Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wu, L.; De Perrot, M. SPARC Gene in Mesothelioma. Encyclopedia. Available online: https://encyclopedia.pub/entry/47335 (accessed on 01 March 2026).
Wu L, De Perrot M. SPARC Gene in Mesothelioma. Encyclopedia. Available at: https://encyclopedia.pub/entry/47335. Accessed March 01, 2026.
Wu, Licun, Marc De Perrot. "SPARC Gene in Mesothelioma" Encyclopedia, https://encyclopedia.pub/entry/47335 (accessed March 01, 2026).
Wu, L., & De Perrot, M. (2023, July 27). SPARC Gene in Mesothelioma. In Encyclopedia. https://encyclopedia.pub/entry/47335
Wu, Licun and Marc De Perrot. "SPARC Gene in Mesothelioma." Encyclopedia. Web. 27 July, 2023.
Copy Citation
The SPARC gene plays multiple roles in extracellular matrix synthesis and cell shaping, associated with tumor cell migration, invasion, and metastasis. The SPARC gene is also involved in the epithelial-mesenchymal transition (EMT) process, which is a critical phenomenon leading to a more aggressive cancer cell phenotype. SPARC gene overexpression has shown to be associated with poor survival in the mesothelioma (MESO) cohort from the TCGA database, indicating that this gene may be a powerful prognostic factor in MESO.
SPARC
epithelial-mesenchymal transition (EMT)
mesothelioma (MESO)
omics
1. SPARC Gene Expression and Its Prognostic Value in Pancancer and Mesothelioma
SPARC gene expression in 32 types of cancer can be found in the TCGA database (http://www.emtome.org/ accessed on 1 May 2022) (Figure 1A). Gene expression of SPARC is associated with overall survival of MESO and pancancer. Survival maps of 32 types of cancer show that SPARC gene is a strong prognostic indicator for MESO (Figure 1B,C). Higher expression of SPARC gene is associated with poorer overall survival (Log rank p = 3.6 × 10−5) and disease-free survival (Log rank p = 0.023) in the MESO cohort (Figure 1D,E) and in pancancer (Figure 1F,G). The prognostic value of the EMT gene signature in MESO has been demonstrated elsewhere and by researchers' group [1].

Figure 1. SPARC gene expression and its association with survival in the MESO cohort and pancancer. (A) SPARC gene expression in the pancancer TCGA database; (B,C) survival maps showing that SPARC gene expression is associated with overall survival (OS) and disease-free survival (DFS) in pancancer; (D,E) SPARC gene expression associated with OS and DFS in the MESO cohort; (F,G) SPARC gene expression associated with OS and DFS in pancancer.
2. Previous Studies of SPARC/Osteonectin on Prognosis in Mesotheliomaand Other Cancers
Previous studies demonstrated the prognostic significance of the secreted protein SPARC, which could be a prognostic biomarker in MESO in a proteomics-based approach [2]. Kao et al. used a proteomics-based approach to identify secreted protein SPARC as a prognostic biomarker in MESO. The SPARC protein, also known as osteonectin (ON, ONT) or basement-membrane protein 40 (BM-40), is a glycoprotein in the bone that binds calcium and collagen [3]. An integrated gene expression analysis was performed on RNA-seq data of MESO patients from the TCGA dataset and from cell lines, and results showed the SPARC gene to be overexpressed in MESO [4].
Another study showed that high tumor cell platelet-derived growth factor receptor beta (PDGFRB) and stromal SPARC expression remained independently associated with shorter survival in MESO, indicating that PDGFRB and SPARC may be potential markers for risk stratification and as targets for therapy [5]. A meta-analysis demonstrated that SPARC expression has prognostic significance in breast cancer [6]. Methylation-mediated SPARC expression was shown to correlate with tumor progression and poor prognosis of breast cancer. Overexpression of SPARC may promote cancer cell migration and invasion, and thus function as an oncogene and a potential therapeutic target for breast cancer [7]. The relationship between stromal-cell-derived SPARC and its clinicopathologic significance was reported in human gastric cancer tissue [8]. The expression of SPARC together with other genes such as FN1 and SERPINE1 may predict poor prognosis of gastric adenocarcinoma [9]. SPARC expression is also associated with tumor metastasis and poor prognosis in head and neck cancers. Exogenous SPARC and SPARC overexpression enhances the EMT signaling pathway via AKT activation and may be associated with tumor progression in head and neck cancers [10]. SPARC is highly expressed in tumor stroma and tumor-associated fibroblasts in pancreatic cancer, and its overexpression in this compartment is associated with poorer prognosis [11].
A systemic research and meta-analysis showed prognostic value of SPARC in hepatocellular carcinoma [12]. In summary, SPARC expression is associated with worse survival in MESO, breast cancer, head and neck cancer, gastric cancer, and hepatocellular carcinoma.
3. SPARC and the Immunosuppressive Tumor Microenvironment
Stromal cells and extracellular matrix are the major components contributing to the interaction of tumor cells with their microenvironment. The immunosuppressive microenvironment drives non-malignant cells to change phenotypic plasticity, which promotes tumor cell aggressiveness and invasion [13]. Myeloid-derived suppressor cells (MDSCs) are well-known key negative regulators of the immune response during tumor growth. SPARC was considered a new MDSC marker licensing suppressive activities. SPARC is identified in both human and mouse MDSCs with immune suppressive capacity and pro-tumoral activities, including the induction of EMT and angiogenesis [14]. TCGA data showed a strong positive correlation between gene co-expression of SPARC and MDSC infiltration in MESO (Figure 2A). Considerable evidence has shown that SPARC mediates the TGF-β1 signaling pathway in different cancer types. TCGA data also showed a strong positive correlation between gene co-expression of SPARC and TGF-β1/TGF-βR1 (Figure 2B,C) in MESO and other cancer types (http://cbiolportal.org/ accessed on 1 August 2022). TGF-β1-induced EMT promotes targeted migration of breast cancer cells towards lymphatic vessels [15]. SPARC is a key mediator of TGF-β1-induced renal cancer metastasis [16]. SPARC acts as a mediator of TGF-β1 in promoting EMT in lung cancer [17].
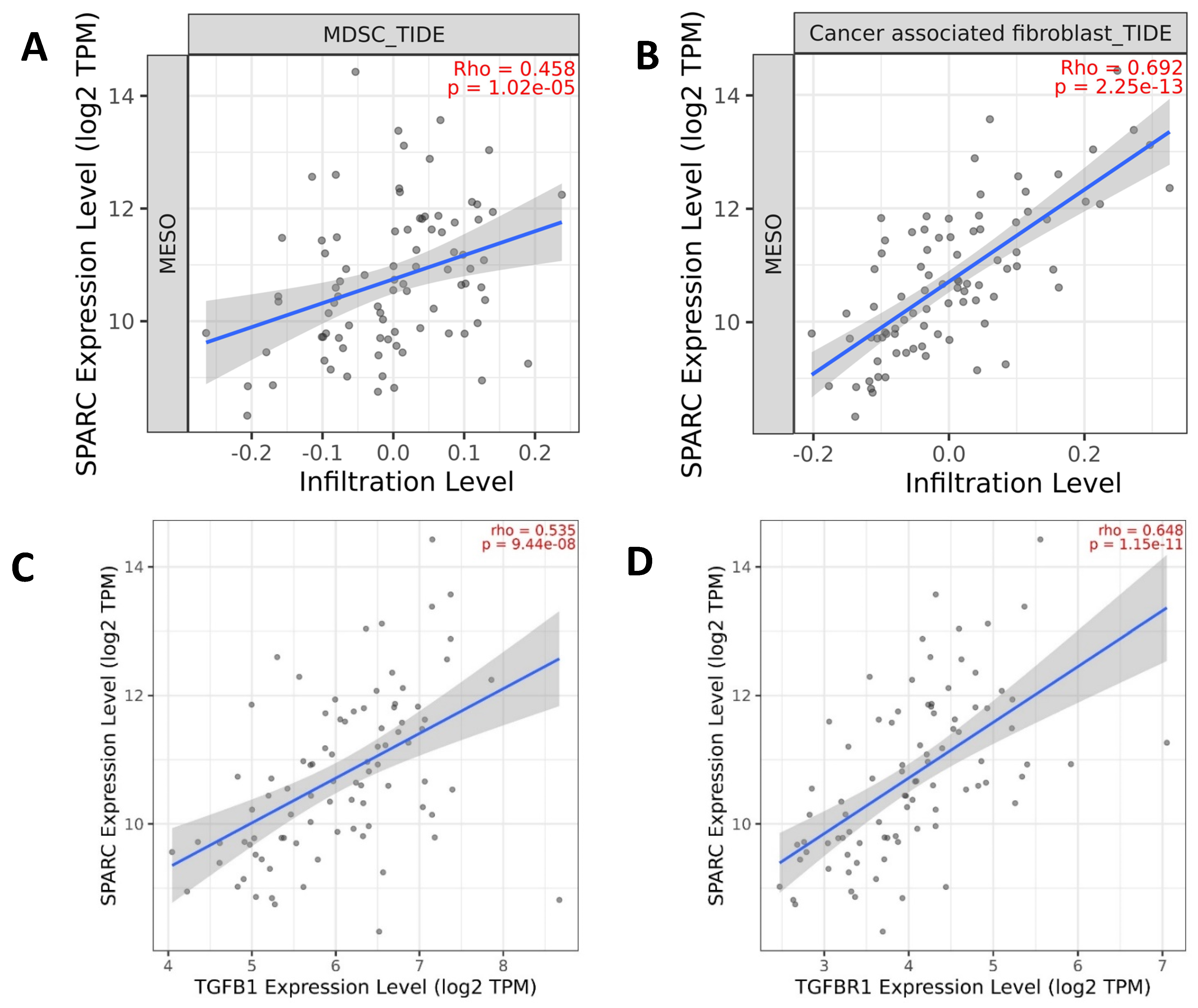
Figure 2. Gene expression of the EMT gene SPARC is correlated with immunosuppressive components. (A,B) Infiltration levels of myeloid-derived suppressor cells (MDSCs) and cancer-associated fibroblasts (CAFs) are positively correlated with SPARC gene expression; (C,D) TGFB1/TGFBR1 gene expression is positively correlated with SPARC gene expression. The graphs were generated based on the TCGA database from www.cbiolportal.org accessed on 1 May 2022 and http://timer.cistrome.org/ accessed on 1 May 2022.
The function of SPARC has been shown to act as a mediator of fibrosis [18]. A recent study demonstrated that SPARC in cancer-associated fibroblasts (CAFs) is an independent indicator for poor prognosis in non-metastatic triple-negative breast cancer and exhibits pro-tumor activity, suggesting that patients with SPARC-expressing CAFs could be eligible for anti-SPARC targeted therapy [19]. TCGA data also showed a strong positive correlation between gene co-expression of SPARC and CAF infiltration in MESO (Figure 2D). TGF-β1-activated CAFs promote breast cancer invasion, metastasis, and EMT [20]. EMT is induced by various signaling pathways, including TGF-β1, NOTCH, and receptor tyrosine kinases. EMT is a crucial mechanism governing the current classification of epithelioid, biphasic, and sarcomatoid diffuse MESO. The mesenchymal epithelial transition factor gene (c-MET) encodes a transmembrane protein c-MET receptor tyrosine, which is primarily activated by its ligand, hepatocyte growth factor (HGF), also known as scatter factor. HGF binding to the c-MET receptor leads to the activation of PI3K/AKT, MAPK/ERK, and STAT pathways involved in cell proliferation, motility, and angiogenesis in MESO and other types of cancer. Upregulation of MET and mutation in the c-MET Semaphorin (SEMA) domain was observed in more aggressive epithelioid MESO [21]. Moreover, the association of MET and SPARC has been observed in different tumors such as esophageal carcinomas, as described by H. Porte et al. [22]. Using single-cell RNA sequencing to investigate EMT signaling cross-talk and gene regulatory networks, Deshmukh et al. found that the NOTCH signaling pathway acts as a key driver of TGF-β-induced EMT [23].
Dysregulated fatty acid metabolism may contribute to tumorigenesis through interaction with oncogenic signaling. Bellenghi M et al. demonstrated that the stearoyl-CoA desaturating enzyme SCD5 overexpresses in a metastatic clone of 4T1 murine breast cancer cells. Their results showed SCD5-driven reprogramming of fatty acid metabolism was able to block SPARC secretion and eventually reverse the EMT process. More intriguingly, variation in the fatty acid profile by SCD5-gene transduction or the direct administration of oleic acid reduced the immune suppressive activity of MDSCs, eventually enhancing T cell activation. A less immunosuppressive microenvironment generated by SCD5 overexpression was enhanced in SPARC-KO mice, indicating that both extracellular and endogenous SPARC additively regulate MDSCs’ suppressive activities [24].
4. SPARC and Cancer Cell Stemness
The SPARC-secreted glycoprotein is produced in various tissues and cells [25]. Studies have shown that SPARC is associated with increased cell motility, invasion, and migration, as well as increased resistance to chemotherapy and radiation [26]. Additionally, SPARC is believed to be involved in the invasion and metastasis of MESO and is known to be upregulated in MESO tumors [8]. Evidence has shown that SPARC promotes self-renewal of limbal epithelial stem cells (LESCs) and ocular surface restoration through JNK and p38-MAPK signaling pathways. Zhou et al. confirmed that the secreted protein SPARC was able to promote the proliferation and suppress the spontaneous differentiation of LESCs in vitro [27]. On the contrary, loss of SPARC could protect hematopoietic stem cells from chemotherapy toxicity by accelerating their return to quiescence [28]. As a matricellular protein SPARC, secreted by a clonal tumor cell subpopulation displaying non-cancer stem cell (CSC) properties in prostate cancer, is a paracrine factor exerted on a distinct tumor cell subpopulation enriched in CSCs. This paracrine interaction enhanced metastatic behavior of the CSC-enriched cancer cell subpopulation. SPARC is expressed in primary prostate cancer cells and metastatic samples, and thus could be a tumor progression biomarker and a therapeutic target in advanced prostate cancer [29].
Mesenchymal stem cells induce tumor stroma formation and epithelial-mesenchymal transition (EMT) through SPARC expression in colorectal cancer [30]. Targeting cancer stem cell signature gene SMOC-2 overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma [31]. However, it remains unknown whether the SPARC gene is a promoter of mesothelioma cell stemness.
5. SPARC May Be a Potential Therapeutic Target in Mesothelioma
The SPARC protein has two calcium binding sites. A study showed that treatment of calcium glucoheptonate resulted in increased proliferation and calcium uptake in the MG-63 cells and elevated expression of osteopontin and osteogenic genes such as collagen-1, SPARC, and osteocalcin [32].
Small molecules such as calcium citrate and calcium phosphate have been used to target SPARC by inducing overexpression (https://go.drugbank.com/drugs/ accessed on 1 May 2022). Therefore, inhibition of SPARC gene by interrupting the EMT process would be a therapeutic approach to MESO treatment.
As stated above, some miRs may target the SPARC gene, leading to an inhibition of its function. For instance, MIR193A and MIR652 genes’ knock-in may function as a tumor suppressor by inhibiting SPARC function in the EMT phenotype of MESO. Neutralization of SPARC function by monoclonal antibody could be another potential MESO treatment [12]
References
- Wu, L.; Yoshihara, K.; Yun, H.; Karim, S.; Shokri, N.; Zaeimi, F.; Man, H.S.J.; Zia, A.; Felley-Bosco, E.; de Perrot, M. Prognostic Value of EMT Gene Signature in Malignant Mesothelioma. Int. J. Mol. Sci. 2023, 24, 4264.
- Kao, S.C.; Kirschner, M.B.; Cooper, A.W.; Tran, T.; Burgers, S.; Wright, C.; Korse, T.; Broek, D.V.D.; Edelman, J.; Vallely, M.; et al. A proteomics-based approach identifies secreted protein acidic and rich in cysteine as a prognostic biomarker in malignant pleural mesothelioma. Br. J. Cancer 2016, 114, 524–531.
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433.
- Morani, F.; Bisceglia, L.; Rosini, G.; Mutti, L.; Melaiu, O.; Landi, S.; Gemignani, F. Identification of Overexpressed Genes in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2021, 22, 2738.
- Ollila, H.; Paajanen, J.; Wolff, H.; Ilonen, I.; Sutinen, E.; Välimäki, K.; Östman, A.; Anttila, S.; Kettunen, E.; Räsänen, J.; et al. High tumor cell platelet-derived growth factor receptor beta expression is associated with shorter survival in malignant pleural epithelioid mesothelioma. J. Pathol. Clin. Res. 2021, 7, 482–494.
- Shi, S.; Ma, H.Y.; Han, X.Y.; Sang, Y.Z.; Yang, M.Y.; Zhang, Z.G. Prognostic Significance of SPARC Expression in Breast Cancer: A Meta-Analysis and Bioinformatics Analysis. BioMed Res. Int. 2022, 2022, 8600419.
- Li, X.L.; Li, J.L.; Qiu, D.J.; Ma, L. Methylation-mediated expression of SPARC is correlated with tumor progression and poor prognosis of breast cancer. Neoplasma 2022, 69, 794–806.
- Gao, Y.; Yin, S.P.; Xie, X.S.; Xu, D.D.; Du, W.D. The relationship between stromal cell derived SPARC in human gastric cancer tissue and its clinicopathologic significance. Oncotarget 2017, 8, 86240–86252.
- Li, L.; Zhu, Z.; Zhao, Y.; Zhang, Q.; Wu, X.; Miao, B.; Cao, J.; Fei, S. FN1, SPARC, and SERPINE1 are highly expressed and significantly related to a poor prognosis of gastric adenocarcinoma revealed by microarray and bioinformatics. Sci. Rep. 2019, 9, 7827.
- Chang, C.H.; Yen, M.C.; Liao, S.H.; Hsu, Y.L.; Lai, C.S.; Chang, K.P.; Hsu, Y.L. Secreted Protein Acidic and Rich in Cysteine (SPARC) Enhances Cell Proliferation, Migration, and Epithelial Mesenchymal Transition, and SPARC Expression is Associated with Tumor Grade in Head and Neck Cancer. Int. J. Mol. Sci. 2017, 18, 1556, Erratum in: Int. J. Mol. Sci. 2018, 19, 2338.
- Vaz, J.; Ansari, D.; Sasor, A.; Andersson, R. SPARC: A Potential Prognostic and Therapeutic Target in Pancreatic Cancer. Pancreas 2015, 44, 1024–1035.
- Yang, X.; Xia, Y.; Wang, S.; Sun, C. Prognostic value of SPARC in hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0273317.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Sangaletti, S.; Talarico, G.; Chiodoni, C.; Cappetti, B.; Botti, L.; Portararo, P.; Gulino, A.; Consonni, F.M.; Sica, A.; Randon, G.; et al. SPARC Is a New Myeloid-Derived Suppressor Cell Marker Licensing Suppressive Activities. Front. Immunol. 2019, 10, 1369.
- Pang, M.F.; Georgoudaki, A.-M.; Lambut, L.; Johansson, E.J.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Nelson, C.M.; et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016, 35, 748–760.
- Bao, J.; Dang, Q.; Lin, C.; Lo, U.; Feldkoren, B.; Dang, A.; Hernandez, E.; Li, F.; Panwar, V.; Lee, C.; et al. SPARC is a key mediator of TGF-β-induced renal cancer metastasis. J. Cell. Physiol. 2021, 236, 1926–1938.
- Sun, W.; Feng, J.; Yi, Q.; Xu, X.; Chen, Y.; Tang, L. SPARC acts as a mediator of TGF-β1 in promoting epithelial-to-mesenchymal transition in A549 and H1299 lung cancer cells. Biofactors 2018, 44, 453–464.
- Trombetta-Esilva, J.; Bradshaw, A.D. The Function of SPARC as a Mediator of Fibrosis. Open Rheumatol. J. 2012, 6, 146–155.
- Alcaraz, L.B.; Mallavialle, A.; Mollevi, C.; Boissière-Michot, F.; Mansouri, H.; Simony-Lafontaine, J.; Laurent-Matha, V.; Chardès, T.; Jacot, W.; Turtoi, A.; et al. SPARC in cancer-associated fibroblasts is an independent poor prognostic factor in non-metastatic triple-negative breast cancer and exhibits pro-tumor activity. Int. J. Cancer 2022, 152, 1243–1258.
- Huang, M.; Fu, M.; Wang, J.; Xia, C.; Zhang, H.; Xiong, Y.; He, J.; Liu, J.; Liu, B.; Pan, S.; et al. TGF-β1-activated cancer-associated fibroblasts promote breast cancer invasion, metastasis and epithelial-mesenchymal transition by autophagy or overexpression of FAP-α. Biochem. Pharmacol. 2021, 188, 114527.
- Belfiore, A.; Busico, A.; Bozzi, F.; Brich, S.; Dallera, E.; Conca, E.; Capone, I.; Gloghini, A.; Volpi, C.C.; Cabras, A.D.; et al. Molecular Signatures for Combined Targeted Treatments in Diffuse Malignant Peritoneal Mesothelioma. Int. J. Mol. Sci. 2019, 20, 5817.
- Porte, H.; Triboulet, J.P.; Kotelevets, L.; Carrat, F.; Prévot, S.; Nordlinger, B.; Digioia, Y.; Wurtz, A.; Comoglio, P.; Gespach, C.; et al. Overexpression of stromelysin-3, BM-40/SPARC, and MET genes in human esophageal carcinoma: Implications for prognosis. Clin. Cancer Res. 1998, 4, 1375–1382.
- Deshmukh, A.P.; Vasaikar, S.V.; Tomczak, K.; Tripathi, S.; Hollander, P.D.; Arslan, E.; Chakraborty, P.; Soundararajan, R.; Jolly, M.K.; Rai, K.; et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc. Natl. Acad. Sci. USA 2021, 118, e2102050118.
- Bellenghi, M.; Talarico, G.; Botti, L.; Puglisi, R.; Tabolacci, C.; Portararo, P.; Piva, A.; Pontecorvi, G.; Carè, A.; Colombo, M.P.; et al. SCD5-dependent inhibition of SPARC secretion hampers metastatic spreading and favors host immunity in a TNBC murine model. Oncogene 2022, 41, 4055–4065.
- Yan, Q.; Sage, E.H. SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. 1999, 47, 1495–1505.
- Bhoopathi, P.; Gorantla, B.; Sailaja, G.S.; Gondi, C.S.; Gujrati, M.; Klopfenstein, J.D.; Rao, J.S. SPARC overexpression inhibits cell proliferation in neuroblastoma and is partly mediated by tumor suppressor protein PTEN and AKT. PLoS ONE 2012, 7, e36093.
- Zhu, J.; Wang, L.-Y.; Li, C.-Y.; Wu, J.-Y.; Zhang, Y.-T.; Pang, K.-P.; Wei, Y.; Du, L.-Q.; Liu, M.; Wu, X.-Y. SPARC promotes self-renewal of limbal epithelial stem cells and ocular surface restoration through JNK and p38-MAPK signaling pathways. Stem Cells 2020, 38, 134–145.
- Ehninger, A.; Boch, T.; Medyouf, H.; Müdder, K.; Orend, G.; Trumpp, A. Loss of SPARC protects hematopoietic stem cells from chemotherapy toxicity by accelerating their return to quiescence. Blood 2014, 123, 4054–4063.
- Mateo, F.; Meca-Cortés, O.; Celià-Terrassa, T.; Fernández, Y.; Abasolo, I.; Sánchez-Cid, L.; Bermudo, R.; Sagasta, A.; Rodríguez-Carunchio, L.; Pons, M.; et al. SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations. Mol. Cancer 2014, 13, 237.
- Naito, T.; Yuge, R.; Kitadai, Y.; Takigawa, H.; Higashi, Y.; Kuwai, T.; Kuraoka, K.; Tanaka, S.; Chayama, K. Mesenchymal stem cells induce tumor stroma formation and epithelial-mesenchymal transition through SPARC expression in colorectal cancer. Oncol. Rep. 2021, 45, 104.
- Lu, H.; Ju, D.-D.; Yang, G.-D.; Zhu, L.-Y.; Yang, X.-M.; Li, J.; Song, W.-W.; Wang, J.-H.; Zhang, C.-C.; Zhang, Z.-G.; et al. Targeting cancer stem cell signature gene SMOC-2 Overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma. EBioMedicine 2019, 40, 276–289.
- Modi, P.K.; Prabhu, A.; Bhandary, Y.P.; Shenoy, P.S.; Hegde, A.; Es, S.P.; Johnson, R.P.; Das, S.P.; Vazirally, S.; Rekha, P.D. Effect of calcium glucoheptonate on proliferation and osteogenesis of osteoblast-like cells in vitro. PLoS ONE 2019, 14, e0222240.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
680
Revisions:
2 times
(View History)
Update Date:
27 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





