filler’s nature and on the preparation method.
3. Nanotubes
A nanotube is a nanometer-scale hollow tube-like structure made of different materials, such as carbon, titania, boron nitride, silicon, silicon carbide, etc. Most research has been focused on carbon nanotubes (CNTs), which exhibit exceptional electrical and thermal conductivity, and exceptional tensile strength and versatility. In addition, they are easily chemically modified and functionalized. CNTs can be single walled (SWCNTs), with a diameter < 1 nm, and multi-walled (MWCNTs), consisting of concentrically interlinked nanotubes with diameters > 100 nm and length exceeding their diameter (μm, or even mm). Just like graphite, carbon nanotubes resist any chemical attack, except if they are simultaneously exposed to oxygen and high temperatures. This property makes them enormously resistant to corrosion. Therefore, they can function as anti-corrosion filler, making them successful candidates as reinforcements in composite corrosion resistant coatings after embedding them in various matrices.
CNTs can fill the holes of metal- and polymer-matrix composites by forming a passive layer on metals and promoting sacrificial protection in zinc-rich polymer (ZRP) coatings [35]. MWCNTs improve the mechanical strength, decrease the porosity of epoxy resin matrices, and increase the adhesion of the coating [36].
The functionalization of CNT surfaces brings great improvement in the CNT properties by decreasing their agglomeration tendency, increasing the interactions with solvent molecules, and, hence, favoring their dispersion in a polymeric matrix [37]. Functionalization of CNTs with ester-containing surfactants led to better anti-corrosion protection of mild steel as a result of further dispersing ability [38]. CNTs doping with other materials such as polydopamine, [39], organic phosphoric acid [40], or rare-earth salts [41] can confer excellent properties and stability to the resulting composites. Moreover, the CNT-doped composites showed promising fatigue resistance and increased adhesion between the coatings and metals.
TiO2 nanotubes also deserve a special mention due to their use in the fabrication of quality biomedical implants. Titania mineralogical types of anatase and rutile are successful materials for the fabrication of resistant coatings on Ti and Ti alloys substrates due to their thermodynamic stability, chemical inertia, and low solubility in body fluids [42]. Various films containing TiO2 nanostructures (nanotubes, nanosheets, nanorods, etc.) are highly hydrophilic, which leads to augmented bioactivity and an improved osseointegration behavior of materials generally used for implants. Where the bone-bonding abilities are concerned, crystalline TiO2 forms overcome the amorphous ones, and nanostructured layers are superior to micro-structured ones. They are obtained on the top of tinny, naturally existing TiO2 on the Ti surface by various methods such as electrolytic deposition, anodic oxidation, sol–gel technique, etc. Different composite layers, including TiO2 nanotubes with good physical and chemical properties and improved surface bioactivity prepared on the surface of Ti-based biomaterials, were also reported [43].
4. Nanocontainers
Materials with hollow, porous, or layered structures and their assemblies are often preferred as nanocontainers to be filled with polymerizable agents or inhibitors. Nanocontainers tailored to specific actions can be incorporated in different matrices (e.g., epoxy, silica etc.), which result in nanocomposite coatings with self-healing properties, especially for corrosion protection of metallic substrates (aluminum, magnesium, steel, and their alloys). During this process, the controlled release of the healing material efficiently repairs cracks that appear in the coatings. A change in the surrounding environment (e.g., pH) can trigger the delivery of the repairing agent or inhibitor from the nanocontainers at the damaged site of the coating. [44].
The encapsulation of corrosion inhibitors into protective shells is the most frequently used technique for incorporation because it presents several advantages over the use of these inhibitors in their free molecular forms. When the core material is unstable, the shell will prevent its premature degradation/altering. The slow release of the corrosion inhibitor from the nanocontainers enables long-term delivery of corrosion inhibitors and the healing of a damaged coating [45]. Organic inhibitors containing nitrogen (e.g., azole groups, amines and amino acids) are preferred [46], but natural compounds such as different plant extracts were also encapsulated in polymeric shells [47][48].
The capsules are prepared mostly from organic polymers, (biopolymers and synthetic polymers), mesoporous silica [49], inorganic clays, and polyelectrolyte multilayers. Microcapsules with the desired properties of thickness, morphology, and sizes could be tailored via proper monitoring of the preparation process parameters.
The most used methods to encapsulate healing agents within nanocapsules are in-situ and interfacial polymerization, multi-stage emulsion polymerization, solvent evaporation, sol–gel, and electro-spraying [48]. The encapsulation procedure must take into consideration the chemical nature of the reactive healing agent to avoid the diffusion of the liquid compound captured inside and out of the capsule shell during storage. At the same time, the microcapsule walls must be sufficiently resistant to processing conditions during their incorporation into the matrix of the host composite [50].
In the last years, multicore microcapsules were prepared. These materials provide the dual action of self-healing and anticorrosion by encapsulating two corrosion inhibitors in cross-linked polymeric shells [51]. Hybrid microcontainers have also been produced (e.g., silica/polymer double-walled hybrid nanocontainers consisting of a hollow cavity, an inner wall of porous SiO2, and outer polymeric wall, which is stimuli-responsive) [52]. The design and preparation of polymeric, inorganic, and hybrid nanocontainers with versatile functionalities represent a challenge, providing great opportunities for the development of a new generation of stimuli responsive smart coatings with extrinsic self-healing properties.
5. Clays and Zeolites
Clay nanomaterials have received attention recently as interesting reinforcement materials to modify polymers for developing low-cost, high-performance protective coatings [53]. The swelling properties of clay minerals come from the hydration of cations in the interlayer space. Because of swelling, the clay minerals exhibit a blocking effect against the water-soluble ions entering the cavities and act as a barrier or sealing material against the surrounding environment. By incorporating clays into the coating materials, the substrate will be protected even when the coating films are damaged by cracks and pinholes [54].
Zeolites are silica–aluminate structures with relatively high chemical reactivity due to the presence of surface silanol groups. The performance of the composite films depends on the high chemical affinity of the filler toward the matrix. Zeolite fillers are usually added in a compatible matrix (e.g., silane) in order to enhance its protective action. The better corrosion resistance of silane–zeolite coating could be explained by condensation of the hydroxyl groups of zeolite surface with silane functional groups, resulting in a crosslinking of the silane network [55][56]. Thanks to their highly porous crystalline structures, zeolites can also act as nano-containers for different types of corrosion inhibitors [57][58]. In these cases, the inhibitor release during time offers a selective self-healing action in corrosion conditions [59]. Recently, halloysite nanotubes (HNTs) and modified HNTs (HNT-NH2 and HNT-NH2-PPy) were successfully introduced in an Ni–P matrix by electroless-deposition, resulting in an adherent protective coating with excellent anticorrosion properties on steel [60].
Zeolites incorporated into Mg composite scaffolds lead to higher compressive strength, corrosion resistance, and bioactivity as compared with Mg scaffolds without zeolite and could be used as a tissue engineering scaffold for possible bone regeneration applications [61]. Zinc-doped hydroxyapatite−zeolite embedded in a polymeric matrix were prepared on magnesium substrates with the aim of diminishing the corrosion rate and improving antibacterial activity [62].
6. Metal–Organic Frameworks (MOFs)
Metal–organic frameworks (MOFs) are novel organic–inorganic, highly porous structures that are composed of metal or metal-cluster-cations (so-called “nodes”) and multidentate anionic or neutral organic molecules (so-called “linkers”) [63]. MOFs possess some exceptional characteristics such as high mechanical and thermal stability, large surface area, permanent porosity, tailorable pore size and pore size distribution, chemical versatility, molecular flexibility, and ease of functionalization [64]. MOFs with 2D or 3D structures have been obtained by traditional solvothermal and non-solvothermal strategies [65]. Metallic ions as copper and manganese, which have unfilled d orbitals in their structures, are easy to coordinate with nitrogen atoms, so they are selected as metal ions for the synthesis of MOFs.
Since most MOF materials have high-affinity interactions with both inorganic and organic compounds, they can easily form composite anticorrosion coatings such as MOF-polymer or MOF-polymer/inorganic compound [66] to protect metal plates like Mg, Al, Zn, and their alloys.
The incorporation of MOFs into a matrix (e.g., an organic polymer) influences the properties of the coating, such as its corrosion resistance, mechanical, and dielectric properties. The presence of hydrophobic MOFs in a polymeric matrix can improve the barrier properties of the coating by hindering the access of corrosive species. MOFs are suitable hosts for corrosion inhibitors, acting as nano-reservoirs involved in self-healing processes via controlling the amount of released corrosion inhibitors.
Various molecules can be grafted on MOF surfaces in order to improve the properties of the coatings in which the MOFs are embedded. For example, water-borne epoxy resin coating with dopamine-grafted MOFs improved the toughness and strength of coating and also enhanced the adhesion force between the coating and metal substrate [67]. Composite MOF–Grafene oxide (GO) coatings can be prepared based on oxygen-containing groups (such as carboxyl, hydroxyl, epoxy, etc.) present on the GO surface, which can combine with unsaturated metal sites of MOFs to form coordination bonds [68].
References
- Radhamani, A.V.; Lau, H.C.; Ramakrishna, S. Nanocomposite coatings on steel for enhancing the corrosion resistance: A review. J. Compos. Mater. 2020, 54, 681–701.
- Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates. Materials 2019, 12, 210.
- Gao, X.; Yan, R.; Xu, L.; Ma, H. Effect of amorphous phytic acid nanoparticles on the corrosion mitigation performance and stability of sol-gel coatings on cold-rolled steel substrates. J. Alloys Compd. 2018, 747, 747–754.
- Ying, L.; Li, L. Understanding the corrosion resistance of nanocrystalline materials: Electrochemical influences. In Corrosion Protection and Control Using Nanomaterials; Saji, V.S., Cook, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Chapter 4; pp. 59–85.
- Chen, F.; Liu, P. Conducting Polyaniline Nanoparticles and Their Dispersion for Waterborne Corrosion Protection Coatings. ACS Appl. Mater. Interfaces 2011, 3, 2694–2702.
- Khodair, Z.T.; Khadom, A.A.; Jasim, H.A. Corrosion protection of mild steel in different aqueous media via epoxy/nanomaterial coating: Preparation, characterization and mathematical views. J. Mater. Res. Technol. 2019, 8, 424–435.
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio- Tribo-Corros. 2015, 1, 28–30.
- Živković, L.j.S.; Jegdić, B.V.; Andrić, V.; Rhee, K.Y.; Bajat, J.B.; Mišković-Stanković, V.B. The effect of ceria and zirconia nanoparticles on the corrosion behaviour of cataphoretic epoxy coatings on AA6060 alloy. Prog. Org. Coat. 2019, 136, 105219.
- Nikoomanzari, E.; Fattah-alhosseini, A.; Pajohi Alamoti, M.R.; Keshavarz, M.K. Effect of ZrO2 nanoparticles addition to PEO coatings on Ti–6Al–4Vsubstrate: Microstructural analysis, corrosion behavior and antibacterial effect of coatings in Hank’s physiological solution. Ceram. Int. 2020, 46, 13114–13124.
- Ashraf, M.A.; Liu, Z.; Peng, W.-X.; Yoysefi, N. Amino acid and TiO2 nanoparticles mixture inserted into sol-gel coatings: An efficient corrosion protection system for AZ91 magnesium alloy. Prog. Org. Coat. 2019, 136, 10529.
- Hoseini, A.; Yarmand, B.; Kolahi, A. Inhibitory effects of hematite nanoparticles on corrosion protection function of TiO2 coating prepared by plasma electrolytic oxidation. Surf. Coat. Technol. 2021, 409, 126938.
- Selegård, L.; Poot, T.; Eriksson, P.; Palisaitis, J.; Persson, P.O.Å.; Hu, Z.; Uvdal, K. In-situ growth of cerium nanoparticles for chrome-free, corrosion resistant anodic coatings. Surf. Coat. Technol. 2021, 410, 126958.
- Kumar, H.K.; Venkatesh, N.; Bhowmik, H.; Kuila, A. Metallic Nanoparticle: A Review. Biomed. J. Sci. Technol. Res. 2018, 4, 3765–3774.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities Arabian. J. Chem. 2019, 12, 908–931.
- Zhaodi, R.; Meng, N.; Shehzad, K.; Xu, Y.; Qu, S.; Yu, B.; Luo, J.K. Mechanical properties of nickel-graphene composites synthesized by electrochemical deposition. Nanotechnology 2015, 26, 065706.
- Liu, C.; Su, F.; Liang, J. Producing cobalt–graphene composite coating by pulse electrodeposition with excellent wear and corrosion resistance. Appl. Surf. Sci. 2015, 351, 889–896.
- Tavandashti, N.P.; Almas, S.M.; Esmaeilzadeh, E. Corrosion protection performance of epoxy coating containing alumina/PANI nanoparticles doped with cerium nitrate inhibitor on Al-2024 substrates. Prog. Org. Coat. 2021, 152, 106133.
- Ngo, N.K.; Shao, S.; Conrad, H.; Sanders, S.F.; D’Souza, F.; Golden, T.D. Synthesis, characterization, and the effects of organo-grafted nanoparticles in nickel coatings for enhanced corrosion protection. Mater. Today Commun. 2020, 25, 101628.
- Shi, X.; Nguyen, T.A.; Suo, Z.; Liu, Y.; Avci, R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf. Coat. Technol. 2009, 204, 237–245.
- Kamburova, K.; Boshkova, N.; Boshkov, N.; Radeva, T. Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125741.
- Samad, U.A.; Alam, M.A.; Anis, A.; Sherif El-Sayed, M.; Al-Mayman, S.I.; Al-Zahrani, S.M. Effect of Incorporated ZnO Nanoparticles on the Corrosion Performance of SiO2 Nanoparticle-Based Mechanically Robust Epoxy Coatings. Materials 2020, 13, 3767.
- Li, X.; Yin, S.; Luo, H. Fabrication of robust superhydrophobic Ni–SiO2 composite coatings on aluminum alloy surfaces. Vacuum 2020, 181, 109674.
- Zamblau, I.; Varvara, S.; Bulea, C.; Muresan, L.M. Corrosion Behavior of Composite Coatings Obtained by ElectrolyticCodeposition of Copper with Al2O3 Nanoparticles. Chem. Biochem. Eng. Q. 2009, 23, 43–52.
- Nemes, P.I.; Zaharescu, M.; Muresan, L.M. Initial corrosion behavior of composite coatings obtained by electrolytic codeposition of zinc with nanoparticles of Ti and.Ce oxides. J. Solid State Electrochem. 2013, 17, 511–518.
- Haddadi, S.A.; Mehmandar, E.; Jabari, H.; ARamazani, S.A.; Mohammadkhani, R.; Yan, N.; Arjmand, M. Zinc-doped silica/polyaniline core/shell nanoparticles towards corrosion protection epoxy nanocomposite coatings. Compos. Part B Eng. 2021, 212, 108713.
- Najjar, R.; Katourani, S.A.; Hosseini, M.G. Self-healing and corrosion protection performance of organic resin core-shell nanoparticles in epoxy/PANI/ZnO nanocomposite coatings on anodized aluminum alloy. Prog. Org. Coat. 2018, 124, 110–121.
- Gu, B.-E.; Huang, C.-Y.; Shen, T.-H.; Lee, Y.-L. Effects of multiwall carbon nanotube addition on the corrosion resistance and underwater acoustic absorption properties of polyurethane coatings. Prog. Org. Coat. 2018, 121, 226–235.
- Kamboj, A.; Raghupathy, Y.; Rekha, M.Y.; Srivastava, C. Morphology, Texture and Corrosion Behavior of Nanocrystalline Copper–Graphene Composite Coatings. JOM 2017, 69, 1149–1154.
- Kumar, M.K.P.; Singh, M.P.; Srivastava, C. Electrochemical behavior of Zn–graphene composite coatings. RSC Adv. 2015, 5, 25603–25608.
- Bai, Z.; Zhang, B. Fabrication of superhydrophobic reduced-graphene oxide/nickel coating with mechanical durability, self-cleaning and anticorrosion performance. Nano Mater. Sci. 2020, 2, 151–158.
- Selim, M.S.; El-Safty, S.A.; Abbas, M.A.; Shenashen, M.A. Facile design of graphene oxide-ZnO nanorod-based ternary nanocomposite as a superhydrophobic and corrosion-barrier coating. Colloids Surf. A Physicochem. Eng. Asp. 2020, 611, 125793.
- Rajitha, K.; Mohana, K.N.S. Synthesis of graphene oxide-based nanofillers and their influence on the anticorrosion performance of epoxy coating in saline medium. Diam. Relat. Mater. 2020, 108, 107974.
- Shi, S.; Zhao, Y.; Zhang, Z.; Yu, L. Corrosion protection of a novel coating for Q235 carbon steel. Prog. Org. Coat. 2019, 132, 227–234.
- Wang, T.; Ge, H.; Zhang, K. A novel core-shell straticulate structured antistatic anticorrosion composite coating. J. Alloys Compd. 2018, 745, 705–715.
- Daneshvar, F. Carbon nanotube/metal corrosion issues for nanotube coatings and inclusions in a matrix. Appl. Phys. 2018, 119212468.
- Ganguli, S.; Bhuyan, M.; Allie, L.; Aglan, H. Effect of multi-walled carbon nanotube reinforcement on the fracture behavior of a tetrafunctional epoxy. J. Mater. Sci. 2005, 40, 3593–3595.
- Deyab, M.A.; Awadallah, A.E. Advanced anticorrosive coatings based on epoxy/functionalized multiwall carbon nanotubes composites. Prog. Org. Coat. 2020, 139, 105423.
- Yousefi, A.; Javadian, S.; Sharifi, M.; Dalir, N.; Motaee, A. An Experimental and Theoretical Study of Biodegradable Gemini Surfactants and Surfactant/Carbon Nanotubes (CNTs) Mixtures as New Corrosion Inhibitor. J. Bio-Tribo-Corros. 2019, 5, 82.
- Hong, M.-S.; Park, Y.; Kim, T.; Kim, K.; Kim, J.-G. Polydopamine/carbon nanotube nanocomposite coating for corrosion Resistance. J. Mater. 2020, 6, 158–166.
- Rui, M.; Zhu, A. The synthesis and corrosion protection mechanisms of PANI/CNT nanocomposite doped with organic phosphoric acid. Prog. Org. Coat. 2021, 153, 106134.
- Montemor, M.F.; Ferreira, M.G.S. Analytical characterisation and corrosion behaviour of bis-aminosilane coatings modified with carbon nanotubes activated with rare-earth salts applied on AZ31 Magnesium alloy. Surf. Coat. Technol. 2008, 202, 4766–4774.
- Muresan, L.M. Corrosion Protective Coatings for Ti and Ti Alloys Used for Biomedical Implants. In Intelligent Coatings for Corrosion Control; Tiwari, A., Rawlins, J., Hihara, L.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 17; pp. 585–603. ISBN 978-0-12-411467-8.
- Gunputh, U.F.; Le, H.; Besinis, A.; Tredwin, C.; Handy, R.D. Multilayered composite coatings of titanium dioxide nanotubes decorated with zinc oxide and hydroxyapatite nanoparticles: Controlled release of Zn and antimicrobial properties against Staphylococcus aureus. Int. J. Nanomed. 2019, 14, 3583–3600.
- Da Cunha, A.B.M.; Leal, D.A.; Santos, L.R.L.; Riegel-Vidotti, I.C.; Marino, C.E.B. pH-sensitive microcapsules based on biopolymers for active corrosion protection of carbon steel at different pH. Surf. Coat. Technol. 2020, 402, 126338.
- Amiri, S.; Rahimi, A. Anticorrosion behavior of cyclodextrins/inhibitor nanocapsule-based self-healing coatings. J. Coat. Technol. Res. 2016, 13, 1095–1102.
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2015, 3, 469–480.
- Bagale, U.D.; Sonawane, S.H.; Bhanvase, B.A.; Kulkarni, R.D.; Gogate, P.R. Green synthesis of nanocapsules for self-healing anticorrosion coating using ultrasound-assisted approach. Green Process. Synth. 2018, 7, 147–159.
- Malekkhouyan, R.; Khorasani, S.N.; Neisiany, R.E.; Torkaman, R.; Koochaki, M.S.; Das, O. Preparation and Characterization of Electrosprayed Nanocapsules Containing Coconut-Oil-Based Alkyd Resin for the Fabrication of Self-Healing Epoxy Coatings. Appl. Sci. 2020, 10, 3171.
- Zea, C.; Barranco-García, R.; Chico, B.; Díaz, I.; Morcillo, M.; de la Fuente, D. Smart Mesoporous Silica Nanocapsules as Environmentally Friendly Anticorrosive Pigments. Int. J. Corros. 2015, 2015, 426397.
- Telegdi, J.; Shaban, A.; Vastag, G. Micro/nano-capsules for anticorrosion coatings. In Fundamentals of Nanoparticles: Classifications, Synthesis Methods, Properties and Characterization-Micro and Nano Technologies, 1st ed.; Butterworth-Heinemann: Oxford, UK; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 17.
- Jadhav, R.S.; Mane, V.; Bagle, A.V.; Hundiwale, D.G.; Mahulikar, P.P.; Waghoo, G. Synthesis of multicore phenol formaldehyde microcapsules and their application in polyurethane paint formulation for self-healing anticorrosive coating. Int. J. Ind. Chem. 2013, 4, 31–39.
- Li, G.L.; Zheng, Z.; Mohwald, H.; Shchukin, D.G. Silica/Polymer Double-Walled Hybrid Nanotubes: Synthesis and Application as Stimuli-Responsive Nanocontainers in Self-Healing Coatings. ACS Nano 2013, 7, 2470–2478.
- Madhup, M.K.; Shah, N.K.; Parekh, N.R. Investigation and improvement of abrasion resistance, water vapor barrier and anticorrosion properties of mixed clay epoxy nanocomposite coating. Prog. Org. Coat. 2017, 102, 186–193.
- Hikasa, A.; Sekino, T.; Hayashi, Y.; Rajagopalan, R.; Niihara, K. Preparation and Corrosion Studies of Self-Healing Multi-Layered Nano Coatings of Silica And Swelling Clay. Mat. Res. Innovat. 2004, 8, 84–88.
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Adhesion aspects of hydrophobic silane zeolite coatings for corrosion protection of aluminium substrate. Prog. Org. Coat. 2014, 77, 1341–1350.
- Rotella, G.; Candamano, S. Fabrication and characterization of zeolite coatings on aluminum and magnesium alloys. Eng. Sci. Technol. Int. J. 2020, 23, 1273–1278.
- Calabrese, L.; Bonaccorsi, L.; Caprì, A.; Proverbio, E. Enhancement of the hydrophobic and anti-corrosion properties of a composite zeolite coating on Al6061 substrate by modification of silane matrix. Corros. Eng. Sci. Technol. 2017, 52, 61–72.
- Rassouli, L.; Naderi, R.; Mahdavian, M. Study of the active corrosion protection properties of epoxy ester coating with zeolite nanoparticles doped with organic and inorganic inhibitors. J. Taiwan Inst. Chem. Eng. 2018, 85, 207–220.
- Calabrese, L.; Proverbio, E. A Brief Overview on the Anticorrosion Performances of Sol-Gel Zeolite Coatings. Coatings 2019, 9, 409.
- Fayyad, E.M.; Jlassi, K.; Sliem, M.H.; Nabhan, F.; Abdullah, A.M. Design of highly anti-corrosive electroless plated Ni–P/modified halloysite nanotubes nanocomposite coating. J. Mater. Res. Technol. 2023, 24, 8014–8034.
- Saheban, M.; Bakhsheshi-Rad, H.R.; Kasiri-Asgarani, M.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Dayaghi, E. Effect of zeolite on the corrosion behavior, biocompatibility and antibacterial activity of porous magnesium/zeolite composite scaffolds. Mater. Technol. 2019, 34, 258–269.
- Iqbal, N.; Iqbal, S.; Iqbal, T.; Bakhsheshi-Rad, H.R.; Alsakkaf, A.; Kamil, A.; Raghav, H.B. Zinc-doped hydroxyapatite—Zeolite/polycaprolactone composites coating on magnesium substrate for enhancing in-vitro corrosion and antibacterial performance. Trans. Nonferr. Met. Soc. China 2020, 30, 123–133.
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14.
- Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials 2020, 13, 2881.
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal–organic frameworks. Polyhedron 2018, 145, 1–15.
- Zhang, M.; Ma, L.; Wang, L.; Sun, Y.; Liu, Y. Insights into the Use of Metal−Organic Framework As High- Performance Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2018, 10, 2259–2263.
- Wang, M.; Yun, H.; Tan, K.; Guo, A.; Ling, J.; Jiang, F.; Shen, X.; Xu, Q. One-step electrochemical synthesis of poly(vinyl pyrrolidone) modified polyaniline coating on stainless steel for high corrosion protection performance. Prog. Org. Coat. 2020, 149, 105908.
- Wei, W.; Liu, Z.; Wei, R.; Han, G.-C.; Liang, C. Synthesis of MOFs/GO composite for corrosion resistance application on carbon steel. RSC Adv. 2020, 10, 29923.
More
Information
Subjects:
Materials Science, Coatings & Films
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
27 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




